Abstract
Lynch syndrome is an autosomal dominant condition caused by pathogenic mutations in the DNA mismatch repair (MMR) genes. Although commonly associated with clinical features such as intellectual disability and congenital anomalies, contiguous gene deletions may also result in cancer predisposition syndromes. We report on a 52-year-old male with Lynch syndrome caused by deletion of chromosome 2p16.3-p21. The patient had intellectual disability and presented with a prostatic adenocarcinoma with an incidentally identified synchronous sigmoid adenocarcinoma that exhibited deficient MMR with an absence of MSH2 and MSH6 protein expression. Family history was unrevealing. Physical exam revealed short stature, brachycephaly with a narrow forehead and short philtrum, brachydactyly of the hands, palmar transverse crease, broad and small feet with hyperpigmentation of the soles. The patient underwent total colectomy with ileorectal anastomosis for a pT3N1 sigmoid adenocarcinoma. Germline genetic testing of the MSH2, MSH6, and EPCAM genes revealed full gene deletions. SNP-array based DNA copy number analysis identified a deletion of 4.8Mb at 2p16.3-p21. In addition to the three Lynch syndrome associated genes, the deleted chromosomal section encompassed genes including NRXN1, CRIPT, CALM2, FBXO11, LHCGR, MCFD2, TTC7A, EPAS1, PRKCE, and 15 others. Contiguous gene deletions have been described in other inherited cancer predisposition syndromes, such as Familial Adenomatous Polyposis. Our report and review of the literature suggests that contiguous gene deletion within the 2p16-p21 chromosomal region is a rare cause of Lynch syndrome, but presents with distinct phenotypic features, highlighting the need for recognition and awareness of this syndromic entity.
Keywords: Lynch syndrome, chromosomal deletion, prostate cancer, colorectal cancer, 2p
INTRODUCTION
Lynch syndrome, an autosomal dominant condition caused by pathogenic mutations in the DNA mismatch repair (MMR) genes (MLH1, MSH2, MSH6, and PMS2) and deletions of the 3′ exons of the EPCAM (TACSTD1) gene, is associated with an increased risk of colorectal, endometrial, ovarian, gastric, and upper urinary tract cancers [1]. The MSH2, MSH6, and EPCAM genes are located on chromosome 2p. The diagnostic process of Lynch syndrome may include tumor immunohistochemical (IHC) staining for MMR protein expression, microsatellite instability (MSI) analysis, and germline genetic testing [1].
Contiguous gene deletion syndromes are the result of a loss of multiple neighboring genes from a particular chromosomal segment. Contiguous gene deletions have been identified in patients with various clinical features such as intellectual disability, developmental delay, and congenital anomalies [2]. Many contiguous gene deletions are the result of de novo events as the associated phenotypes are often complex and lead to decreased reproductive fitness [2].
Although previously reported, contiguous gene deletions are a rare cause of cancer predisposition syndromes [3]. Here, we report on a patient with Lynch syndrome caused by a contiguous gene deletion at chromosome 2p16.3-p21 encompassing the MSH2, MSH6 and EPCAM genes. Along with a review of past reports, we highlight the need for awareness of contiguous gene deletion syndromes as the underlying cause of developmental anomalies and the need to consider cancer risk and management of affected individuals as they age.
METHODS
Clinical Presentation
A 52-year-old male with a history of intellectual disability of unknown etiology was diagnosed with Gleason 7 prostatic adenocarcinoma upon biopsy after a screening PSA measured 22 ng/mL. Bone scan was negative. External beam radiation therapy was planned, but not performed since the patient could not comply with remaining still during the radiation. Upon presenting to our institution for surgical management, a one-month history of bright red blood per the rectum was reported by the patient’s caretaker. Pre-surgical evaluation whole body and prostate magnetic resonance imaging (MRI) revealed concentric thickening of the distal sigmoid colon suspicious for a primary tumor and confined prostatic disease. Flexible sigmoidoscopy identified a partially circumferential non-obstructing large mass in the sigmoid colon; biopsy confirmed invasive poorly differentiated carcinoma. IHC staining for the MMR proteins demonstrated an absence of MSH6. Staging CT of chest, abdomen and pelvis showed no evidence of distant metastasis.
