Abstract
Substance use disorders continue to impose increasing medical, financial and emotional burdens on society in the form of morbidity and overdose, family disintegration, loss of employment and crime, while advances in prevention and treatment options remain limited. Importantly, not all individuals exposed to abused substances effectively develop the disease. Genetic factors play a significant role in determining addiction vulnerability and interactions between innate predisposition, environmental factors and personal experiences are also critical. Thus, understanding individual differences that contribute to the initiation of substance use as well as on long-term maladaptations driving compulsive drug use and relapse propensity is of critical importance to reduce this devastating disorder. In this paper, we discuss current topics in the field of addiction regarding individual vulnerability related to behavioral endophenotypes, neural circuits, as well as genetics and epigenetic mechanisms. Expanded knowledge of these factors is of importance to improve and personalize prevention and treatment interventions in the future.
Keywords: drug abuse, genetics, epigenetics, endophenotypes, striatum, prodynorphin, dopamine D2 receptor
1. Introduction
Addiction is a chronic, relapsing disorder characterized by craving, compulsive drug use, and loss of control over limiting drug intake. It has been estimated that, depending on the substance, approximately 20-40% of people who experiment with drugs of abuse go on to develop addiction (Van Etten and Anthony, 1999; Vsevolozhskaya and Anthony, 2016). This individual vulnerability to addiction is a complex phenomenon influenced by behavioral, cellular, molecular and genetic contributors. Importantly, the ways in which these factors interact remain to be established and harnessed into a framework. This will help identify individuals and sub-populations at elevated risk in order to implement evidence-based prevention strategies.
For decades, the “Nature vs. Nurture” debate has played a significant role in psychology and behavioral sciences (Dick et al., 2010) including the field of addiction. Classically, the field has been shaped by two main schools of thought. On one hand, researchers focused their attention on genetic traits linked to addiction vulnerability with clinical studies demonstrating that the presence of specific genetic markers and associated traits provides an important contribution to the development of this pathological condition (Cloninger et al., 1981; Goldman et al., 2005; Hart and Kranzler, 2015; Kendler et al., 2012; Kendler et al., 1997b). Although debates currently exist regarding reproducibility of the findings from small sample sizes in many human genetic studies as well as the need for genome-wide association assessments, preclinical animal models have also provided multiple examples for genetic links (Bubier et al., 2014; Crabbe, 2016; Rubinstein et al., 1997; Zhou et al., 2013). For instance, in the alcohol field genetic selection was used to segregate vulnerability traits in specific rat and mouse strains (Ciccocioppo, 2013). On the other hand, it is also clear that the unfolding of clinical drug dependence is influenced by a number of additional elements generically defined as “environmental factors” (Belcher et al., 2014; Goldman et al., 2005). These factors include, for example, stress, lifestyle, education, and individual experiences (Enoch, 2012). Further evidence for the role of the environment comes from twin adoption studies demonstrating additional effect of environmental factors in identical genetic backgrounds (Kendler et al., 2012; Kendler et al., 1997b; Lopez-Quintero et al., 2011).
As a result of this complexity, investigators have spent substantial efforts in trying to discover specific genetic, molecular and behavioral traits that reliably identify vulnerable subgroups within the general population. The contribution of genetics and environmental factors has been examined for certain endophenotypes such as impulsivity and novelty seeking (Belin et al., 2008; Kendler et al., 1997a; Kendler et al., 2003; Krueger, 1999; Tarter et al., 2003) as well as individual differences in the way people respond to initial drug exposure (de Wit and Phillips, 2012; Schuckit, 1980). Based on the individual constellation of such factors, people could in theory be categorized as having low, moderate or high risk for developing substance use disorders. Identifying at-risk individuals or populations could significantly advance targeted prevention efforts and diminish the increasing prevalence of substance use disorders. Additionally, targeted manipulations of risk-conferring molecular and cellular impairments could, in the future, help to develop critically needed novel treatments for substance use disorders and even to reduce the transition from occasional use to pathological compulsive drug intake.
In this article, we highlight some of our recent approaches to investigate the complex interplay between genetics and environmental factors that contribute to the range of behavioral traits conferring addiction risk in individuals at the pathological end of the spectrum. In addition, we discuss new molecular insights that provide a mechanistic link between genetic and epigenetic regulation of discrete neuronal pathways underlying individual variation of addiction-related phenotypes.
2. Individual addiction vulnerability in genetically homogenous populations
In the last few years, with the development of epigenetics, the “Nature vs. Nurture” dichotomy has been gradually dissipating (Traynor and Singleton, 2010; Weaver, 2007). It is now clear that the environment can influence the expression of genetic traits and that genome and epigenome interact with each other to shape endophenotypes (Crews et al., 2014). This explains why individual variability can exist in otherwise genetically highly segregated populations (Freund et al., 2013). We explored this phenomenon by looking at alcohol abuse-related individual variability in genetically selected alcohol preferring Marchigian Sardinian (msP) rats. This rat line was derived from Wistar rats by artificial selection for high alcohol preference and excessive drinking over a period of more than 25 years (Ciccocioppo et al., 2006). The genetic pressure applied to this line for more than 80 generations has stably segregated alcohol abuse-related traits such as excessive drinking, high motivation for alcohol consumption and high vulnerability to relapse (Ciccocioppo et al., 2006; Ciccocioppo et al., 1999).
In a recent series of experiments comparing msP and Wistar rats, we analysed individual data distribution in relation to operant alcohol self-administration and relapse to alcohol seeking (Ayanwuyi et al., 2013; Cippitelli et al., 2008). As depicted in Figure 1A, msP rats show increased self-administration of a 10% alcohol solution compared to Wistar rats, and enhanced motivation for alcohol taking measured by progressive ratio schedule (Figure 1B). Using a classical operant self-administration training-extinction/reinstatement paradigm we also observed that, compared to Wistars, msP rats reach a higher level of relapse elicited by re-exposure to cues previously paired with alcohol availability (Figure 1C). Intriguingly, however, no differences were observed when relapse was elicited by delivering the pharmacological stressor yohimbine (Figure 1D), emphasizing the complex nature of the various mechanisms that can mediate relapse. Previously, we have shown that msP rats are highly sensitive to stress and respond with freezing when exposed to foot-shock or yohimbine (Ayanwuyi et al., 2013; Cippitelli et al., 2015; Hansson et al., 2006). This coping behavior contrasts active lever responding and thus reduces stress-induced relapse rates in these models. Differences in response to various types of stress might be important contributors to relapse liability. In addition, analysis of the individual data points revealed a high level of individual variability both in msP and Wistar rats (Figure 1).
Figure 1. Individual differences exist in genetically highly homogeneous populations.
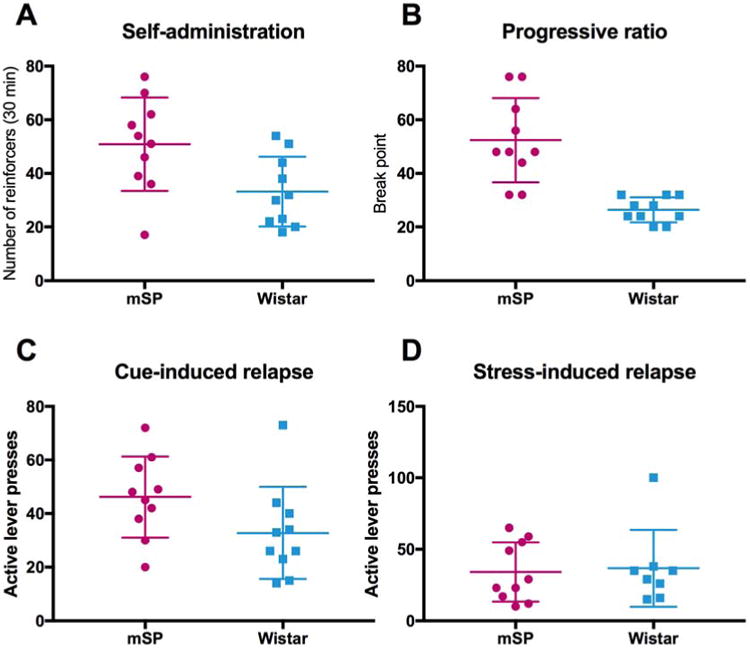
Scatter plot depicting individual differences within alcohol preferring Marchigian-Sardininan (msP) and Wistar rat populations in (A) operant alcohol self-administration under fixed ratio condition, (B) break point under progressive ratio contingency, (C) relapse to alcohol seeking elicited by exposure to environmental cues previously associated to alcohol self-administration, and (D) relapse elicited by the pharmacological stressor yohimbine (1.25 mg/kg, i.p.) in rats previously trained to self-administer 10% alcohol solution.
