Abstract
HIV and alcohol use are two serious and co-existing problems in sub-Saharan Africa. We examined the relationship between spirituality and/or religiousness (SR) and unhealthy alcohol use among treatment-naïve HIV-infected adults attending the HIV clinic in Mbarara, Uganda. Unhealthy alcohol was defined as having either an Alcohol Use Disorders Identification Test – Consumption score of ≥4 for men or ≥3 for women, or having a phosphatidylethanol level of ≥50ng/ml based on analysis of dried blood-spot specimens. Of the 447 participants, 67.8% were female; the median age was 32 years (interquartile range [IQR]: 27–40). About half reported being Protestant (49.2%), 35.1 % Catholic, and 9.2% Muslim. The median SR score was high (103 [IQR: 89–107]); 43.3% drank at unhealthy levels. Higher SR scores were associated with lower odds of unhealthy drinking (adjusted odds ratio [aOR]: 0.83 per standard deviation [SD] increase; 95% confidence interval [CI] 0.66–1.03). The “religious behavior” SR sub-scale was significantly associated with unhealthy alcohol use (aOR: 0.72 per SD increase; 95% CI 0.58–0.88). Religious institutions, which facilitate expression of religious behavior, may be helpful in promoting and maintaining lower levels of alcohol use.
Keywords: Spirituality/Religiousness, Alcohol, HIV, phosphatidylethanol, Uganda
INTRODUCTION
HIV/AIDS and high levels of alcohol consumption are 2 serious and co-existing problems in sub-Saharan African (SSA); the effect of alcohol use on the HIV epidemic has been described as “adding fuel to the fire” (1). Many studies conducted in the region have shown that drinking alcohol, particularly at high levels and in a sexual context, is associated with an increased risk of acquiring HIV infection (2). Those who use alcohol may be more likely to delay HIV testing and treatment initiation (3), and for those who are on highly active anti-retroviral treatment (HAART), alcohol use has been associated with decreased adherence to medication (4).
Among Ugandan adults who drink, the level of alcohol use is on par with the highest levels of consumption in the world, based on World Health Organization estimates (5). The average consumption of pure alcohol per person per year among drinkers is 23.7 liters (5), comparable to drinkers in Russia (22.3 liters) and greater than those in the USA (13.3 liters) (5). Uganda also suffers from an HIV epidemic, with 7.3% of Ugandan adults estimated to be infected based on a 2011 sero-behavioral survey (6). A long term longitudinal study of rural Ugandans demonstrated that those who were HIV-infected were more likely to consume alcohol than those who were not (7). Another study from Uganda showed that among HIV-infected adults who drank alcohol, 64% did so at unhealthy levels (8).
Given that alcohol use is a potentially modifiable behavior, interventions that try to reduce drinking may also help decrease HIV acquisition and transmission, and improve HIV care and treatment (9). Understanding the characteristics of Ugandans who engage in unhealthy drinking would facilitate the design of effective interventions to reduce alcohol consumption. Male sex, lower socio-economic status, having symptoms of depression, and being Catholic have been positively associated with drinking alcohol in several studies in SSA, including in Uganda (7,10–13). It is not known whether the association between some religions and lower levels of drinking is because those religions prohibit or discourage alcohol use, or from other factors such as spirituality and/or religiousness (SR). Spirituality is defined as a connection to that which is considered sacred, while religiousness or religiosity is generally thought of as maintaining beliefs or enacting practices and rituals that express one’s relationship with a higher being (14). Both spirituality and religiosity have been shown to be associated with lower alcohol use in HIV un-infected populations (14).
In Uganda, religion is an important component of life. Almost all (99%) adults belong to an organized religion (15), with 85% identifying as Christian and 12% as Muslim. Therefore, the influence of religion, spirituality, and religiousness on alcohol use is important to explore when designing interventions to reduce levels of drinking. However, we are aware of only 2 studies from Uganda that have evaluated the relationship between SR and alcohol use, and none have been conducted among persons with HIV (16,17). One study found that among Muslim adults, higher levels of religiosity were associated with a decreased likelihood of drinking (16). An evaluation of fisher-folk, a population at particularly high risk for HIV in Uganda, found that engagement in religious activities such as church attendance was associated with less drinking (17).
To better understand and identify potentially effective interventions to reduce drinking in the HIV-infected population in Uganda, we examined the role of SR on unhealthy alcohol use. We used the Ironson-Woods SR index, a scale that has been shown to be correlated with HIV disease outcomes (18,19) to evaluate the relationship between SR and unhealthy alcohol use. We used data from a longitudinal cohort study of treatment-naïve HIV-infected adults. Because depression has been found to be related both to SR (20,21) and to alcohol use (22,23), we also explored whether depression might mediate the relationship between SR and unhealthy drinking.
