Abstract
Background
Observational studies have demonstrated an association between nephrolithiasis and hypertension. The aim of this meta-analysis was to summarize all available evidence.
Methods
PubMed, EMBASE, the Cochrane Central Register of Controlled Trials databases, and the reference lists of relevant articles were searched to identify observational studies that reported study-specific risk estimates comparing the risk of hypertension in patients with nephrolithiasis. We used a random-effect model to pool the study-specific risk estimates. We also assessed the potential heterogeneity by subgroup analyses, meta-regression analyses, and sensitivity analyses.
Results
A total of 7 articles including 9 studies (n = 313,222 participants) were eventually identified in this meta-analysis. In comparison with the patients who did not have nephrolithiasis, nephrolithiasis significantly increased the risk of hypertension (OR, 1.43; 95% CI, 1.30–1.56), with significant heterogeneity between these studies (I 2 = 83.5%, P <0.001). The heterogeneity reduced in subgroups of cohort studies, USA, large sample size trials, men, and adjustment for confounding factors ≥ 5. Sensitivity analysis further demonstrated the results to be robust.
Conclusions
Nephrolithiasis is associated with increased risk of hypertension. Future randomized, high-quality clinical trials are encouraged to definitively clarify the relationship between nephrolithiasis and hypertension, which may influence clinical management and primary prevention of hypertension in nephrolithiasis patients.
Electronic supplementary material
The online version of this article (10.1186/s12882-017-0762-8) contains supplementary material, which is available to authorized users.
Keywords: Kidney stone, Nephrolithiasis, Hypertension, Meta-analysis
Background
Nephrolithiasis is a common condition, with the prevalence varying by age and sex. The disease usually presents in men aged 60 to 69, with a prevalence rate of approximately 1.7 to 8.8% worldwide [1, 2]. Hypertension is defined as persistent elevation of systematic arterial blood pressure (systolic pressure ≥ 140 mmHg and/or diastolic pressure ≥ 90 mmHg). It is an extremely common cardiovascular disease, affecting over 30% of young adults and 70% of elderly individuals [3]. Hypertension is also a silent yet dangerous disease. Despite intensive studies aimed at identifying risk factors for hypertension, the exact pathogenic mechanisms of hypertension often are unclear. Nevertheless, there is growing evidence supporting that nephrolithiasis largely contribute to the occurrence of hypertension. Since the association between arterial hypertension and nephrolithiasis was described in 1965 for the first time by Tibblin [4], much effort has been devoted to this field. Data from several observational studies suggested a risk of hypertension in nephrolithiasis patients of 1.24–1.96 compared to the general population [5–11]. A previous review performed by Cupisti et al. [12] has shown the current understanding of the potential link between nephrolithiasis and the occurrence of hypertension, but no meta-analysis has been used to examine the relationship.
Given the fact that individual studies may have insufficient statistical power because of sample size, we performed a meta-analysis to collect all beneficial evidence to assess the risk of hypertension among nephrolithiasis patients, which may emphasize the importance of considering additional intervention methods in this area.
Methods
Our study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement checklist [13].
Search strategy
PubMed, EMBASE, and Cochrane Library databases were searched for observational studies to March 18, 2017. The used search terms were as follows: “renal stones” or “renal stone” or “kidney stones” or “kidney stone” or “nephrolithiasis” or “calculi” and “hypertension” or “blood pressure” and “risk” or “incidence” or “epidemiology”. Furthermore, we searched reference lists of all included studies for additional eligible studies. Two of the authors (WS and YL) independently screened titles and abstracts, analyzed full-text articles, and ascertained the final eligible records. Conflicting results were resolved by discussion. We merged retrieved citations using EndNote X7.