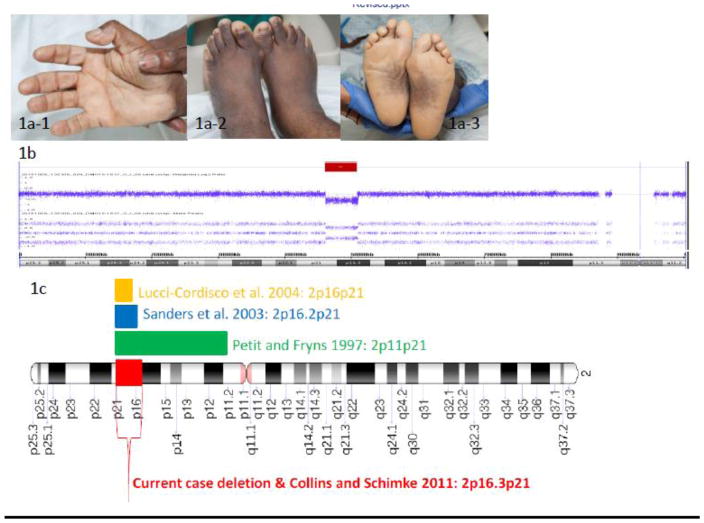
Based on the abnormal MMR IHC result, the patient was referred to the Clinical Genetics Service. A four-generation pedigree was unrevealing for cancer, colorectal polyps, and developmental disorders. The patient has no children. Three brothers and two sisters are alive and well in their 40s/50s. Mother and father are alive and well in their 70s. Consanguinity was denied. The family is of African American ancestry. The patient was born with intellectual disability. Language comprehension is limited to simple commands; verbal expression is limited to a handful of words. He lives with his parents and requires help in all self-care activities. He attends a daily adult program and needs daily supervision and support. Physical exam revealed short stature (4′8″), brachycephaly with a narrow forehead and short philtrum, brachydactyly, palmar transverse crease, fleshy pads at the tips of the fingers, broad and small feet with hyperpigmentation of the soles (Figure 1a). Written informed consent was obtained from the patient’s parent/guardian for use of photographs for publication.
Fig 1.
a) Photographs of the proband demonstrating brachydactyly of fingers and toes (a-1 and a-2), palmar transverse crease (a-1), broad and small feet with hyperpigmentation of the soles (a-3); b) DNA copy number (log2 ratio) and allele peak profile across the p arm of chromosome 2; red bar indicates the interstitial deletion (~4.8Mb) at 2p16.3-p21; c) Chromosome 2 animation demonstrating identified contiguous gene deletions in the current and previously reported cases
Genetic Evaluation
Based upon the presence of MMR-deficiency in the colon tumor, the possibility of Lynch syndrome was raised. A possible association between Lynch syndrome and prostate cancer was also noted. The patient’s mother provided informed consent for germline genetic testing of the MSH6, MSH2, and EPCAM genes and a germline DNA specimen was obtained. Germline genetic testing of the MSH6, MSH2, and EPCAM genes was performed in the MSK Diagnostic Molecular Genetics Laboratory (sequencing and multiplex ligation-dependent probe amplification). Following initial test results, the patient’s mother provided additional informed consent for SNP-array based DNA copy number analysis (Affymetrix CytoScan HD Assay) which was performed at the MSK Clinical Cytogenetics Laboratory.
GENETIC TEST RESULTS
Genetic testing revealed heterozygous full gene deletions of the MSH6, MSH2, and EPCAM genes. Based on this finding, a SNP-array based DNA copy number analysis was performed and identified a deletion of 4.8Mb at 2p16.3-p21. In addition to the three Lynch syndrome associated genes, the deleted chromosomal section includes the following 24 genes: PRKCE, EPAS1, TMEM247, ATP6V1E2, RHOQ, PIGF, CRIPT, SOCS5, LOC388948, LOC100134259, MCFD2, TTC7A, C2orf61, CALM2, MIR559, KCNK12, FBXO11, FOXN2, PPP1R21, STON1, GTF2A1L, LHCGR, FSHR, and NRXN1 (Figure 1b).