METHODS
Study design and participants
We analyzed data collected from a parent longitudinal study examining the effect of unhealthy alcohol use on HIV disease progression among HIV-infected adults not yet initiated on HAART. The Alcohol Drinking Effects Prior to Treatment (ADEPT) Study is part of the Uganda-Russia-Boston Alcohol Network for Alcohol Research Collaboration on HIV/AIDS (URBAN ARCH) Consortium. Participants were recruited between 2011 and 2014 from the Mbarara Regional Referral Hospital (MRRH) Immune Suppression Syndrome (ISS) clinic in south-western Uganda. Patients were eligible for the parent study if they were ≥18 years, fluent in English or Runyankole (the local language), lived within 60 kilometers of the clinic, and were not yet eligible to initiate HAART. During the enrollment period, the national guidelines on eligibility for HAART changed in February 2014 from having a CD4 cell count of ≤ 350 cells/mm3, to having a CD4 cell count of ≤ 500 cells/mm3. During the last year of enrollment for the parent study (October 2013-August 2014), we preferentially began recruiting participants who reported drinking alcohol in the prior year.
ADEPT follow-up study visits occurred every 6 months, for a maximum of 36 months. If participants became eligible for HAART during the study, they completed a final interview and exited the cohort. The ISS clinic physicians determined HAART eligibility and provided HIV clinical care independently of study activities. Counseling on alcohol use is not a routine part of care at the ISS clinic, and it is emphasized only at clinic enrolment. There was no alcohol use counseling provided by study staff; however, if a participant requested help to decrease their alcohol use or scored ≥20 on the Alcohol Use Disorders Identification Test (24) they were referred to a mental health counselor in the Psychiatry Department at MRRH. We only used data collected during the first year of follow-up for this analysis.
The institutional review boards of all the participating universities approved the study. Participants provided signed informed consent, and were given lunch and reimbursement for transportation at each study visit.
Measurements
At each study visit, participants completed an interviewer-administered structured questionnaire in English or Runyankole, and underwent blood draws.
Primary Outcome
The primary outcome for this analysis was unhealthy alcohol use in the prior 3 months; we created a composite variable based on both self-report and a biomarker of recent alcohol use, phosphatidylethanol (PEth). We used this composite measure because we previously found patients from the ISS clinic frequently under-reported their alcohol consumption (8,25). Self-reported drinking was measured using the Alcohol Use Disorders Identification Test – Consumption (AUDIT-C) (26,27), which includes 3 questions about alcohol consumption over the past year, each scaled from 0–4; we modified the AUDIT-C to ask about a reference period of the prior 3 months. PEth is a sensitive and specific biomarker that reflects the amount of alcohol consumed during the previous 2–3 weeks. We chose a cut-off value of ≥50 ng/ml. This cut-off value has previously been shown to be 93% sensitive and 83% specific in detecting consumption of ≥2 drinks per day in a sample of 222 patients with liver disease (28); we have also used this cut-off for unhealthy drinking in our previous studies (8,29). Those who had a score of ≥4 for men and ≥3 for women on the AUDIT-C, or who had a PEth result ≥50 ng/ml, were considered to drink at unhealthy levels.
Main Independent Variable
Spirituality and religiousness (SR), the main independent variable, was measured at baseline using the short version of the Ironson-Woods Spirituality/Religiousness Index (18), which includes 22 scaled items divided into 4 domains or sub-scales. The total score on the SR Index can range from 22–110, with higher scores indicating greater spirituality and/or religiousness. The SR scale consists of 4 sub-scales or domains. The domain “sense of peace” is created from 9 items (score range 9–45), that assess the level of comfort, strength, and meaning to life gained from one’s beliefs, the feeling of being connected to a higher power, and the belief in an afterlife. The “faith in God” domain is based on 6 items (score range 6–30) that assess one’s view of God, and beliefs related to the role God may play in recovery from illness. Five items (score range 5–25) form the “religious behavior” domain that measures levels of attendance at religious services, prayer, meditation, participation in religious activities and sharing of beliefs with fellow members. The “compassionate view of others” domain is created from 2 items (score range 2–10) that assess levels of compassion for other people (18). In our study, the Cronbach’s alpha for the overall SR index was 0.95, and as follows for the each of the sub-scales: sense of peace, 0.90; faith in God, 0.90; religious behavior, 0.89; compassionate view of others, 0.93.
Covariates
Demographic characteristics were collected during the study interview, including gender, age, education, employment, marital status, and religious affiliation. Symptoms of depression were measured using the Center for Epidemiologic Studies Depression (CES-D) scale, which contains 20 questions each scaled from 0–3 (30) (Cronbach’s alpha =0.88). A score of ≥16 is considered to identify those at risk for clinical depression. Symptoms of HIV disease during the prior 3 months and lasting at least 4 weeks were assessed by using a standardized HIV symptom checklist; the checklist is based on the 9 most common HIV disease-related symptoms (31). We categorized participants into whether they reported any of these symptoms or not. Social support was measured by using a modified 11-item version of the Duke University-University of North Carolina social support scale (32) (Cronbach’s alpha =0.83). A mean score of < 3 was considered to indicate low perceived levels of social support. The level of physical functioning was evaluated by using the Physical Functioning Scale (PFS) of the Medical Outcomes Study-HIV (MOS-HIV) health survey (33). The PFS consists of 6 items that assess the presence of minor to severe physical limitations; questions were modified to be culturally relevant to Uganda (Cronbach’s alpha =0.85). Raw scores on the PFS were transformed to range from 0 to 100 (34). Baseline values of all covariates were used in the analyses.