Inclusion and exclusion criteria
The inclusion criteria were: (1) the study design was a cross-sectional, case–control, or cohort study; (2) identified nephrolithiasis as exposure, including medical records, questionnaire, direct interview etc.; (3) the outcome measure was hypertension, including medical records, questionnaire, blood pressure measurement, direct interview etc.; and (4) odds ratio (OR) or hazard ratio (HR) or risk ratio (RR), and the corresponding 95% confidence interval (CI) were reported or could be calculated. Reviews, letters, case reports, and animal studies were excluded.
Data extraction
Study characteristics were extracted by two authors (WS and YL) separately as follows: first author’s name, publication year, country origin, study design, sample, average age, proportion of men, method of nephrolithiasis and hypertension diagnosis, and adjustment factors. When needed, we contacted the original author for clarification.
Quality assessment
We evaluated the quality of studies using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [14]. Two authors (WS and YR) performed the quality assessment independently and disagreements were resolved by discussion.
Statistical analyses
The study-specific most adjusted HR, RR or OR was used to compute a summary OR and its 95%CI. HRs and RRs were directly considered as ORs [15]. Heterogeneity of ORs among studies was assessed using the Chi-squared based on Q-statistic test (P < 0.10) and quantified by I2 statistic. I2 values were considered to represent insignificant (0–25%), low (26–50%), moderate (51–75%), and high (>75%) heterogeneity [16]. The random-effects model was used to calculate the combined risk estimates. Subgroup analysis and univariable random effects meta-regression were further conducted to explore the potential source of heterogeneity. Stratified analyses were conducted based on study design (cohort or cross-sectional), region (USA or non-USA), sample size (<35,000 or ≥35,000), gender (men or women), and the number of confounders adjusted for (<5 or ≥5). We conducted sensitivity analyses to assess the influence of a study on the pooled effect estimate by recalculating the pooled OR with removal of one study in each turn. Reporting bias was evaluated using Egger’s test [17]. All meta-analyses were performed by the STATA (version 10.0, Stata Corporation, College Station, TX, USA). P < 0.05 in 2-tailed test was considered to be statistically significant.
Results
Study selection, characteristics, and quality
As shown in Fig. 1, our literature search returned 2327 results for relevant articles, and the full text retrieved for 85 articles. Finally, we identified 9 observational studies, based on 7 articles.
Fig. 1.
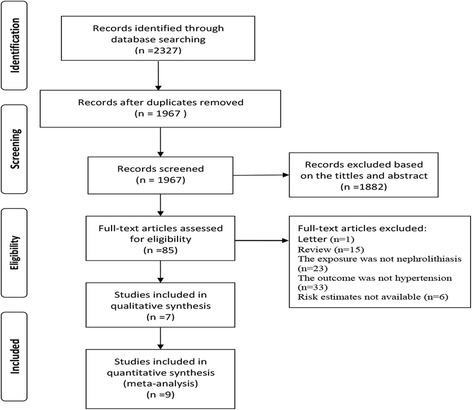
Flow chart of study selection
The main characteristic of the studies included are presented in Table 1. Included studies were published during 1998–2017. These articles included 4 cohort studies, and 5 cross-sectional studies. Of these studies, six were conducted in United States, one in Italy, one in Portugal, and one in Japan. The primary analysis included data for 313,222 participants derived from 9 observational studies that reported an association between nephrolithiasis and the risk of hypertension.
Table 1.