CLINICAL SEQUELA
Based upon the initial finding of full gene deletions of the MSH6, MSH2, and EPCAM genes, the diagnosis of Lynch syndrome was made. Given the increased risk of metachronous colorectal cancer and the difficulty of compliance with bowel preparation for colonoscopy, the option of total colectomy with ileorectal anastomosis was discussed, and after consideration of the risks and benefits of different surgical approaches, accepted by the family.
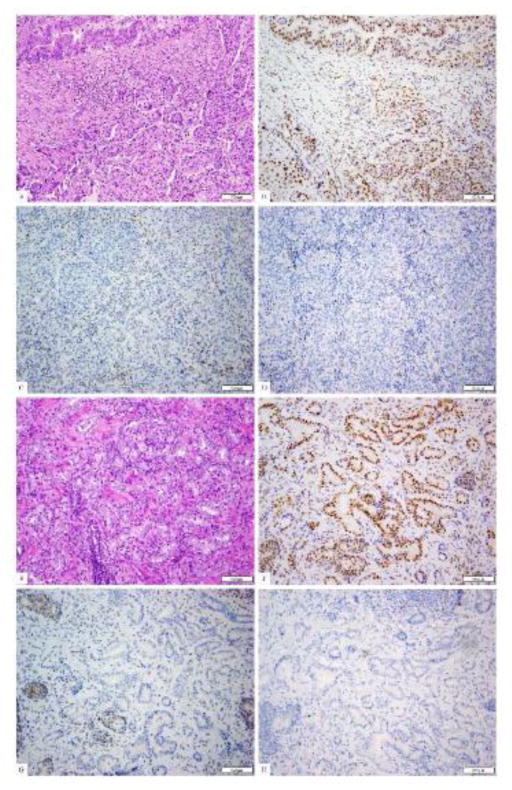
The patient underwent a robotic total abdominal colectomy with partial rectal resection and ileo-mid rectal anastomosis. Surgical pathology confirmed a pT3N1M0 poorly differentiated rectosigmoid adenocarcinoma with lymphovascular invasion, and increased tumor infiltrating lymphocytes; 2 out of 42 lymph nodes were positive for disease. A separate tubulovillous adenoma with intramucosal carcinoma was also noted. IHC staining demonstrated absence of MSH2 and MSH6 protein expression, but intact expression of the MLH1 and PMS2 proteins (Figure 2a–d). The patient had an uneventful surgical recovery and received adjuvant chemotherapy including capecitabine and oxaliplatin (CAPEOX). Chemotherapy was prematurely discontinued due to cumulative side effects including thrombocytopenia and anemia.
Fig 2.
The patient’s colorectal adenocarcinoma (a, H&E) shows mixed histological patterns with a gland-forming component (upper left) and a solid component. By immunohistochemistry, the tumor shows the presence of nuclear staining for PMS2 (b) and MLH1 (picture not shown), but absence of staining for MSH2 (c) and MSH6 (d). The patient’s prostatic adenocarcinoma shows typical acinar units histologically (e, H&E). By immunohistochemistry, this prostatic carcinoma also shows the presence of nuclear staining for PMS2 (f) and MLH1 (picture not shown), but absence of staining for MSH2 (g) and MSH6 (h).
The patient eventually underwent retropubic radical prostatectomy with nerve sparing bilateral lymphadenectomy. Pathology revealed a pT3a multicentric Gleason 7 (3+4) invasive adenocarcinoma with 5 benign lymph nodes. IHC staining demonstrated absence of MSH2 and MSH6 protein expression, but intact expression of the MLH1 and PMS2 proteins (Figure 2e–h).