Laboratory testing
Venous blood samples were collected at each visit and tested for CD4 cell counts. Viral load testing was done on only baseline samples. Dried blood spot (DBS) cards were prepared from venous blood draws performed at each study visit. DBS testing for PEth was conducted using liquid chromatography and tandem mass spectrometry (LC-MS/MS) (35) at the United States Drug Testing Laboratory in Des Plaines, Illinois. All participants were tested for PEth at baseline. Those who had undetectable levels of PEth (<8 ng/ml) at baseline and who reported no alcohol use at all study visits were not re-tested for PEth at follow-up visits; those with detectable PEth or self-reported alcohol use at any visit were tested for PEth at all visits.
Analysis
Because the SR index was only assessed at baseline, we analyzed unhealthy alcohol use data collected at the first 3 visits completed within the first year of the study to retain temporal proximity to report of SR.
We describe the characteristics of the overall sample at baseline using frequency distributions for categorical variables, and medians and inter-quartile ranges (IQR) for continuous variables. We also calculated the median and IQR of the SR score and sub-scale scores for the entire sample, and stratified by baseline characteristics.
We examined the association between baseline SR scores and unhealthy alcohol use measured at each visit using generalized estimating equation (GEE) logistic regression models to account for correlation due to including multiple observations from participants. The models used a compound symmetry working correlation; robust standard errors are reported for all models. We fit bivariate and multivariable GEE models for unhealthy alcohol use; individual models included either the baseline overall SR scale score or 1 of the 4 SR sub-scale scores as the main predictor of interest. The following baseline covariates were included a priori in all multivariable models because they have previously been shown to be associated with unhealthy alcohol use and/or SR: gender, age, religion, employment, depression, low social support, and MOS-HIV PFS (7,14,36). While alcohol use and SR have both been associated with CD4 cell count and viral load (19,37), we did not include CD4 cell count and HIV viral load in the models because we did not think they were causal predictors of unhealthy alcohol use or SR. As the SR index and sub-scales consist of a different number of items and thus have varying ranges for their raw scores, for the purposes of regression analyses we used z-scores to standardize the scales to have a mean of zero and standard deviation of 1 for comparability between scales. Thus, all regression models of SR and unhealthy alcohol use include SR z-scores rather than raw scores.
We were interested in exploring whether symptoms of depression may be a potential mediator rather than a confounder of the relationship between SR and unhealthy alcohol use. Thus, we additionally fit a multivariable model without the depression variable to assess how inclusion of this factor impacts the association between SR and unhealthy alcohol use.
RESULTS
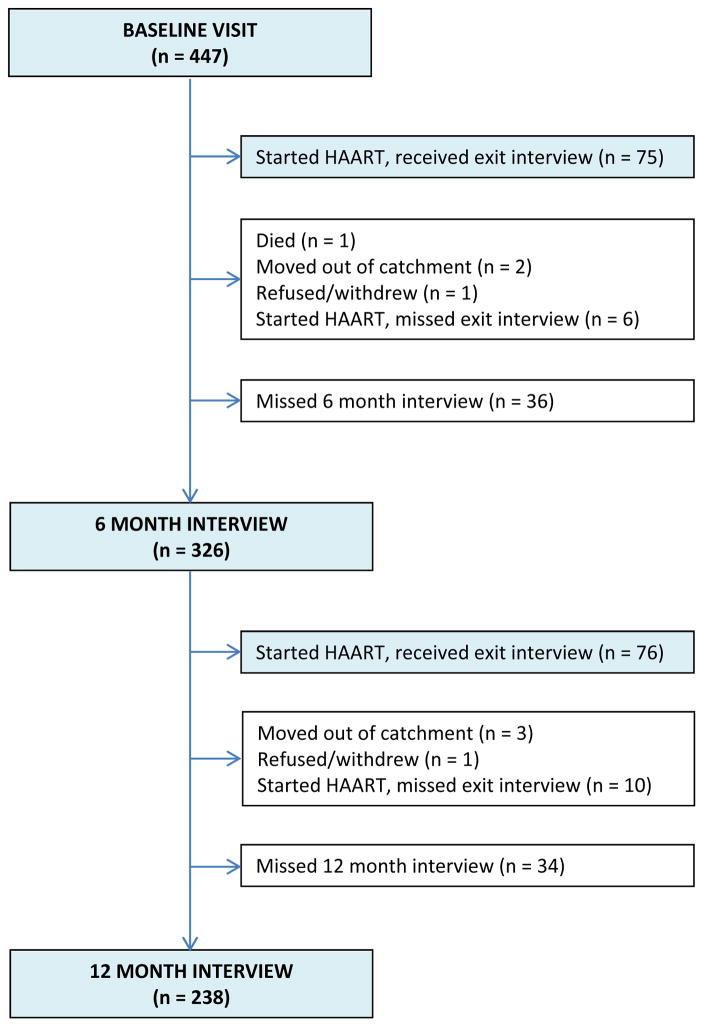
A total of 447 participants completed baseline interviews between September 2011 and August 2014. During the first 12 months of follow-up, 1 participant died, 5 moved away, 2 withdrew, and 167 participants were exited from the parent study as they became eligible for HAART. Seventy-five (75) participants completed an exit interview prior to 6 months, 326 persons completed the 6-month interview, 76 completed an exit interview between 6 and 12 months, and 238 completed the 12-month interview (Figure 1). The median number of interviews included per participant in these analyses was 3 (IQR: 2–3).