Characteristics of the identified studies
| First author Year | Country | Design | Sample | Average age (y) | Men (%) | Definition of Kidney stones | Confirmation of Hypertension | Adjustments |
|---|---|---|---|---|---|---|---|---|
| Madore et al. (1) 1998(cohort) | USA | cohort | 67,745 | 51.5 | 0.0% | medical records | physician diagnosed hypertension | age (in 5-year categories),BMI (in quintiles), dietary intake of calcium, sodium, potassium, magnesium, and caffeine (in quintiles), and intake of alcohol (in eight categories) |
| Madore et al. (1) 1998(cross-sectional) | USA | cross-sectional | 89,376 | NA | 0.0% | medical records | physician diagnosed hypertension | age |
| Madore et al. (2) 1998(cohort) | USA | cohort | 37,809 | 53.2 | 100.0% | initial questionnaire | physician diagnosed hypertension | age, BMI and the intake of calcium, sodium, potassium, magnesium, and alcohol |
| Madore et al. (2) 1998(cross-sectional) | USA | cross-sectional | 51,529 | NA | 100.0% | initial questionnaire | physician diagnosed hypertension | age |
| Strazzullo et al. 2001 [7] | Italy | cohort | 381 | 45.1 | 100.0% | fixed-sequence questionnaire | examination | age |
| Gillen et al. 2005 [8] | USA | cross-sectional | 20,029 | 43.6 | 46.6% | patient reported | self-reported previous diagnosis of hypertension, SBP, DBP, and pulse pressure calculated as the difference between SBP and DBP | age, sex, race (African American versus other), BMI, history of CVD (myocardial infarction, stroke, congestive heart failure), diabetes, and smoking status (ever versus never), dietary intake, insurance status, alcohol use, household income, and marital status. |
| Domingos et al. 2011 [9] | Portugal | cross-sectional | 23,349 | NA | NA | direct interview | direct interview | age and BMI |
| Ando et al. 2012 [10] | Japan | cross-sectional | 20,990 | NA | NA | NA | NA | overweight, hypertension and hyperuricemia |
| Kittanamongkolchai et al. 2017 [11] | USA | cohort | 2014 | 41.5 | 52.9% | chart review | medical records | age, sex, BMI, serum creatinine, CKD, diabetes, gout, coronary artery disease, dyslipidemia, tobacco use, and alcohol abuse |
Note: NA not avaliable, BMI body mass index, CVD cardiovascular disease, CKD chronic kidney disease, SBP systolic BP, DBP diastolic BP
According to the STROBE, all but one included studies were of high quality (Additional file 1: Table S1).
Nephrolithiasis and risk of hypertension
As shown in Fig. 2, the multivariate-adjusted OR of hypertension within the 9 individual study populations ranged between 1.24 and 1.96, with an overall multivariate-adjusted OR of 1.43 (95% CI, 1.30–1.56). Significant heterogeneity was observed (I 2 = 83.5%, P <0.001).
Fig. 2.
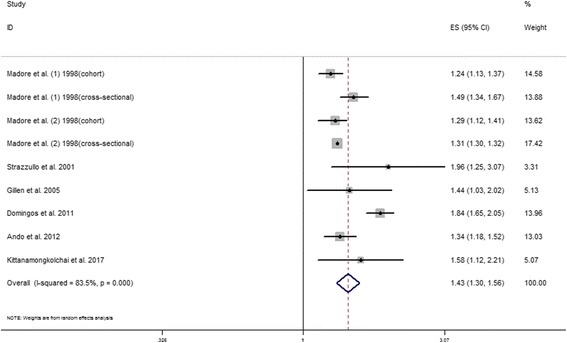
Risk of hypertension in nephrolithiasis compared with controls
Subgroup analyses
In most cases, low-to-high heterogeneity was still present in stratified analyses unless adjustment for confounding factors was more than 5 (I 2 = 0%). We used meta-regression to explore the sources of heterogeneity and found that study design, resign, sample size, gender, and adjustment for confounding factors may be potential sources of heterogeneity (Table 2).
Table 2.