In a follow-up clinical genetic counseling consultation, it was discussed that the contiguous gene deletion is presumed to be responsible for both the patient’s intellectual disability and his Lynch syndrome. Moreover, given the absence of MSH2 and MSH6 protein expression in both the colorectal cancer and prostate cancer, both cancers are attributed to Lynch syndrome. While the identified contiguous gene deletion is presumably of de novo origin, individualized genetic risk assessment was recommended for the patient’s first-degree relatives.
DISCUSSION
Contiguous gene deletions have previously been described in the setting of inherited cancer predisposition syndromes, such as Familial Adenomatous Polyposis [4, 5]. Our report describes an adult male with a history of intellectual disability and new synchronous cancer diagnoses who was found to have Lynch syndrome as a result of a contiguous gene deletion. From review of the literature, a small number of individuals with similar deletions encompassing 2p16p21 have been previously described [6, 7, 8,9, 10] (Figure 1c, Table 1). Our patient’s physical features and level of intellectual disability are similar to past reports of individuals with 2p16.2p21 deletions, although he is the oldest affected individual to be described.
Table 1.
Reported cases of deletions encompassing 2p16p21 areas
| Reference | Deletion | Phenotypic description |
|---|---|---|
| Petit and Fryns 1997 | 2p11p21 + acefr | Mild dysmorphism and moderate mental retardation |
| Sanders et al. 2003 | 2p16.2p21 | Atrial septal defect, mild hypotelorism, prominent nasal bridge, long smooth philtrum, mild developmental delay |
| Lucci-Cordisco et al. 2004 | 2p16p21 | Developmental delay, premature puberty, short stature, brachycephalic skull with narrow forehead, large and horizontal eyes with thick eyebrows, prominent columella with hypoplastic nostrils, short philtrum, simplified ears, hirsutism, generalized obesity, brachydactyly, cutaneous syndactyly between the second and third toes bilaterally, moderate mental retardation, colon adenocarcinoma at 37 years |
| Collins and Schimke 2011 | 2p16.3p21 | Developmental delay, mental retardation, short stature, microcephaly, hypotelorism, essentially normal colonoscopy at age 21 years (only an inflammatory pseudopolyp was identified) |
| Morton et al. 2011 | 17.6 Mb (SIX3, MSH2, and MSH6) | Fetus with holoprosencephaly |
| Morton et al. 2011 | 192 kb (MSH6 and FBX011) | Developmental delay and dysmorphic features |
| Morton et al. 2011 | 537 kb (MSH6 and FBX011) | Developmental delay and dysmorphic features |
| Current report | 2p16.3p21 (4.8Mb) | Intellectual disability, short stature, brachycephaly with a narrow forehead and short philtrum, brachydactyly, broad and small feet with hyperpigmentation of the soles |
On review of the genes encompassed by the 4.8 Mb deletion, potential medical implications were noted in a number of genes (Table 2). Mutations in the EPAS1, MCFD2, and CALM2 genes have been described to be associated with Erythrocytosis Familial type 4, Factor V and Factor VIII combined deficiency, and Long-QT syndrome, respectively. Our patient demonstrated normochromic normocytic anemia likely due to chronic kidney disease with a normal erythropoietin level without evidence of Erythrocytosis Familial type 4. Electrocardiogram did not reveal Long-QT syndrome. We hypothesize that genotype-phenotype mechanisms may explain why the patient does not express these conditions. Erythrocytosis Familial type 4 has been associated with activating, as opposed to deleterious mutations in EPAS1 (HGNC ID #3374). Similarly, described alterations in the MCFD2 and CALM2 genes have included splice site, frameshift and missense mutations, but not loss of function deletions (HGNC ID # 18451 and 1445, respectively). Given that previously described patients with 2p16p21 deletions also experienced development delay/intellectual disability and that at least two of the genes, CRIPT and NRXN1, found to be deleted in our patient have been linked to central nervous system defect, it is believed that this patient’s history of intellectual disability is likely related to the identified contiguous gene deletion. Of note, while the deletion identified in our patient incurred a loss of 27 genes, the SIX3 gene was not included. In some of the previous reports, SIX3 was noted to be associated with holoprosencephaly.