Figure 1.
Study interview completion of the first three study interviews completed within 12 months of study enrollment, ADEPT Study, Mbarara, Uganda.
Table 1 describes baseline characteristics of the study participants. Approximately two-thirds (67.8%) were female, the median age was 32 years (IQR: 27–40), half (49.0%) were married, and about two-thirds (69.4%) had a primary education or less. Participants reported belonging to Protestant (49.2%), Catholic (35.1%), Muslim (9.2%), or other (Pentecostal or Seventh Day Adventist) denominations (6.5%). One person, also included in the “other” category, reported having no religious affiliation. At baseline, 43.3% of participants drank alcohol at unhealthy levels. Among participants recruited prior to October 2013, when drinking was not an entry criterion (n = 331), 32.3% used alcohol at unhealthy levels; the prevalence among participants recruited after October 2013 (n = 116), when those who had used alcohol in the prior year were selectively recruited, was 74.8%.
Table i.
Baseline characteristics of ADEPT study participants and Spirituality and Religiousness (SR) Index scores, Mbarara, Uganda (N = 447).
| Baseline characteristics | Overall SR Index Score | SR Index sub-scales | ||||
|---|---|---|---|---|---|---|
| Sense of Peace | Faith in God | Compassionate View of Others | Religious Behaviors | |||
| N (%) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |
| Overall | 447 (100.0) | 103 (89–107) | 42 (36–45) | 29 (24–30) | 10 (8–10) | 22 (20–25) |
| Gender | ||||||
| Female | 303 (67.8) | 103 (88–108) | 42 (36–45) | 29 (24–30) | 10 (8–10) | 22 (20–25) |
| Male | 144 (32.2) | 102 (91–107) | 42 (37–45) | 29 (25–30) | 10 (8–10) | 22 (20–24) |
| Age (years) | 32 (27–40) | |||||
| 18–29 | 164 (36.7) | 102 (88–107) | 42 (36–45) | 28 (24–30) | 10 (8–10) | 21 (20–24) |
| 30–38 | 143 (32.0) | 102 (89–107) | 41.5 (36–45) | 29 (24–30) | 10 (8–10) | 21 (20–24) |
| 39–65 | 140 (31.3) | 104 (90–108) | 43 (36–45) | 30 (25–30) | 10 (8–10) | 23 (20–25) |
| Current marital status | ||||||
| Married | 219 (49.0) | 104 (91–108) | 43 (38–45) | 29.5 (25–30) | 10 (8–10) | 23 (20–25) |
| Not married | 228 (51.0) | 102 (88–107) | 41 (36–45) | 28.5 (24–30) | 10 (8–10) | 21 (20–24) |
| Employment | ||||||
| Paid Employment | 168 (37.8) | 103 (89–107) | 42 (37–45) | 29 (25–30) | 10 (8–10) | 22 (20–24) |
| Business | 78 (17.5) | 101 (91–108) | 43 (36–45) | 29 (25–30) | 10 (8–10) | 21 (20–25) |
| Agriculture | 173 (38.9) | 103 (89–108) | 42 (36–45) | 29 (25–30) | 10 (8–10) | 22.5 (20–25) |
| Unemployed | 26 (5.8) | 88 (88–102) | 36 (36–42) | 24 (24–29) | 8 (8–10) | 20 (20–23) |
| Education | ||||||
| Primary or less | 310 (69.4) | 101 (88–107) | 42 (36–45) | 29 (24–30) | 10 (8–10) | 21 (20–24) |
| More than primary | 137 (30.7) | 104 (92–108) | 43 (37–45) | 29 (25–30) | 10 (8–10) | 23 (20–25) |
| Religion | ||||||
| Protestant | 220 (49.2) | 102 (88–107) | 42 (36–45) | 29 (24–30) | 10 (8–10) | 21.5 (20–24) |
| Catholic | 157 (35.1) | 103 (88–108) | 42 (36–45) | 29 (24–30) | 10 (8–10) | 22 (20–25) |
| Muslim | 41 (9.2) | 103 (93–108) | 43 (37–45) | 29 (26–30) | 10 (9–10) | 23 (20–25) |
| Other | 29 (6.5) | 106 (93–109) | 42 (39–45) | 29 (25–30) | 10 (8–10) | 24 (20–25) |
| AUDIT-C positive | ||||||
| Yes | 133 (30.0) | 102.5 (88.5–107) | 41.5 (36–45) | 29 (24.5–30) | 10 (8–10) | 21 (20–23) |