Subgroup analyses of hypertension in patients with kidney stones
| Subgroup | No. of studies | OR (95% CI) | I2 (%) | P a | P b |
|---|---|---|---|---|---|
| Study design | |||||
| Cohort | 4 | 1.33 (1.18, 1.49) | 43.8 | 0.148 | 0.518 |
| Cross-sectional | 5 | 1.47 (1.28, 1.70) | 90.7 | <0.001 | |
| Region | |||||
| USA | 5 | 1.33 (1.26, 1.40) | 37.8 | 0.154 | 0.106 |
| Non-USA | 3 | 1.64 (1.26, 2.14) | 86.3 | 0.001 | |
| Sample size | |||||
| < 35,000 | 5 | 1.59 (1.32, 1.92) | 73.4 | 0.005 | 0.079 |
| ≥ 35,000 | 4 | 1.32 (1.25, 1.40) | 54.3 | 0.087 | |
| Gender | |||||
| Men | 4 | 1.31 (1.25, 1.37) | 13.4 | 0.326 | 0.547 |
| Women | 3 | 1.43 (1.21, 1.69) | 78.2 | 0.010 | |
| Adjustment for confounding factors | |||||
| < 5 | 5 | 1.51 (1.30, 1.76) | 91.2 | <0.001 | 0.275 |
| ≥ 5 | 4 | 1.32 (1.14, 1.52) | 0 | 0.497 | |
OR odds ratio, CI confidence interval
a P value for heterogeneity among studies assessed with Cochran’s Q test
b P value for interaction evaluated by meta-regression models
Sensitivity analyses and reporting bias
Sensitivity analyses were performed by excluding one study at a time. And they indicated that the omission of any of the studies led to changes in estimates between 1.34 (95% CI: 1.27–1.40) and 1.47 (95% CI: 1.32–1.64). The changes were not significant. However, deletion of the Domingos et al.’s study reduced the heterogeneity from high to low levels (Additional file 2: Table S2). The P value of Egger’s test was 0.134, suggesting that there was no publication bias statistically.
Discussion
To the best of our knowledge, this study is the first meta-analysis to present hypertension risk in patients with a history of nephrolithiasis. We confirmed nephrolithiasis was associated with an increased risk of hypertension. The risk of hypertension remained pronounced in all subgroups.
As with other published meta-analyses of this type [18, 19], our study has a high level of heterogeneity. We constructed subgroup analyses and meta-regression analyses to explore sources of heterogeneity. In subgroup analyses, the heterogeneity reduced in patients within cohort studies, USA, large sample size trials, men, and adjustment for confounding factors ≥ 5. This conclusion is supported by the results of the meta-regression, which showed that study design, resign, sample size, gender, and adjustment for confounding factors may be potential sources of heterogeneity. In addition, different follow-up time and adjust factors may be also the source of heterogeneity.
Note that female patients with kidney stones showed much higher risk for hypertension than male patients in our study. The underlying pathophysiology remains unclear. However, the differences by sex are not infrequent. A similar finding has also been observed in a meta-analysis on the association of nephrolithiasis and risk of incident chronic kidney disease (CKD) [18]. Similarly, a review also demonstrated a statistically significant increased risk of coronary heart disease in female patients with prior nephrolithiasis, but there was no significant association in male patients with prior nephrolithiasis [20]. Of note, Madore et al.’s study indicated that both women and men with hypertension at baseline were not more likely to develop nephrolithiasis during the follow-up [5, 6]. Due to the limited data, further studies are needed to direct the sex difference in hypertension response to nephrolithiasis.
The relation between nephrolithiasis and hypertension is rather unclear, but after our complete literature retrieval, we found several potential reasons which may explain the observed associations. First, alterations in calcium metabolism maybe have an important role in the pathogenesis of both nephrolithiasis and hypertension [21, 22]. Second, the traits of metabolic syndrome are factors highly prevalent in hypertensives as well as in kidney stone formers, so insulin resistance may be a common pathophysiological mechanism [23, 24]. Third, CKD is a condition which may occur more frequently in nephrolithiasis patients and in hypertensive patients. Therefore, CKD may be another factor involved in the linkage between nephrolithiasis and hypertension. Finally, inflammation and oxidative stress have been recently hypothesized as possible links between stone disease and hypertension [25]. Obviously, all of these potential reasons are comorbidities in nephrolithiasis and hypertension. However, more medical research is needed to explore and test the relevant presumption.