Table 2.
Selected genes with potential medical implications for the current patient
| Deleted gene | HGNC (HUGO Gene Nomenclature Committee) ID number | Associated phenotype | Phenotype expressed in current patient |
|---|---|---|---|
| EPAS1 | 3374 | Erythrocytosis Familial type 4 (usually associated with activating mutations) | No |
| CRIPT | 14312 | Postnatal growth deficiency, microcephaly, dysmorphic facies, ocular abnormalities, developmental delay, recurrent infections, central nervous system defects | Postnatal growth deficiency, dysmorphic facies, ocular abnormalities, developmental delay, central nervous system defects |
| MCFD2 | 18451 | Factor V and Factor VIII combined deficiency | No |
| TTC7A | 19750 | Early onset inflammatory bowel disease and intestinal atresia | No |
| CALM2 | 1445 | Long-QT syndrome | No |
| EPCAM, MSH2, MSH6 | 11529 7325 7329 |
Lynch syndrome | Lynch syndrome colon cancer and prostate cancer |
| LHCGR | 6585 | Male secondary sexual development disorders | Not evaluated by our service |
| NRXN1 | 8008 | Pitt-Hopkins-like syndrome 2 | Intellectual disability; psychomotor delay; limited speech; impaired communication and socialization skills; thin eyebrows; a prominent nose; a wide mouth; thick ears; happy, excitable demeanor with frequent smiling, laughter, and hand-flapping movements; strabismus; short stature; small hands and feet; flat feet (pes planus); unusually fleshy pads at the tips of the fingers and toes |
In regards to Lynch syndrome, the current case also highlights the importance of informed decision-making with respect to surgical management given risk of metachronous colorectal cancer. Parry et al. found no metachronous cancers after “extensive colon resection” (defined as “total colectomy with ileorectal anastomosis and subtotal colectomy with ileosigmoid anastomosis”) in a cohort of 50 subjects with MMR gene mutations compared to 74 metachronous colorectal cancers in 332 mutation carriers who had segmental resections [11]. In this case, the family’s decision to proceed with total colectomy included considerations of future cancer risk, need for on-going annual colonoscopies and quality of life considerations. Another point exemplified by the current case is the association of the prostate cancer with Lynch syndrome. Although conflicting data exists, a study by Raymond et al. calculated an overall hazard ratio for prostate cancer of 1.99 (95% CI, 1.31 to 3.03; P = 0.0013) in Lynch syndrome mutation carriers compared to the general population [12]. Indeed, immunohistochemical staining of the DNA mismatch repair proteins in the patient’s prostate cancer tissue revealed absence of the MSH2 and MSH6 proteins implicating that the Lynch syndrome gene deletions played a role in the patient’s prostate cancer development. Lastly, while the patient’s initial rectosigmoid adenocarcinoma biopsy demonstrated absence of only the MSH6 protein, it is believed that the reported retention of MSH2 on the biopsy specimen was likely due to a technical issue, such as edge artifact. MMR IHC analysis on the patient’s rectosigmoid adenocarcinoma resection specimen demonstrated absence of both the MSH2 and MSH6 proteins, reflecting the patient’s identified gene deletions.
Our report of a contiguous gene deletion at chromosome 2p16.3-p21 exhibits strong similarities to previously documented cases. Investigation for an etiology of this patient’s intellectual disability was not previously undertaken. Only more recently developed technologies, which were not available when the patient was a child, have provided the ability to detect interstitial chromosome deletions. The identified contiguous gene deletion syndrome has provided a likely explanation for the patient’s intellectual disability, dysmorphic features, and cancer diagnoses. As more children with developmental delay, intellectual disability and congenital anomalies are being assessed with advanced genetic technologies, additional cases of chromosome rearrangements involving cancer predisposition genes will likely be identified. It is important for healthcare providers, patients and their caretakers to recognize the associated cancer risks in the setting of such diagnoses so that appropriate medical management, such as intense cancer surveillance, prophylactic procedures, and chemoprevention, can be considered at appropriate ages.