| No | 311 (70.1) | 103 (89–108) | 42 (36–45) | 29 (24–30) | 10 (8–10) | 22 (20–25) |
| PEth≥50 (ng/ml) | ||||||
| Yes | 154 (34.5) | 101 (88–107) | 41 (36–45) | 29 (24–30) | 10 (8–10) | 20 (20–24) |
| No | 292 (65.5) | 103 (89–108) | 42 (36–45) | 29 (25–30) | 10 (8–10) | 22 (20–25) |
| Unhealthy alcohol use (AUDIT-C+ and/or PEth≥50) | ||||||
| Yes | 193 (43.3) | 102 (88–107) | 41 (36–45) | 29 (24–30) | 10 (8–10) | 21 (20–24) |
| No | 253 (56.7) | 103 (89–108) | 42 (37–45) | 29 (25–30) | 10 (8–10) | 23 (20–25) |
| Symptoms of depression (CES-D≥16) | ||||||
| Yes | 141 (33.4) | 100 (88–107) | 41 (36–44) | 29 (24–30) | 10 (8–10) | 21 (20–24.5) |
| No | 281 (66.6) | 103 (91–108) | 42 (37–45) | 29 (25–30) | 10 (8–10) | 22 (20–25) |
| Low social support | ||||||
| Yes | 133 (30.0) | 101 (88–107) | 41 (36–44) | 29 (24–30) | 10 (8–10) | 21 (20–25) |
| No | 311 (70.1) | 103 (89–108) | 43 (37–45) | 29 (25–30) | 10 (8–10) | 22 (20–25) |
| Presence of HIV symptoms | ||||||
| Yes | 285 (63.8) | 103 (91–108) | 43 (37–45) | 30 (25–30) | 10 (8–10) | 22 (20–25) |
| No | 162 (36.2) | 100.5 (88–106) | 41 (36–44) | 28 (24–30) | 10 (8–10) | 21 (20–24) |
| Physical functioning score, median (IQR) | 100 (88.9–100) | |||||
| CD4 count (cells/mm3) | 550 (416–685) | |||||
| <500 | 180 (40.3) | 103 (90–107) | 42 (36–45) | 29 (25–30) | 10 (8–10) | 22 (20–24) |
| ≥500 | 267 (59.7) | 103 (88–108) | 42 (36–45) | 29 (24–30) | 10 (8–10) | 21 (20–25) |
Based on a CES-D of ≥16, 33.4% of participants were at risk for clinical depression at baseline. Thirty-six percent of participants reported no HIV symptoms in the 4 weeks prior to baseline, and the median CD4 count was 550 cells/mm3 (IQR: 416–685). Almost one-third (30.0%) reported low levels of social support.
The overall median SR score was 103 (IQR: 89–107). The median scores for the subscales were as follows: “sense of peace”: 42 (IQR: 36–45); “faith in God”: 29 (IQR: 24–30); “compassionate view of others”: 10 (IQR: 8–10); and “religious behaviors”: 22 (IQR: 20–25). The distribution of the overall SR and sub-scale median scores by sample characteristics are also shown in Table 1. We note relatively low variability in scores overall, and by sample characteristics.
Table 2 shows unadjusted odds ratios (OR) for the associations between these baseline characteristics and unhealthy alcohol use measured over 1 year of follow-up; this analysis included 1162 observations from 447 study participants. Higher overall SR z-scores appeared to be associated with lower odds of unhealthy alcohol use (OR: 0.83 per 1 standard deviation [SD] increase; 95% Confidence Interval [CI] 0.69, 1.00). Exploratory analyses showed that higher religious behavior sub-scale z-scores were associated with reduced odds of unhealthy drinking (OR: 0.68 per SD increase; 95% CI 0.56, 0.82), while the associations of the other sub-scales with unhealthy alcohol use were not statistically significant. In unadjusted analyses, the odds of engaging in unhealthy drinking among men were 2.7 times higher than those among women (OR: 2.68; 95% CI 1.85, 3.89). Muslims (OR: 0.31; 95% CI 0.15, 0.65) and those belonging to the ‘other’ religion category (mainly Pentecostals and Seventh Day Adventists) (OR: 0.16; 95% CI 0.06, 0.46) had lower odds of unhealthy drinking compared to Protestants.
Table ii.