Several limitations of this meta-analysis should be pointed out. First, significant heterogeneity was detected in the nephrolithiasis and hypertension, the differences in characteristics of populations, study designs, sample size, men (%), diagnostic criteria, and adjusted confounders may contribute to the high heterogeneity. For example, the diagnosis of hypertension was inferred from self-reported blood pressure or patient questionnaire in two cross-sectional studies (Gillen et al. [8] and Domingos et al. [9]), which may bias the true incidence of hypertension. Second, we had no access to the information on the total number or type of nephrolithiasis. Therefore, we could not evaluate the association between different types of nephrolithiasis and hypertension. Third, most of the studies included were partially representatives of western countries, and thus extrapolating results to other parts of the world should be interpreted cautiously. Fourth, no publication bias was detected statistically in our study, but potential publication bias could not be completely ignored, given the fact that studies with null results tend not to be published. Finally, although all the included studies controlled for several known risk factors for hypertension, residual confounding cannot be excluded because the results of our study were based on observational studies.
Conclusions
Our study demonstrates that nephrolithiasis is significantly associated with increased risk of hypertension. Well-designed randomized controlled trials are necessary to elucidate the underlying mechanism and will provide more effective preventive and therapeutic measures. Our study has important implications for public health, which emphasizes that clinicians pay attention to the potential association between nephrolithiasis and hypertension.
Additional files
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement scores of the included published studies. (DOCX 17 kb)
Sensitivity analysis. (DOC 28 kb)
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Availability of data and materials
All data that support the conclusions of this manuscript are included within the article.
Abbreviations
- CI
95% confidence
- HR
Hazard ratio
- NOS
Newcastle-ottawa scale
- OR
Odds ratio
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- RR
Risk ratio
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
Authors’ contributions
DJW conceived and designed the study. SWF, RYL, and LYY screened the abstract and full text, extracted data, assessed studies and drafted the manuscript. SWF, LYY, YY, and RYL performed statistical analyses. LH, and DJW revised the manuscript. All authors read the manuscript and approved the final version.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12882-017-0762-8) contains supplementary material, which is available to authorized users.
Contributor Information
Weifeng Shang, Email: 18771031327@163.com.
Yuanyuan Li, Email: 18207190110@163.com.
Yali Ren, Email: rylkong@126.com.
Yi Yang, Email: yangyi0427@126.com.
Hua Li, Email: 15327238286@163.com.
Junwu Dong, Email: junwudongwuhan@163.com.
References
- 1.Lieske JC, Pena de la Vega LS, Slezak JM, Bergstralh EJ, Leibson CL, Ho KL, Gettman MT. Renal stone epidemiology in Rochester, Minnesota: an update. Kidney Int. 2006;69(4):760–764. doi: 10.1038/sj.ki.5000150. [DOI] [PubMed] [Google Scholar]
- 2.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abel N, Contino K, Jain N, Grewal N, Grand E, Hagans I, Hunter K, Roy S. Eighth joint National Committee (JNC-8) guidelines and the outpatient Management of Hypertension in the African-American population. N Am J Med Sci. 2015;7(10):438–445. doi: 10.4103/1947-2714.168669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tibblin G. A population study of 50-year-old men. An analysis of the non-participation group. Acta Med Scand. 1965;178(4):453–459. doi: 10.1111/j.0954-6820.1965.tb04290.x. [DOI] [PubMed] [Google Scholar]
- 5.Madore F, Stampfer MJ, Willett WC, Speizer FE, Curhan GC. Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis. 1998;32(5):802–807. doi: 10.1016/S0272-6386(98)70136-2. [DOI] [PubMed] [Google Scholar]