The selection criteria applied to msP rats through generations were excessive home cage drinking and high preference for 10% alcohol (Ciccocioppo et al., 2006). These two phenotypic traits have been robustly segregated and all adult msP rats are highly homogeneous in alcohol preference and intake. Remarkably, however, substantial individual variability was observed when msP rats were monitored for alcohol intake under operant self-administration condition (Figure 1A). This observation indicates that while genetic pressure has successfully reduced individual vulnerability for the exact traits used to select animals, individual differences persist for other, closely related behaviors (i.e. drinking under operant condition). Consequently, genetic selection based on a specific addiction-like trait does not necessarily determine the expression of other, closely related vulnerability traits. For multifaceted psychiatric diseases like addiction, it is therefore debatable whether innate vulnerability to develop the disorder can be effectively predicted based on the expression of one or a few phenotypic traits. In addition, despite the genotypic identity of a homogeneous population, the trajectory to develop addiction-like traits can be subject to individual variability. This finding recapitulates clinical observations from twin adoption studies showing that, in addition to the genetic background, individual vulnerability to develop addiction is influenced by subjective experiences and environmental factors (Kendler et al., 2012; Kendler et al., 1997b; Lopez-Quintero et al., 2011). Epigenetic regulation may represent the primary mechanism through which the environment can regulate gene expression and function (Robison and Nestler, 2011). However, it is also worth noting that in our experiments rats were maintained in as similar an environment as possible, having been subjected to the same daily training, exposed to the same food and water, and being handled by the same experimenter. Nevertheless, they developed marked individual differences in alcohol taking. This may suggest that complex cellular and molecular factors, including epigenetic regulation, resulting from minor environmental perturbations might contribute to variability in addiction-related behavioral endophenotypes and the development of individual vulnerability in homogeneous populations.
There are intriguing possibilities that deserve to be further explored. For instance, it would be interesting to evaluate the social behavior of genetically heterogeneous animals and measure potential correlations between social experience and the propensity to develop addiction. At birth, a subset of individuals from a litter could be subjected to maternal neglect or exposed to social isolation or variable levels of competition with other pups. Similarly, during development individuals may gain different social status with some rats being dominant and others submissive. Through epigenetic mechanisms these conditions may heavily influence, for example, brain development, stress system function and hormonal responses, which may then shape the expression of distinct endophenotypes (Brake et al., 2004; Choi et al., 2009; Gerritsen et al., 2012; Green et al., 2010; Moffett et al., 2007; Pruessner et al., 2004; Tyrka et al., 2009) and thereby influence addiction vulnerability.
3. Impulsivity, novelty seeking, reward sensitivity and other quantitative traits in addiction vulnerability
A robust set of relationships between clinical addictions, risk for addictions and variation in reward sensitivity, novelty seeking and impulsivity have been demonstrated in human subjects and laboratory animal models alike (Belin et al., 2008; Ersche et al., 2013; Ersche et al., 2012; Jentsch et al., 2014; Jentsch and Taylor, 1999; Jupp and Dalley, 2014; Kreek et al., 2005; Perry and Carroll, 2008). The constructs of reward sensitivity and impulsivity are intimately related in the sense that impulses are affective reward-anticipation states that drive pursuit or consumption of reward. Experiences of novel contexts and stimuli can also serve as reinforcers (Gancarz et al., 2012), and novelty seeking and preference may therefore represent one manifestation of relatively heightened reward sensitivity or impulsivity. Impulsivity is, in turn, a term referring to the propensity of an individual to exhibit a higher than expected level of these behaviors, hypothetically resulting from either greater urgency of the reward anticipation processes or compromised ability to engage in top-down inhibitory control over impulsive feelings and actions in a context-appropriate way (Jentsch and Pennington, 2014; Jentsch and Taylor, 1999).
A large body of data supports the idea that heightened impulsivity and novelty seeking co-occurs with problematic substance use. Some of the earliest evidence of this relationship derives from the observations that people with substance use disorders endorse more of these traits when completing personality questionnaires/inventories (Bardo et al., 2013; Belcher et al., 2014; Ersche et al., 2012; Jentsch et al., 2014; Kreek et al., 2005; Moeller et al., 2001; Terracciano and Costa, 2004; Verdejo-Garcia et al., 2008). Laboratory-based behavioral measures (including the stop-signal reaction time, reversal learning and delay discounting tasks) similarly reveal a heightened tendency to act impulsively or make impulsive choices in substance users (Clark et al., 2006; Courtney et al., 2012; Ersche et al., 2008; Monterosso et al., 2005). Thus, there are multiple manifestations of impulsive behavior and novelty seeking that associate with problematic substance use and that could contribute directly to the loss of control over drug intake that is a prominent characteristic of relapsing use disorders (Tang et al., 2015).
Although there is evidence indicating that experience with alcohol or addictive drugs can, in some circumstances, elicit diminished impulse control (Groman and Jentsch, 2013; Jentsch et al., 2014; Lopez-Caneda et al., 2014), the association between impulsivity and addictive behaviors also seems to run in the opposite direction – namely, a propensity for impulsivity is a quantitative indicator of risk for developing a problematic pattern of substance use. Longitudinal studies in human youth, particularly in those at high risk for drug/alcohol abuse, indicate that heightened impulsive behavior precedes, and to some degree predicts, the initiation of hazardous substance use (Bardo et al., 2013; Henges and Marczinski, 2012; Nigg et al., 2004; Norman et al., 2011; Rubio et al., 2008; Schweinsburg et al., 2004). Further evidence supporting this relationship is the heightened risk for addiction in people affected by childhood onset externalizing disorders involving impulsive behaviors, particularly attention deficit/hyperactivity disorder (Groman et al., 2009).
Controlled, prospective studies in animal models were instrumental in dissecting the relationship between impulsive behaviors, novelty seeking/preference and substance use. Studies of genetically heterogeneous animals have demonstrated that individual differences in behavioral responses to novelty predict the magnitude of the reinforcing and/or rewarding effects of drugs and alcohol (Arenas et al., 2016; Bardo et al., 2013; Belin et al., 2016; Carroll et al., 2009; Deroche-Gamonet and Piazza, 2014). Similarly, a proclivity for impulsive behavior is positively correlated with variation in the acquisition or escalation of drug self-administration and/or the reinstatement of that behavior after extinction (Anker et al., 2009; Dalley et al., 2007; Diergaarde et al., 2008; Diergaarde et al., 2012; Perry et al., 2005; Perry et al., 2008). As these studies used outbred rodents, both genetic variation and unique environmental exposures could contribute to the observed individual phenotypic differences.