Association of baseline characteristics, and Spirituality and Religiousness (SR) Index scores, with unhealthy alcohol use, ADEPT Study, Mbarara, Uganda (N = 447).
| Baseline | Longitudinal Regression Model | |||
|---|---|---|---|---|
| Unhealthy alcohol use median (IQR) or N (%)ǂ | Unadjusted Odds Ratio (95% Confidence Interval)§ | z-statistic (p-value) | ||
| Baseline characteristics | No N=253 |
Yes N=193 |
||
| Spirituality and Religiousness (SR) Indexa | ||||
| Overall SR Index | 103 (89–108) | 102 (88–107) | 0.83 (0.69, 1.00) | −1.99 (0.05) |
| Sense of peace | 42 (37–45) | 41 (36–45) | 0.85 (0.71, 1.02) | −1.74 (0.08) |
| Faith in God | 29 (25–30) | 29 (24–30) | 1.00 (0.83, 1.20) | −0.01 (1.00) |
| Compassionate view of others | 10 (8–10) | 10 (8–10) | 0.91 (0.76, 1.09) | −0.98 (0.33) |
| Religious behaviors | 23 (20–25) | 21 (20–24) | 0.68 (0.56, 0.82) | −4.06 (<0.01) |
| Gender | ||||
| Female | 194 (64.2) | 108 (35.8) | 1.00 | |
| Male | 59 (41.0) | 85 (59.0) | 2.68 (1.85, 3.89) | 5.19 (<0.01) |
| Age (years) | ||||
| 18–29 | 98 (59.8) | 66 (40.2) | 1.00 | |
| 30–38 | 76 (53.2) | 67 (46.9) | 1.20 (0.79, 1.82) | 0.87 (0.39) |
| 39–65 | 79 (56.8) | 60 (43.2) | 1.08 (0.70, 1.65) | 0.34 (0.73) |
| Current marital status | ||||
| Married | 122 (56.0) | 96 (44.0) | 1.00 | |
| Not married | 131 (57.5) | 97 (42.5) | 0.91 (0.64, 1.29) | −0.55 (0.59) |
| Employment | ||||
| Paid Employment | 88 (52.4) | 80 (47.6) | 1.00 | |
| Business | 43 (55.1) | 35 (44.9) | 1.01 (0.61, 1.67) | 0.04 (0.97) |
| Agriculture | 102 (59.3) | 70 (40.7) | 0.78 (0.52, 1.16) | −1.23 (0.22) |
| Unemployed | 20 (76.9) | 6 (23.1) | 0.37 (0.16, 0.85) | −2.32 (0.02) |
| Education | ||||
| Primary or less | 172 (55.5) | 138 (44.5) | 1.00 | |
| More than primary | 81 (59.6) | 55 (40.4) | 0.93 (0.64, 1.35) | −0.38 (0.70) |
| Religion | ||||
| Protestant | 117 (53.4) | 102 (46.6) | 1.00 | |
| Catholic | 77 (49.0) | 80 (51.0) | 1.20 (0.82, 1.75) | 0.95 (0.34) |
| Muslim | 33 (80.5) | 8 (19.5) | 0.31 (0.15, 0.65) | −3.10 (<0.01) |
| Other | 26 (89.7) | 3 (10.3) | 0.16 (0.06, 0.46) | −3.41 (<0.01) |
| Symptoms of depression (CES-D≥16) | ||||
| Yes | 81 (57.5) | 60 (42.6) | 1.00 | |
| No | 158 (56.4) | 122 (43.6) | 1.12 (0.76, 1.65) | 0.59 (0.56) |
| Low social support | ||||
| Yes | 72 (54.6) | 60 (45.5) | 1.00 | |
| No | 178 (57.2) | 133 (42.8) | 0.89 (0.61, 1.29) | −0.63 (0.53) |
| Presence of HIV symptoms | ||||
| Yes | 155 (54.6) | 129 (45.4) | 1.00 | |
| No | 98 (60.5) | 64 (39.5) | 0.75 (0.52, 1.08) | −1.53 (0.13) |
| Physical functioning score, median (IQR) | 100 (88.9 – 100) | 100 (88.9 – 100) | 0.99 (0.98, 1.01) | −1.15 (0.25) |
| CD4 count (cells/mm3) | ||||
| <500 | 102 (56.7) | 78 (43.3) | 1.00 | |
| ≥500 | 151 (56.8) | 115 (43.2) | 1.02 (0.72, 1.46) | 0.13 (0.89) |
z-scores of the SR Index are included in the GEE models, ORs correspond to 1 standard deviation increase in score
Frequencies and medians are from the baseline visit
odds ratios and 95% confidence intervals are from GEE models, including 1162 study visits from 447 participants
Separate multivariable models were used to examine the association of the overall SR scale and of each of the SR sub-scales with unhealthy alcohol use during the first year of follow-up (Table 3). Similar to unadjusted analyses, overall SR z-scores appeared to be associated with lower odds of unhealthy alcohol use; however, this association was no longer statistically significant in the multivariable model (Model 1; adjusted OR [aOR]: 0.83 per SD increase; 95% CI 0.66, 1.03). In the multivariable analyses, only the association of the “religious behavior” sub-scale z-score with unhealthy alcohol use reached statistical significance (Model 5; aOR: 0.72 per SD increase; 95% CI 0.58, 0.88). In the multivariable model that included the overall SR score (Model 1), the odds of unhealthy alcohol use were significantly higher for men (aOR: 2.80; 95% CI 1.84, 4.24) compared to women, and significantly lower for Muslim participants (aOR: 0.26; 95% CI 0.12, 0.58), and for those in the ‘other’ religion category (aOR: 0.13; 95% CI 0.04, 0.41), compared to Protestants. The associations of these covariates with unhealthy alcohol use were consistent across all multivariable models. In a model excluding the depression variable, the adjusted odds of unhealthy alcohol use per SD increase in the overall SR score was 0.80 (95% CI 0.66, 0.98), suggesting that having symptoms of depression does not mediate the relationship between SR and unhealthy alcohol use.