- 6.Madore F, Stampfer MJ, Rimm EB, Curhan GC. Nephrolithiasis and risk of hypertension. Am J Hypertens. 1998;11(1 Pt 1):46–53. doi: 10.1016/S0895-7061(97)00371-3. [DOI] [PubMed] [Google Scholar]
- 7.Strazzullo P, Barba G, Vuotto P, Farinaro E, Siani A, Nunziata V, Galletti F, Mancini M, Cappuccio FP. Past history of nephrolithiasis and incidence of hypertension in men: a reappraisal based on the results of the Olivetti prospective heart study. Nephrol Dial Transplant. 2001;16(11):2232–2235. doi: 10.1093/ndt/16.11.2232. [DOI] [PubMed] [Google Scholar]
- 8.Gillen DL, Coe FL, Worcester EM. Nephrolithiasis and increased blood pressure among females with high body mass index. Am J Kidney Dis. 2005;46(2):263–269. doi: 10.1053/j.ajkd.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 9.Domingos F, Serra A. Nephrolithiasis is associated with an increased prevalence of cardiovascular disease. Nephrol Dial Transplant. 2011;26:864–868. doi: 10.1093/ndt/gfq501. [DOI] [PubMed] [Google Scholar]
- 10.Ando R, Nagaya T, Suzuki S, Okada A, Yasui T, Tozawa K, Takahashi H, Kawai M, Kohri K. Positive associations of current and past history of kidney stones with overweight, hypertension, hyperuricemia and chronic kidney disease in a screened population. Urology. 2012;1:S51. [Google Scholar]
- 11.Kittanamongkolchai W, Mara KC, Mehta RA, Vaughan LE, Denic A, Knoedler JJ, Enders FT, Lieske JC, Rule AD. Risk of hypertension among first-time symptomatic kidney stone formers. Clin J Am Soc Nephrol. 2017;12(3):476–482. doi: 10.2215/CJN.06600616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cupisti A, D'Alessandro C, Samoni S, Meola M, Egidi MF. Nephrolithiasis and hypertension: possible links and clinical implications. J Nephrol. 2014;27(5):477–482. doi: 10.1007/s40620-014-0068-x. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England) 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 15.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Research ed) 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research ed) 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang W, Li L, Ren Y, Ge Q, Ku M, Ge S, Xu G. History of kidney stones and risk of chronic kidney disease: a meta-analysis. PeerJ. 2017;5:e2907. doi: 10.7717/peerj.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi HB, Tang B, Liu YW, Wang XF, Chen GJ. Alzheimer disease and cancer risk: a meta-analysis. J Cancer Res Clin Oncol. 2015;141(3):485–494. doi: 10.1007/s00432-014-1773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferraro PM, Taylor EN, Eisner BH, Gambaro G, Rimm EB, Mukamal KJ, Curhan GC. History of kidney stones and the risk of coronary heart disease. JAMA. 2013;310(4):408–415. doi: 10.1001/jama.2013.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strazzullo P, Nunziata V, Cirillo M, Giannattasio R, Ferrara LA, Mattioli PL, Mancini M. Abnormalities of calcium metabolism in essential hypertension. Clin Sci (London, England : 1979) 1983;65(2):137–141. doi: 10.1042/cs0650137. [DOI] [PubMed] [Google Scholar]
- 22.Strazzullo P, Mancini M. Hypertension, calcium metabolism, and nephrolithiasis. Am J Med Sci. 1994;307(Suppl 1):S102–S106. [PubMed] [Google Scholar]
- 23.Obligado SH, Goldfarb DS. The association of nephrolithiasis with hypertension and obesity: a review. Am J Hypertens. 2008;21(3):257–264. doi: 10.1038/ajh.2007.62. [DOI] [PubMed] [Google Scholar]
- 24.Kohjimoto Y, Sasaki Y, Iguchi M, Matsumura N, Inagaki T, Hara I. Association of metabolic syndrome traits and severity of kidney stones: results from a nationwide survey on urolithiasis in Japan. Am J Kidney Dis. 2013;61(6):923–929. doi: 10.1053/j.ajkd.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 25.Khan SR. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol Res. 2012;40(2):95–112. doi: 10.1007/s00240-011-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement scores of the included published studies. (DOCX 17 kb)
Sensitivity analysis. (DOC 28 kb)
Data Availability Statement
All data that support the conclusions of this manuscript are included within the article.