Phenotyping of inbred rodents represents a powerful approach for analyzing the degree to which the observed association of two or more phenotypes (such as impulsivity and drug taking) is due to genetic correlation – that is, whether a common set of alleles influence the expression of both phenotypes, producing a pattern of phenotypic co-variation (Civelek and Lusis, 2014). Because all subjects drawn from a particular inbred strain have, to the greatest extent possible, the same genotypes, it is possible to observe genetic correlations between two or more phenotypes across a common panel of strains, even when those phenotypes are measured in different subjects, by different research groups and/or at different times. Thus, a genetic correlation between two or more phenotypes is revealed by a strain-level statistical pattern of correlation between them. That said, it is also known that phenotype measurements vary even within inbred and genetically homogeneous animals (Crabbe et al., 1999). Clearly, non-genetic sources of variance (which include both environment and experimental error) also need to be considered alongside genetic factors. Nonetheless, using this approach, heritable variation in impulsive behavior has been shown to genetically correlate with intravenous cocaine self-administration (Cervantes et al., 2013) and alcohol intake (Loos et al., 2013) in recombinant inbred mice. Complementing these findings is the observation that selective breeding for high alcohol intake also selects for heightened impulsivity (Oberlin and Grahame, 2009). Both approaches reveal compelling evidence that the association between impulsive and addiction-relevant behaviors likely results, at least in part, from their co-heritability.
The neuroanatomical and molecular mechanisms that underlie impulsivity and addiction risk remain incompletely understood, but the dopamine D2-like receptor in striatal circuitry appears to be one functionally relevant determinant (Buckholtz et al., 2010b; Dalley and Roiser, 2012; Groman and Jentsch, 2012; Jentsch and Pennington, 2014; London, 2016; Volkow and Morales, 2015). Relatively low dopamine D2-like receptors have been consistently linked with clinically-impairing substance use disorders (Koob and Volkow, 2010), and this molecular change is directly linked with heightened impulsivity and impulsive behaviors in drug dependent people (Ballard et al., 2015; Kohno et al., 2015; Lee et al., 2009; London, 2016; Robertson et al., 2015). Altered dopamine D2 signaling co-varies with impulsivity in healthy humans as well (Buckholtz et al., 2010b) and low dopamine D2-like receptor density in the forebrain has been correlated with impulsive behaviors in non-human primates (Groman et al., 2011), rats (Dalley et al., 2007) and inbred mice (Laughlin et al., 2011). Thus, there appears to be a highly conserved relationship between individual variation in D2-like receptor signaling in striatal circuits, impulsivity and associated risk for addiction (Jentsch and Pennington, 2014). These findings indicate that D2R signaling is a promising target for pharmacological or behavioral interventions aimed at mitigating the vulnerability of some people to develop particularly problematic patterns of substance use.
Until recently, little compelling data was available to link specific genetic variants to dysregulated dopamine D2-like receptor signaling and to the associated problems with impulse control and heightened addiction risk. To fill this gap in the literature, we employed a panel of 51 recombinant inbred mouse strains derived from an intercross of the C57Bl/6J and DBA/2J parental strains (Williams et al., 2001). These subjects were screened for heritable variation in impulsivity using an operant reversal learning task (Izquierdo and Jentsch, 2012; Laughlin et al., 2011). In this paradigm, an action is paired with a specific outcome and, after achieving a learning criterion, the contingencies are reversed. Successful reversal learning depends on the subject's ability to stop, inhibit or change the initially-trained responses, as well as to learn to overcome the learned irrelevance of the non-reinforced behavior. As such, this paradigm is thought to be a measure of behavioral flexibility with links to impulse control (Izquierdo and Jentsch, 2012). Using this approach, we identified a large-effect quantitative trait locus (QTL) on mouse chromosome 10 that explained approximately 1/3 of the genetic variance in the reversal learning phenotype. This QTL is syntenic to portions of human chromosomes 12 and 22. We further sought to identify genes expressed from this QTL that exhibited a cis-regulated pattern of expression (showing that genetic variants within the QTL influenced expression) and heritable differences of expression in certain brain regions that were genetically correlated with reversal learning abilities (Gaglani et al., 2009; Overall et al., 2009; Rosen et al., 2009). Expression results were derived from datasets stored in GeneNetwork.org (Illumina beadchip WG-6v1 assay). These analyses identified Syn3, the gene encoding the synapsin III protein (Kao et al., 1998), which belongs to a family of synaptic phosphoproteins acting to regulate the size of the ready-releasable pool (Hilfiker et al., 1999). As assessed in our operant test (Laughlin et al., 2011), Syn3 relative expression in hippocampus, dorsal striatum, nucleus accumbens (NAc) and neocortex is negatively correlated with impulsivity (striatal correlation shown in Figure 2A). Interestingly, Syn3 is expressed in dopaminergic neurons of the ventral midbrain, where it plays a non-redundant role in governing the magnitude of action potential-dependent dopamine release (Kile et al., 2010). Based on these results, we hypothesized that genetic variation near the Syn3 locus influences expression of the gene, with low expression-associated alleles leading to relatively larger changes in synaptic dopamine concentration in response to action potentials than do high expression-associated alleles (Figure 2B). This excess of phasic, activity-dependent dopamine release could be a neurochemical substrate of impulsivity and addiction risk, with the reduced availability of D2-like receptors often correlated with impulsivity representing an apparent compensation to the heightened presynaptic release. Notably, other states of heightened impulsivity and addiction risk, including the adolescent developmental stage, have also been linked with increased phasic dopamine release (Wong et al., 2013) suggesting that multiple biological pathways that predispose for this neurochemical mechanism may quantitatively influence impulsivity and substance use.
Figure 2. Syn3 impairments underlie heightened impulsivity and addiction risk.
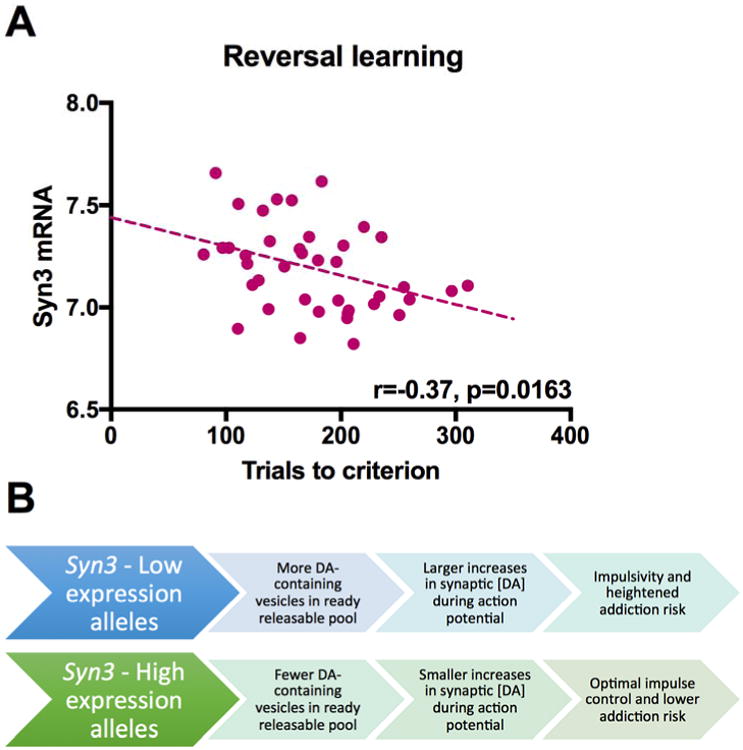
(A) Expression of Syn3 in tissue from the striatum of 40 strains of BXD mice exhibits a pattern of genetic correlation with reversal learning abilities. BXD strains demonstrating relatively low Syn3 expression in the striatum exhibit heightened impulsivity in the reversal learning test. (B) Hypothetical mechanistic relationship between alleles influencing Syn3 expression and impulse control and addiction risk phenotypes. DA: dopamine.