Table iii.
Multivariable models* evaluating the association between Spirituality and Religiousness (SR) Index scores and baseline factors with unhealthy alcohol use, ADEPT Study, Mbarara, Uganda (N = 447).
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline characteristics |
aOR (95% CI) | z-statistic (p-value) |
aOR (95% CI) | z-statistic (p-value) |
aOR (95% CI) | z-statistic (p-value) |
aOR (95% CI) | z-statistic (p-value) |
aOR (95% CI) | z-statistic (p-value) |
| Overall SR Index | 0.83 (0.66, 1.03) | −1.67 (0.10) | - | - | - | - | - | - | - | - |
| SR Index subscales a | ||||||||||
| Sense of peace | - | - | 0.83 (0.67, 1.03) | −1.67 (0.09) | - | - | - | - | - | - |
| Faith in God | - | - | - | - | 1.02 (0.83, 1.24) | 0.15 (0.88) | - | - | - | - |
| Compassionate view of others | - | - | - | - | - | - | 0.90 (0.73, 1.11) | −1.00 (0.32) | - | - |
| Religious behaviors | - | - | - | - | - | - | - | - | 0.72 (0.58, 0.88) | −3.15 (<0.01) |
| Gender | ||||||||||
| Female | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| Male | 2.80 (1.84, 4.24) | 4.83 (<0.01) | 2.81 (1.86, 4.26) | 4.87 (<0.01) | 2.84 (1.87, 4.31) | 4.90 (<0.01) | 2.85 (1.88, 4.31) | 4.94 (<0.01) | 2.64 (1.73, 4.03) | 4.49 (<0.01) |
| Age (years) | ||||||||||
| 18–29 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| 30–38 | 0.99 (0.61, 1.60) | −0.05 (0.96) | 0.99 (0.61, 1.60) | −0.03 (0.97) | 0.97 (0.60, 1.56) | −0.14 (0.89) | 0.98 (0.61, 1.58) | −0.09 (0.93) | 0.99 (0.61, 1.61) | −0.03 (0.98) |
| 39–65 | 1.07 (0.65, 1.76) | 0.25 (0.80) | 1.06 (0.64, 1.74) | 0.22 (0.83) | 1.03 (0.62, 1.70) | 0.11 (0.92) | 1.04 (0.63, 1.71) | 0.15 (0.88) | 1.14 (0.68, 1.89) | 0.50 (0.62) |
| Employment | ||||||||||
| Paid Employment | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| Business | 1.04 (0.60, 1.81) | 0.15 (0.88) | 1.05 (0.61, 1.82) | 0.17 (0.86) | 1.05 (0.61, 1.82) | 0.17 (0.87) | 1.04 (0.60, 1.80) | 0.15 (0.89) | 1.02 (0.58, 1.79) | 0.08 (0.94) |
| Agriculture | 0.79 (0.50, 1.25) | −1.01 (0.31) | 0.80 (0.51, 1.25) | −0.98 (0.33) | 0.81 (0.51, 1.27) | −0.93 (0.35) | 0.79 (0.50, 1.25) | −1.01 (0.31) | 0.80 (0.50, 1.26) | −0.97 (0.33) |
| Unemployed | 0.56 (0.21, 1.51) | −1.15 (0.25) | 0.56 (0.21, 1.51) | −1.15 (0.25) | 0.62 (0.23, 1.66) | −0.95 (0.34) | 0.58 (0.22, 1.55) | −1.08 (0.28) | 0.57 (0.21, 1.54) | −1.11 (0.27) |
| Religion | ||||||||||
| Protestant | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| Catholic | 1.12 (0.74, 1.71) | 0.54 (0.59) | 1.12 (0.74, 1.70) | 0.53 (0.59) | 1.10 (0.73, 1.68) | 0.46 (0.64) | 1.11 (0.73, 1.69) | 0.49 (0.62) | 1.12 (0.73, 1.71) | 0.53 (0.60) |
| Muslim | 0.26 (0.12, 0.58) | −3.29 (<0.01) | 0.26 (0.11, 0.58) | −3.27 (<0.01) | 0.25 (0.11, 0.56) | −3.38 (<0.01) | 0.26 (0.11, 0.58) | −3.30 (<0.01) | 0.26 (0.12, 0.56) | −3.38 (<0.01) |
| Other | 0.13 (0.04, 0.41) | −3.47 (<0.01) | 0.13 (0.04, 0.41) | −3.48 (<0.01) | 0.13 (0.04, 0.41) | −3.48 (<0.01) | 0.13 (0.04, 0.40) | −3.51 (<0.01) | 0.14 (0.04, 0.42) | −3.46 (<0.01) |
| Symptoms of depression (CES-D≥16) | ||||||||||
| Yes | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| No | 1.17 (0.75, 1.81) | 0.70 (0.49) | 1.18 (0.76, 1.82) | 0.72 (0.47) | 1.12 (0.73, 1.73) | 0.52 (0.60) | 1.15 (0.74, 1.77) | 0.62 (0.53) | 1.14 (0.73, 1.78) | 0.58 (0.56) |
| Low social support | ||||||||||
| Yes | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| No | 0.80 (0.52, 1.22) | −1.04 (0.30) | 0.80 (0.52, 1.23) | −1.00 (0.32) | 0.76 (0.49, 1.15) | −1.30 (0.20) | 0.78 (0.51, 1.20) | −1.12 (0.26) | 0.80 (0.52, 1.24) | −0.99 (0.32) |
| Physical functioning score | 0.99 (0.97, 1.00) | −1.50 (0.13) | 0.99 (0.97, 1.00) | −1.58 (0.11) | 0.99 (0.97, 1.00) | −1.45 (0.15) | 0.99 (0.97, 1.00) | −1.42 (0.16) | 0.99 (0.97, 1.01) | −1.40 (0.16) |
aOR = adjusted odds ratio
95%CI = 95% confidence interval
z-scores of the SR Index are included in the GEE models, ORs correspond to 1 standard deviation increase in score
models adjusted for the variables listed in the table, and include 1162 study visits from 447 participants
DISCUSSION
We evaluated the level of spirituality and religiousness among HIV-infected HAART-naive adults in Uganda and its relationship to unhealthy alcohol use. Overall, SR scores were extremely high among all study participants, regardless of demographic characteristics, presence of depression, levels of social support, or symptoms of HIV disease. SR scores did not appear to vary by religious affiliation or denomination.