Acknowledgments
This research was funded in part through The Romeo Milio Lynch Syndrome Foundation, the Kate and Robert Niehaus Clinical Genetics Initiative, and the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of interest: None
References
- 1.Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76(1):1–18. doi: 10.1111/j.1399-0004.2009.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nussbaum RL, McInnes RR, Williard HF. Thompson and Thompson Genetics in Medicine. 6. Saunders/Elsevier; Philadelphia: 2004. pp. 164–165. [Google Scholar]
- 3.Adams SA, Coppinger J, Saitta SC, Stroud T, Kandamurugu M, Fan Z, Ballif BC, Shaffer LG, Bejjani BA. Impact of genotype-first diagnosis: the detection of microdeletion and microduplication syndromes with cancer predisposition by aCGH. Genet Med. 2009;11(5):314–322. doi: 10.1097/GIM.0b013e3181a028a5. [DOI] [PubMed] [Google Scholar]
- 4.Herrera L, Kakati S, Gibas L, Pietrzak K, Sandberg AA. Gardner syndrome in a man with an interstitial deletion of 5q. Am J Med Genet. 1986;25(3):473–476. doi: 10.1002/ajmg.1320250309. [DOI] [PubMed] [Google Scholar]
- 5.Raedle J, Friedl W, Engels H, Koenig R, Trojan J, Zeuzem S. A de novo deletion of chromosome 5q causing familial adenomatous polyposis, dysmorphic features, and mild mental retardation. Am J Gastroenterol. 2001;96(10):3016–3020. doi: 10.1111/j.1572-0241.2001.04674.x. [DOI] [PubMed] [Google Scholar]
- 6.Petit P, Fryns JP. Interstitial deletion 2p accompanied by marker chromosome formation of the deleted segment resulting in a stable acentric marker chromosome. Genet Couns. 1997;8(4):341–343. [PubMed] [Google Scholar]
- 7.Sanders SR, Dawson AJ, Vust A, Hryshko M, Tomiuk M, Riordan D, Prasad C. Interstitial deletion of chromosome 2p16.2p21. Clin Dysmorphol. 2003;12(3):183–185. doi: 10.1097/01.mcd.0000065051.36236.e9. [DOI] [PubMed] [Google Scholar]
- 8.Lucci-Cordisco E, Zollino M, Baglioni S, Mancuso I, Lecce R, Gurrieri F, Crucitti A, Papi L, Neri G, Genuardi M. A novel microdeletion syndrome with loss of the MSH2 locus and hereditary non-polyposis colorectal cancer. Clin Genet. 2004;67(2):178–182. doi: 10.1111/j.1399-0004.2004.00390.x. [DOI] [PubMed] [Google Scholar]
- 9.Collins DL, Schimke RN. Regarding cancer predisposition detected by CHG arrays. Genet Med. 2011;13(11):982. doi: 10.1097/gim.0b013e31823552a8. [DOI] [PubMed] [Google Scholar]
- 10.Morton SA (Adams), Coppinger J, Ballif BC, Shaffer LG, Ellison JW, Saitta SC, Stroud T, Manickam K, Fan Z. Response to the letter by Collins and Schimke. Genet Med. 2011;13(11):982–983. [Google Scholar]
- 11.Parry S, Win AK, Parry B, Macrae FA, Gurrin LC, Church JM, Baron JA, Giles GG, Leggett BA, Winship I, Lipton L, Young GP, Young JP, Lodge CJ, Southey MC, Newcomb PA, Le Marchand L, Haile RW, Lindor NM, Gallinger S, Hopper JL, Jenkins MA. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut. 2011;60(7):950–957. doi: 10.1136/gut.2010.228056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raymond VM, Mukherjee B, Wang F, Huang SC, Stoffel EM, Kastrinos F, Syngal S, Cooney KA, Gruber SB. Elevated risk of prostate cancer among men with Lynch syndrome. J Clin Oncol. 2013;31(14):1713–1718. doi: 10.1200/JCO.2012.44.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]