4. Genetic and epigenetic interface in discrete striatal pathways associated with behavioral phenotypes of addiction vulnerability
A central goal of neurobiological research efforts over the past decades has been to delineate the specific cells and pathways within complex neuronal circuits that transform genetically encoded information to behavioral action conferring individual vulnerability to addiction. Several neuronal circuits have been highly implicated in mediating addiction-related behavioral traits with key areas of focus on the ventral midbrain dopaminergic projections, ventral and dorsal striatum, medial prefrontal cortex, and extended amygdala (Koob and Volkow, 2010). One particular hub that has maintained considerable attention in the field is the striatum which integrates dopaminergic and glutamatergic input from the midbrain and cortex to modulate emotion, motivation, reward, and goal-directed behavior (Everitt and Robbins, 2013) and has been strongly implicated in addiction to various substances (Calipari et al., 2016; Corbit et al., 2012; Egervari et al., 2017; Szutorisz et al., 2014; Wang et al., 2014). Several human and non-human primate studies have documented that in vivo differences in striatal activity are correlated with individual differences in sensation- and novelty-seeking (Abler et al., 2006; McClure et al., 2003; Montague et al., 2004), both behavioral traits that underlie substance use vulnerability (Belin et al., 2011; Belin et al., 2008). In addition, individual differences in striatal dopamine release (Boileau et al., 2006; Buckholtz et al., 2010a; Buckholtz et al., 2010b; Treadway et al., 2012) and in vivo fMRI BOLD signal (Beaver et al., 2006; Bjork et al., 2008) correlate with reward seeking and impulsivity traits (see section 3).
The biochemical phenotypes of striatal neurons are well characterized. Both the nucleus accumbens and the dorsal striatum consist predominantly of GABAergic medium spiny neurons (MSNs) that can be subdivided into two major populations (Ena et al., 2011). While these populations can not be distinguished based on cell morphology and are comprised by a similar number of MSNs (Surmeier et al., 2007), they give rise to anatomically, functionally and biochemically distinct pathways (Albin et al., 1989; Alexander and Crutcher, 1990; Graybiel, 2000). One population of MSNs projects directly to the globus pallidus internus/substantia nigra pars reticularis (GPi/SNpr) in the mesencephalon, thereby inhibiting basal ganglia output, which in turn leads to increased activity in thalamocortical circuits. This striatonigral or direct pathway expresses dopamine D1 receptors (D1R), prodynorphin (PDYN) and substance P (Gerfen et al., 1990; Graybiel, 2000; Surmeier et al., 2007). Analogous neurons of the ventral striatum project to the ventral tegmental area and medial substantia nigra pars compacta forming the striatomesencephalic (SM) pathway. Another subset of striatal MSNs project to the globus pallidus externus, which in turn projects to the GPi/SNpr leading to its disinhibition. This striatopallidal (SP) or indirect pathway is characterized by dopamine D2 receptor (D2R), proenkephalin (PENK) and adenosine A2A receptor expression (Gerfen et al., 1990; Graybiel, 2000; Surmeier et al., 2007). Ventral striatal SP neurons project to the ventral pallidum. Due to their distinct molecular and anatomical characteristics, direct and indirect pathway MSNs contribute differentially to the behavioral output of the ventral and dorsal striatum (Hikida et al., 2010; Kravitz et al., 2012). In general, direct pathway activity seems to be more connected to positive reinforcement and ‘Go’ behavior, while indirect pathway activity regulates negative reinforcement and ‘No-Go’ behavior (Egervari et al., 2016; Frank et al., 2007; Hikida et al., 2010; Hikida et al., 2013; Jutras-Aswad et al., 2012), both of which strongly influence substance use disorders. Interestingly, selective genetic and optogenetic manipulation of D1R or D2R expressing MSNs can shift the balance of direct and indirect pathway activity and thereby affect rewarding properties of abused substances. Increased excitability of SM neurons in the NAc, for example, increases cocaine reward, while increased excitability of SP neurons desensitizes the rewarding effects of the drug (Lobo et al., 2010). More recently, Neumeier and colleagues showed that inhibition of SM neurons using designer receptors exclusively activated by designer drugs decreased, while inhibition of SP neurons increased amphetamine-induced locomotor responses (Ferguson et al., 2011).
Individual differences in direct and indirect pathway activity in part underlie addiction-related behavioral endophenotypes and contribute significantly to individual addiction vulnerability (Ferguson et al., 2011; Hikida et al., 2010; Hikida et al., 2013; Kravitz et al., 2012; Lobo et al., 2010). This contribution has been well documented both in human and in translational animal models, especially in the case of the striatopallidal pathway. Striatal D2R signaling, for example, is strongly linked to impulsivity and addiction risk (see section 3). In animal models, a series of studies using a visual attention task (5-choice serial reaction time task) documented that impulsive behavior, together with low D2 receptor availability in the NAc, predicts escalation of drug intake (Dalley et al., 2007), frequency of relapse following abstinence (Economidou et al., 2012) and continued responding for the drug despite associated negative consequences (i.e. the delivery of punishers contingent on lever pressing for the drug) (Everitt et al., 2008).
Human genetic studies have also significantly expanded our knowledge regarding striatopallidal contribution to addiction vulnerability and underlying behavioral traits. For example, carriers of the TaqIA A1 allele, exhibiting markedly decreased striatal D2R expression (Ritchie and Noble, 2003; Stice et al., 2010), are significantly over-represented in populations affected by substance use disorders (Lawford et al., 2000; Noble et al., 2000). In addition, genetic variants of the PENK gene, showing enriched expression in SP neurons, have also been shown to be associated with risk for opioid abuse (Comings et al., 1999; Nikoshkov et al., 2008) and cannabis dependence (Jutras-Aswad et al., 2012). Intriguingly, the link between PENK single nucleotide polymorphisms (SNPs) and cannabis dependence was related to a strong synergistic interaction with neuroticism/anxiety temperamental trait: a combination of the high-risk allele and high neuroticism trait resulted in a 9-fold increase in cannabis dependence risk, while the high-risk allele with low neuroticism, or low risk allele with high neuroticism manifested in about 1.3-1.8-fold increased risk (Jutras-Aswad et al., 2012). Importantly, the same SNPs were related to PENK mRNA expression in the striatum as well as the central amygdala, a key region regulating negative affect, emphasizing the functional relevance of these variants (Jutras-Aswad et al., 2012). It is important to note that not only does PENK genetically associate with cannabis dependence, but exposure to THC (Δ9-tetrahydrocannabinol, the main psychoactive component of marijuana) itself leads to long-lasting alterations in NAc Penk expression via epigenetic mechanisms, which in turn contribute to enhanced opiate vulnerability (Tomasiewicz et al., 2012). Thus, the complex interaction of genetics and drug exposure regulate the expression of genes within specific neuronal circuits linked to addiction risk.
While the field has predominantly focused on the indirect striatal pathway, emerging data in recent years emphasize the significant contribution of direct pathway-related impairments to addiction vulnerability, as well. For example, rats with enhanced Pdyn levels in the NAc exhibit increased vulnerability for alcohol intake and novelty seeking behavioral traits (Guitart-Masip et al., 2006). Moreover, high responder rats to novelty stressor (exposure to low-light, low-noise, novel environment) show enhanced drug self-administration and are characterized by elevated NAc Pdyn mRNA expression (Lucas et al., 1998). In addition, genetic variants in the 3′ un-translated region (3′UTR) of the human PDYN gene were shown to be associated with cocaine, alcohol and heroin use disorders (Clarke et al., 2012; Clarke et al., 2009; Taqi et al., 2011; Yuferov et al., 2009). Interestingly, the 3′UTR variants are in high linkage disequilibrium and comprise a haplotype block associated with striatal PDYN mRNA levels as well as cocaine dependence and combined cocaine/alcohol co-dependence (Yuferov et al., 2009). The association between 3′UTR variants and drug addiction is particularly fascinating since this region of the gene was not previously considered to have a functional role in regulating gene expression. It is now well acknowledged that microRNAs, small non-coding RNAs of ∼22 nucleotides, are an important epigenetic mechanism that bind the 3′UTR of target mRNAs to regulate gene expression post-transcriptionally through affecting translation and mRNA degradation (Ambros, 2004; Bartel, 2004).
Recently, we described a genetically influenced mRNA-microRNA relationship between a PDYN 3′UTR variant and miR-365 that contributes to certain behavioral endophenotypes relevant to addiction vulnerability (Egervari et al., 2016). We found that rs2235749 and other SNPs in high linkage disequilibrium with rs2235749 were associated with novelty seeking and positive reinforcement based decision-making in humans. On the molecular level, rs2235749 impaired the binding of miR-365 to the PDYN 3′UTR. The disruption of miR-365 binding directly increased PDYN mRNA levels in vitro and the SNP was associated with elevated PDYN expression in the NAc in post-mortem human brains (Egervari et al., 2016). Strikingly, inhibiting miR-365 function in the NAc in a rat model (using miRZip-365, a short oligonucleotide with complementary sequence to miR-365 that essentially acts as a chemical sponge and prevents miR-365's binding to its endogenous targets – Figure 3A) led to increased Pdyn mRNA in this region, and strongly affected novelty seeking (Figure 3B) and reward learning (Figure 3C) phenotypes (Egervari et al., 2016). These findings highlight a novel mechanism in which genetic variants of PDYN disrupt its epigenetic regulation and thereby influence gene expression and the manifestation of behavioral endophenotypes that contribute to addiction risk. Similar mechanistic studies are of critical importance to expand knowledge about the underlying functional impairments associated with genetic risk alleles that could help to identify specific molecular targets for future treatment interventions.
Figure 3. A genetically influenced microRNA-mRNA interaction affects novelty seeking and reward learning.
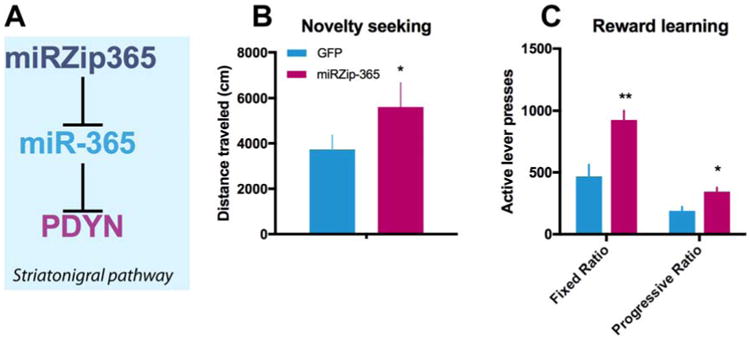
(A) Specific expression of miRZip-365 in nucleus accumbens striatonigral neurons results in inhibition of miR-365's binding to its endogenous targets including Prodynorphin. miRZip-365 leads to significant increases in (B) novelty seeking in Open Field and (C) positive reinforcement-based decision making in fixed ratio and progressive ratio food self-administration paradigms. PDYN: prodynorphin, * p<0.05, **p<0.01.
5. Conclusions
Exposure to addictive substances does not trigger the same psychopathological progression to substance use disorder in everyone. Rather, emerging evidence discussed in this review points to a more complex model of addiction vulnerability in which genetically determined and environmental factors interact at multiple levels of molecular, neuronal and circuit organization (Figure 4)., On the genetic level, polymorphisms of genes related to multiple components of the brain reward circuitry play a critical role. For example, variants of reward-related genes have been shown to be associated with addiction vulnerability (Figure 4A). Importantly, many of these SNPs have been proven to be functionally relevant and shown to underlie molecular impairments with respect to the synthesis and function of endogenous opioid neuropeptides, dopamine and other neurotransmitters. These relationships are often mediated by epigenetic mechanisms. For instance, as discussed in Section 4, polymorphisms can affect DNA methylation or the binding of transcription factors and regulatory microRNAs and thereby influence gene expression (Figure 4A). Given that the expression levels of neurotransmitters and neuropeptides directly and critically affect neuronal communication, these molecular differences can in turn result in functional changes of the mesocorticolimbic circuitry (Figure 4B). The resulting circuit-level alterations play an important role in the development of specific behavioral endophenotypes (Figure 4C), such as impulsivity, novelty seeking or enhanced reward sensitivity and diminished executive control, which can underlie an increased likelihood of experimenting with abused substances or of developing a compulsive, habitual pattern of substance use. These three major dimensions (genetic/molecular, circuit-level and behavioral) of vulnerability are strongly intertwined and together determine individual risk for developing addiction (Figure 4). Importantly, exposure to drugs and other environmental insults can further influence this complex system via dynamic epigenetic mechanisms. Consequently, it is now acknowledged that multiple individual and environmental factors contribute to substance use by way of underlying neuronal/molecular/epigenetic mechanisms that induce the range of low to high risk addiction phenotypes.
Figure 4. Individual addiction vulnerability.
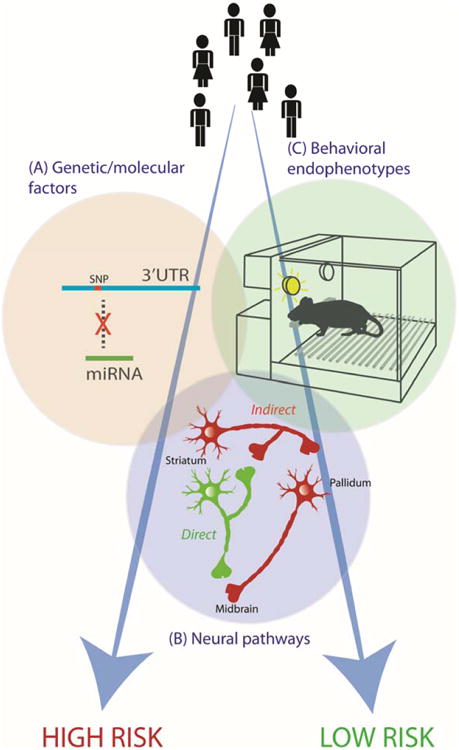
High and low risk for developing substance use disorders is determined by a complex interaction of (A) genetic/molecular factors (e.g. genetic variants affecting microRNA binding and epigenetic regulation of gene expression), (B) neural pathways (e.g. changes in direct and indirect striatal pathway activity), (C) behavioral endophenotypes (e.g. heightened impulsivity and novelty seeking), and environmental factors. SNP: single nucleotide polymorphism, 3′UTR: 3′ untranslated region, miRNA: microRNA.
This complex and multidimensional nature of addiction risk has long been an important challenge for clinical prevention interventions and medication development.. Importantly, significant advances based on preclinical research discussed above have now begun to enable the dissection of discrete behaviors, neuroanatomical circuits and molecular underpinnings driving individual differences in addiction-related behaviors. The accumulating evidence reviewed here emphasizes the complex nature of addiction vulnerability. Importantly, the different components (Figure 4) need to be integrated in future preclinical models in order to expand neurobiological insights into addiction risk.
Although genetic and epigenetic complexities increase the challenge of delineating the molecular mechanisms underlying novelty seeking, impulsivity, reward sensitivity and multiple other behavioral traits linked to addiction, focused research attention on this topic is essential considering the tremendous and increasing economic and emotional burden addiction places on the individual, their families and society. The ability to identify at-risk individuals could lead to targeted prevention solutions to counter the increasing prevalence of substance use disorders. The fact that the long-term course of substance use disorders often has its onset during adolescence or young adulthood also emphasizes the profound impact of prevention strategies. Expanded neurobiological information about genetic and epigenetic factors is also relevant for the development of novel treatments that would enable targeted therapeutic manipulations of risk-conferring molecular and cellular impairments to alleviate problematic drug use in specific subgroups. Overall, personalized prevention and treatment interventions in the future are dependent on research today that delves deeper into the complex interplay of genetic and epigenetic factors impacting individual addiction risk.
Highlights.
Only a subset of people exposed to abused substances go on to develop addiction
Individual differences in vulnerability exist in highly homogenous populations
Key factors include behavioral phenotypes, genetics and epigenetics
Knowledge of individual risk helps to target prevention and treatment efforts
Acknowledgments
This work was supported by National Institute of Health grants DA15446 (YLH), DA031852 and DA039841 (JDJ) as well as AA014351 and AA017447 (RC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage. 2006;31:790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Gliddon LA, Carroll ME. Performance under a Go/No-go task in rats selected for high and low impulsivity with a delay-discounting procedure. Behav Pharmacol. 2009;20:406–414. doi: 10.1097/FBP.0b013e3283305ea2. [DOI] [PubMed] [Google Scholar]
- Arenas MC, Aguilar MA, Montagud-Romero S, Mateos-Garcia A, Navarro- Frances CI, Minarro J, Rodriguez-Arias M. Influence of the Novelty-Seeking Endophenotype on the Rewarding Effects of Psychostimulant Drugs in Animal Models. Curr Neuropharmacol. 2016;14:87–100. doi: 10.2174/1570159X13666150921112841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayanwuyi LO, Carvajal F, Lerma-Cabrera JM, Domi E, Bjork K, Ubaldi M, Heilig M, Roberto M, Ciccocioppo R, Cippitelli A. Role of a genetic polymorphism in the corticotropin-releasing factor receptor 1 gene in alcohol drinking and seeking behaviors of marchigian sardinian alcohol-preferring rats. Front Psychiatry. 2013;4:23. doi: 10.3389/fpsyt.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ME, Mandelkern MA, Monterosso JR, Hsu E, Robertson CL, Ishibashi K, Dean AC, London ED. Low Dopamine D2/D3 Receptor Availability is Associated with Steep Discounting of Delayed Rewards in Methamphetamine Dependence. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu119. pyu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher AM, Volkow ND, Moeller FG, Ferre S. Personality traits and vulnerability or resilience to substance use disorders. Trends Cogn Sci. 2014;18:211–217. doi: 10.1016/j.tics.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Everitt BJ, Dalley JW. In search of predictive endophenotypes in addiction: insights from preclinical research. Genes Brain Behav. 2016;15:74–88. doi: 10.1111/gbb.12265. [DOI] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High- novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:569–579. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Hommer DW. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103:1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. The European journal of neuroscience. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Bubier JA, Jay JJ, Baker CL, Bergeson SE, Ohno H, Metten P, Crabbe JC, Chesler EJ. Identification of a QTL in Mus musculus for alcohol preference, withdrawal, and Ap3m2 expression using integrative functional genomics and precision genetics. Genetics. 2014;197:1377–1393. doi: 10.1534/genetics.114.166165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Cole D, Kessler RM, Zald DH. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nature neuroscience. 2010a;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic network differences in human impulsivity. Science. 2010b;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Pena CJ, Walker DM, Pirpinias ST, Guise KG, Ramakrishnan C, Deisseroth K, Nestler EJ. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:2726–2731. doi: 10.1073/pnas.1521238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend. 2009;104(1):S70–78. doi: 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Cervantes MC, Laughlin RE, Jentsch JD. Cocaine self-administration behavior in inbred mouse lines segregating different capacities for inhibitory control. Psychopharmacology. 2013;229:515–525. doi: 10.1007/s00213-013-3135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological psychiatry. 2009;65:227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R. Genetically selected alcohol preferring rats to model human alcoholism. Curr Top Behav Neurosci. 2013;13:251–269. doi: 10.1007/7854_2012_199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol- preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addiction biology. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Froldi R, Colombo G, Gessa GL, Massi M. Antidepressant-like effect of ethanol revealed in the forced swimming test in Sardinian alcohol-preferring rats. Psychopharmacology. 1999;144:151–157. doi: 10.1007/s002130050988. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Ayanwuyi LO, Barbier E, Domi E, Lerma-Cabrera JM, Carvajal F, Scuppa G, Li H, Ubaldi M, Heilig M, Roberto M, Ciccocioppo R. Polymorphism in the corticotropin-releasing factor receptor 1 (CRF1-R) gene plays a role in shaping the high anxious phenotype of Marchigian Sardinian alcohol- preferring (msP) rats. Psychopharmacology. 2015;232:1083–1093. doi: 10.1007/s00213-014-3743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology. 2008;198:449–460. doi: 10.1007/s00213-008-1104-0. [DOI] [PubMed] [Google Scholar]
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet. 2014;15:34–48. doi: 10.1038/nrg3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in current and former substance users. Biological psychiatry. 2006;60:515–522. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Clarke TK, Ambrose-Lanci L, Ferraro TN, Berrettini WH, Kampman KM, Dackis CA, Pettinati HM, O'Brien CP, Oslin DW, Lohoff FW. Genetic association analyses of PDYN polymorphisms with heroin and cocaine addiction. Genes Brain Behav. 2012;11:415–423. doi: 10.1111/j.1601-183X.2012.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Krause K, Li T, Schumann G. An association of prodynorphin polymorphisms and opioid dependence in females in a Chinese population. Addiction biology. 2009;14:366–370. doi: 10.1111/j.1369-1600.2009.00151.x. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Comings DE, Blake H, Dietz G, Gade-Andavolu R, Legro RS, Saucier G, Johnson P, Verde R, MacMurray JP. The proenkephalin gene (PENK) and opioid dependence. Neuroreport. 1999;10:1133–1135. doi: 10.1097/00001756-199904060-00042. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biological psychiatry. 2012;72:389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Arellano R, Barkley-Levenson E, Galvan A, Poldrack RA, Mackillop J, Jentsch JD, Ray LA. The relationship between measures of impulsivity and alcohol misuse: an integrative structural equation modeling approach. Alcohol Clin Exp Res. 2012;36:923–931. doi: 10.1111/j.1530-0277.2011.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC. Progress With Nonhuman Animal Models of Addiction. J Stud Alcohol Drugs. 2016;77:696–699. doi: 10.15288/jsad.2016.77.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Crews D, Gillette R, Miller-Crews I, Gore AC, Skinner MK. Nature, nurture and epigenetics. Mol Cell Endocrinol. 2014;398:42–52. doi: 10.1016/j.mce.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42–8. doi: 10.1016/j.neuroscience.2012.03.065. [DOI] [PubMed] [Google Scholar]
- de Wit H, Phillips TJ. Do initial responses to drugs predict future use or abuse? Neurosci Biobehav Rev. 2012;36:1565–1576. doi: 10.1016/j.neubiorev.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Piazza PV. Psychobiology of cocaine addiction: Contribution of a multi-symptomatic animal model of loss of control. Neuropharmacology. 2014;76 Pt B:437–449. doi: 10.1016/j.neuropharm.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Dick DM, Riley B, Kendler KS. Nature and nurture in neuropsychiatric genetics: where do we stand? Dialogues Clin Neurosci. 2010;12:7–23. doi: 10.31887/DCNS.2010.12.1/ddick. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biological psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, van Mourik Y, Pattij T, Schoffelmeer AN, De Vries TJ. Poor impulse control predicts inelastic demand for nicotine but not alcohol in rats. Addiction biology. 2012;17:576–587. doi: 10.1111/j.1369-1600.2011.00376.x. [DOI] [PubMed] [Google Scholar]
- Economidou D, Theobald DE, Robbins TW, Everitt BJ, Dalley JW. Norepinephrine and dopamine modulate impulsivity on the five-choice serial reaction time task through opponent actions in the shell and core sub-regions of the nucleus accumbens. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2057–2066. doi: 10.1038/npp.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egervari G, Jutras-Aswad D, Landry J, Miller ML, Anderson SA, Michaelides M, Jacobs MM, Peter C, Yiannoulos G, Liu X, Hurd YL. A Functional 3′UTR Polymorphism (rs2235749) of Prodynorphin Alters microRNA-365 Binding in Ventral Striatonigral Neurons to Influence Novelty Seeking and Positive Reward Traits. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41:2512–2520. doi: 10.1038/npp.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egervari G, Landry J, Callens J, Fullard JF, Roussos P, Keller E, Hurd YL. Striatal H3K27 Acetylation Linked to Glutamatergic Gene Dysregulation in Human Heroin Abusers Holds Promise as Therapeutic Target. Biological psychiatry. 2017;81:585–594. doi: 10.1016/j.biopsych.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ena S, de Kerchove d'Exaerde A, Schiffmann SN. Unraveling the differential functions and regulation of striatal neuron sub-populations in motor control, reward, and motivational processes. Frontiers in behavioral neuroscience. 2011;5:47. doi: 10.3389/fnbeh.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The influence of gene-environment interactions on the development of alcoholism and drug dependence. Curr Psychiatry Rep. 2012;14:150–158. doi: 10.1007/s11920-011-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Smith DG, Bullmore ET, Robbins TW. Distinctive personality traits and neural correlates associated with stimulant drug use versus familial risk of stimulant dependence. Biological psychiatry. 2013;74:137–144. doi: 10.1016/j.biopsych.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology. 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Chamberlain SR, Muller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. The American journal of psychiatry. 2012;169:926–936. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nature neuroscience. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16311–16316. doi: 10.1073/pnas.0706111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund J, Brandmaier AM, Lewejohann L, Kirste I, Kritzler M, Kruger A, Sachser N, Lindenberger U, Kempermann G. Emergence of individuality in genetically identical mice. Science. 2013;340:756–759. doi: 10.1126/science.1235294. [DOI] [PubMed] [Google Scholar]
- Gaglani SM, Lu L, Williams RW, Rosen GD. The genetic control of neocortex volume and covariation with neocortical gene expression in mice. BMC Neurosci. 2009;10:44. doi: 10.1186/1471-2202-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancarz AM, Robble MA, Kausch MA, Lloyd DR, Richards JB. Association between locomotor response to novelty and light reinforcement: sensory reinforcement as a rodent model of sensation seeking. Behav Brain Res. 2012;230:380–388. doi: 10.1016/j.bbr.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Tendolkar I, Franke B, Vasquez AA, Kooijman S, Buitelaar J, Fernandez G, Rijpkema M. BDNF Val66Met genotype modulates the effect of childhood adversity on subgenual anterior cingulate cortex volume in healthy subjects. Mol Psychiatry. 2012;17:597–603. doi: 10.1038/mp.2011.51. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Curr Biol. 2000;10:R509–511. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, James AS, Jentsch JD. Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2009;33:690–698. doi: 10.1016/j.neubiorev.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Jentsch JD. Cognitive control and the dopamine D(2)-like receptor: a dimensional understanding of addiction. Depress Anxiety. 2012;29:295–306. doi: 10.1002/da.20897. [DOI] [PubMed] [Google Scholar]
- Groman SM, Jentsch JD. Identifying the molecular basis of inhibitory control deficits in addictions: neuroimaging in non-human primates. Curr Opin Neurobiol. 2013;23:625–631. doi: 10.1016/j.conb.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Lee B, London ED, Mandelkern MA, James AS, Feiler K, Rivera R, Dahlbom M, Sossi V, Vandervoort E, Jentsch JD. Dorsal striatal D2-like receptor availability covaries with sensitivity to positive reinforcement during discrimination learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7291–7299. doi: 10.1523/JNEUROSCI.0363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart-Masip M, Gimenez-Llort L, Fernandez-Teruel A, Canete T, Tobena A, Ogren SO, Terenius L, Johansson B. Reduced ethanol response in the alcohol-preferring RHA rats and neuropeptide mRNAs in relevant structures. The European journal of neuroscience. 2006;23:531–540. doi: 10.1111/j.1460-9568.2005.04556.x. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AB, Kranzler HR. Alcohol Dependence Genetics: Lessons Learned From Genome-Wide Association Studies (GWAS) and Post-GWAS Analyses. Alcohol Clin Exp Res. 2015;39:1312–1327. doi: 10.1111/acer.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henges AL, Marczinski CA. Impulsivity and alcohol consumption in young social drinkers. Addict Behav. 2012;37:217–220. doi: 10.1016/j.addbeh.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Hikida T, Yawata S, Yamaguchi T, Danjo T, Sasaoka T, Wang Y, Nakanishi S. Pathway-specific modulation of nucleus accumbens in reward and aversive behavior via selective transmitter receptors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:342–347. doi: 10.1073/pnas.1220358110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P. Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci. 1999;354:269–279. doi: 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology. 2012;219:607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Annals of the New York Academy of Sciences. 2014;1327:1–26. doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Pennington ZT. Reward, interrupted: Inhibitory control and its relevance to addictions. Neuropharmacology. 2014;76 Pt B:479, 486. doi: 10.1016/j.neuropharm.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jupp B, Dalley JW. Behavioral endophenotypes of drug addiction: Etiological insights from neuroimaging studies. Neuropharmacology. 2014;76 Pt B:487, 497. doi: 10.1016/j.neuropharm.2013.05.041. [DOI] [PubMed] [Google Scholar]
- Jutras-Aswad D, Jacobs MM, Yiannoulos G, Roussos P, Bitsios P, Nomura Y, Liu X, Hurd YL. Cannabis-dependence risk relates to synergism between neuroticism and proenkephalin SNPs associated with amygdala gene expression: case-control study. PloS one. 2012;7:e39243. doi: 10.1371/journal.pone.0039243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao HT, Porton B, Czernik AJ, Feng J, Yiu G, Haring M, Benfenati F, Greengard P. A third member of the synapsin gene family. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4667–4672. doi: 10.1073/pnas.95.8.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Chen X, Dick D, Maes H, Gillespie N, Neale MC, Riley B. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nature neuroscience. 2012;15:181–189. doi: 10.1038/nn.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Davis CG, Kessler RC. The familial aggregation of common psychiatric and substance use disorders in the National Comorbidity Survey: a family history study. Br J Psychiatry. 1997a;170:541–548. doi: 10.1192/bjp.170.6.541. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Neale MC, Pedersen NL. Temperance board registration for alcohol abuse in a national sample of Swedish male twins, born 1902 to 1949. Arch Gen Psychiatry. 1997b;54:178–184. doi: 10.1001/archpsyc.1997.01830140090015. [DOI] [PubMed] [Google Scholar]
- Kile BM, Guillot TS, Venton BJ, Wetsel WC, Augustine GJ, Wightman RM. Synapsins differentially control dopamine and serotonin release. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:9762–9770. doi: 10.1523/JNEUROSCI.2071-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Ghahremani DG, Morales AM, Robertson CL, Ishibashi K, Morgan AT, Mandelkern MA, London ED. Risk-taking behavior: dopamine D2/D3 receptors, feedback, and frontolimbic activity. Cereb Cortex. 2015;25:236–245. doi: 10.1093/cercor/bht218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature neuroscience. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature neuroscience. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Laughlin RE, Grant TL, Williams RW, Jentsch JD. Genetic dissection of behavioral flexibility: reversal learning in mice. Biological psychiatry. 2011;69:1109–1116. doi: 10.1016/j.biopsych.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawford BR, Young RM, Noble EP, Sargent J, Rowell J, Shadforth S, Zhang X, Ritchie T. The D(2) dopamine receptor A(1) allele and opioid dependence: association with heroin use and response to methadone treatment. Am J Med Genet. 2000;96:592–598. doi: 10.1002/1096-8628(20001009)96:5<592::aid-ajmg3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED. Impulsivity, Stimulant Abuse, and Dopamine Receptor Signaling. Adv Pharmacol. 2016;76:67–84. doi: 10.1016/bs.apha.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Loos M, Staal J, Smit AB, De Vries TJ, Spijker S. Enhanced alcohol self- administration and reinstatement in a highly impulsive, inattentive recombinant inbred mouse strain. Frontiers in behavioral neuroscience. 2013;7:151. doi: 10.3389/fnbeh.2013.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Caneda E, Rodriguez Holguin S, Cadaveira F, Corral M, Doallo S. Impact of alcohol use on inhibitory control (and vice versa) during adolescence and young adulthood: a review. Alcohol Alcohol. 2014;49:173–181. doi: 10.1093/alcalc/agt168. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, Perez de los Cobos J, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug Alcohol Depend. 2011;115:120–130. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas LR, Angulo JA, Le Moal M, McEwen BS, Piazza PV. Neurochemical characterization of individual vulnerability to addictive drugs in rats. The European journal of neuroscience. 1998;10:3153–3163. doi: 10.1046/j.1460-9568.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38:339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. The American journal of psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ. Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol. 2007;73:321–330. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, Puttler LI, Adams KM, Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism: findings in early adolescence. J Abnorm Psychol. 2004;113:302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Nikoshkov A, Drakenberg K, Wang X, Horvath MC, Keller E, Hurd YL. Opioid neuropeptide genotypes in relation to heroin abuse: dopamine tone contributes to reversed mesolimbic proenkephalin expression. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:786–791. doi: 10.1073/pnas.0710902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EP, Zhang X, Ritchie TL, Sparkes RS. Haplotypes at the DRD2 locus and severe alcoholism. Am J Med Genet. 2000;96:622–631. doi: 10.1002/1096-8628(20001009)96:5<622::aid-ajmg7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol ClinExpRes. 2009;33:1294–1303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall RW, Kempermann G, Peirce J, Lu L, Goldowitz D, Gage FH, Goodwin S, Smit AB, Airey DC, Rosen GD, Schalkwyk LC, Sutter TR, Nowakowski RS, Whatley S, Williams RW. Genetics of the hippocampal transcriptome in mouse: a systematic survey and online neurogenomics resource. Front Neurosci. 2009;3:55. doi: 10.3389/neuro.15.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self- administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol. 2008;16:165–177. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochem Res. 2003;28:73–82. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Ishibashi K, Mandelkern MA, Brown AK, Ghahremani DG, Sabb F, Bilder R, Cannon T, Borg J, London ED. Striatal D1- and D2-type dopamine receptors are linked to motor response inhibition in human subjects. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:5990–5997. doi: 10.1523/JNEUROSCI.4850-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen GD, Pung CJ, Owens CB, Caplow J, Kim H, Mozhui K, Lu L, Williams RW. Genetic modulation of striatal volume by loci on Chrs 6 and 17 in BXD recombinant inbred mice. Genes Brain Behav. 2009;8:296–308. doi: 10.1111/j.1601-183X.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. doi: 10.1016/s0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- Rubio G, Jimenez M, Rodriguez-Jimenez R, Martinez I, Avila C, Ferre F, Jimenez-Arriero MA, Ponce G, Palomo T. The role of behavioral impulsivity in the development of alcohol dependence: a 4-year follow-up study. Alcohol Clin Exp Res. 2008;32:1681–1687. doi: 10.1111/j.1530-0277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. J Stud Alcohol. 1980;41:242–249. doi: 10.15288/jsa.1980.41.242. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Annals of the New York Academy of Sciences. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage. 2010;50:1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine- receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Szutorisz H, DiNieri JA, Sweet E, Egervari G, Michaelides M, Carter JM, Ren Y, Miller ML, Blitzer RD, Hurd YL. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:1315–1323. doi: 10.1038/npp.2013.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Posner MI, Rothbart MK, Volkow ND. Circuitry of self-control and its role in reducing addiction. Trends Cogn Sci. 2015;19:439–444. doi: 10.1016/j.tics.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Taqi MM, Bazov I, Watanabe H, Sheedy D, Harper C, Alkass K, Druid H, Wentzel P, Nyberg F, Yakovleva T, Bakalkin G. Prodynorphin CpG-SNPs associated with alcohol dependence: elevated methylation in the brain of human alcoholics. Addiction biology. 2011;16:499–509. doi: 10.1111/j.1369-1600.2011.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. The American journal of psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Terracciano A, Costa PT., Jr Smoking and the Five-Factor Model of personality. Addiction. 2004;99:472–481. doi: 10.1111/j.1360-0443.2004.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasiewicz HC, Jacobs MM, Wilkinson MB, Wilson SP, Nestler EJ, Hurd YL. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biological psychiatry. 2012;72:803–810. doi: 10.1016/j.biopsych.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor BJ, Singleton AB. Nature versus nurture: death of a dogma, and the road ahead. Neuron. 2010;68:196–200. doi: 10.1016/j.neuron.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biological psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten ML, Anthony JC. Comparative epidemiology of initial drug opportunities and transitions to first use: marijuana, cocaine, hallucinogens and heroin. Drug Alcohol Depend. 1999;54:117–125. doi: 10.1016/s0376-8716(98)00151-3. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Vsevolozhskaya OA, Anthony JC. Transitioning from First Drug Use to Dependence Onset: Illustration of a Multiparametric Approach for Comparative Epidemiology. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41:869–876. doi: 10.1038/npp.2015.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shang S, Kang X, Teng S, Zhu F, Liu B, Wu Q, Li M, Liu W, Xu H, Zhou L, Jiao R, Dou H, Zuo P, Zhang X, Zheng L, Wang S, Wang C, Zhou Z. Modulation of dopamine release in the striatum by physiologically relevant levels of nicotine. Nat Commun. 2014;5:3925. doi: 10.1038/ncomms4925. [DOI] [PubMed] [Google Scholar]
- Weaver IC. Epigenetic programming by maternal behavior and pharmacological intervention. Nature versus nurture: let's call the whole thing off. Epigenetics. 2007;2:22–28. doi: 10.4161/epi.2.1.3881. [DOI] [PubMed] [Google Scholar]
- Williams RW, Gu J, Qi S, Lu L. The genetic structure of recombinant inbred mice: high-resolution consensus maps for complex trait analysis. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-11-research0046. RESEARCH0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WC, Ford KA, Pagels NE, McCutcheon JE, Marinelli M. Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:4913–4922. doi: 10.1523/JNEUROSCI.1371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Ji F, Nielsen DA, Levran O, Ho A, Morgello S, Shi R, Ott J, Kreek MJ. A functional haplotype implicated in vulnerability to develop cocaine dependence is associated with reduced PDYN expression in human brain. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1185–1197. doi: 10.1038/npp.2008.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Karlsson C, Liang T, Xiong W, Kimura M, Tapocik JD, Yuan Q, Barbier E, Feng A, Flanigan M, Augier E, Enoch MA, Hodgkinson CA, Shen PH, Lovinger DM, Edenberg HJ, Heilig M, Goldman D. Loss of metabotropic glutamate receptor 2 escalates alcohol consumption. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16963–16968. doi: 10.1073/pnas.1309839110. [DOI] [PMC free article] [PubMed] [Google Scholar]