Those with higher levels of SR appeared to have lower odds of unhealthy drinking, but this association did not reach statistical significance in adjusted analyses. Those with higher scores on the religious behavior sub-scale—which includes participation in religious rituals, attending religious services, praying, and discussion of religious beliefs with others—had lower odds of drinking at unhealthy levels. In contrast, the scores on the SR sub-scales measuring “sense of peace,” “belief in God,” and “compassionate view of others” were not related to unhealthy alcohol use.
The mean overall SR score was higher among participants in our study (99.0, SD 10.6), compared to scores measured among a group of HIV-infected adults in the US (89.33, SD 19.38), both evaluated using the Ironson–Woods SR Index (18). The high SR scores that we found may not be surprising, given that religion plays an important role in the lives of most Ugandans. Nearly everyone in the country reports being affiliated with an organized religion (15), in contrast to residents of the US and other western countries, where religious affiliation is much less prevalent (38). Being diagnosed with HIV infection might also enhance feelings of spirituality and religiousness (19). Engaging in religious practices could serve as an alternative method of dealing with stress or other problems, rather than resorting to drinking. Some studies have shown that those who use alcohol as a means of coping rather than drinking for social reasons are more likely to suffer from alcohol problems (39,40).
We also found that those who were Muslim or who were Pentecostal or Seventh Day Adventist were much less likely to drink heavily. These religions dictate against the use of alcohol or actively discourage its use. In addition to overt institutional proscriptions against drinking, attending religious services and gatherings may provide peer support for and reinforce norms around minimal or no alcohol use.
Other studies have shown that those who are spiritual and/or religious are less likely to have symptoms of depression (14), and that those who drink alcohol are more likely to be depressed (22,23). Based on these observations, we explored whether depression might mediate the relationship between SR and unhealthy alcohol use. However, when we excluded symptoms of depression from the multivariable model, the aOR for the association of SR and unhealthy alcohol use was very similar to the model in which the CES-D score was included. This indicates that depression is unlikely to mediate the relationship between SR and unhealthy alcohol use in our study.
This study had several limitations. The parent study only included participants who were not yet eligible for HAART. Therefore, our findings may not be applicable to HIV-infected persons on treatment, particularly as alcohol consumption may decrease when patients begin HAART (8,36,41). Alcohol counseling is not routine at the ISS Clinic; however, some patients may have received alcohol counseling at the clinic even prior to HAART initiation. Our findings may also not be applicable to HIV uninfected adults. We collected information on SR only at baseline, assuming that it would remain constant throughout the one-year period analyzed here. The Ironson-Woods SR Index has not been validated for use among Ugandans, and may not measure aspects of SR that are important to or expressed by Ugandans. However, we conducted cognitive interviewing of the SR scale as part of a previous study (8), and found good understanding of the questions. Also, we found good scale reliability in this study, as measured by Cronbach’s alpha. However, testing the validity of the scale among populations in sub-Saharan Africa is needed if this measurement tool is to be used widely.
Another limitation of our findings is that we did not ask any questions about religious prohibitions of drinking alcohol, or whether the religion to which they reported affiliation actively discouraged or banned alcohol use. Additionally, because religion is considered to be important in Uganda, the high SR index scores might have been the result of social desirability bias, and the relatively narrow range in SR scores may have limited the power of our study to detect an effect of SR on the outcome. Performing qualitative interviews to better understand SR in the Ugandan setting and to help clarify the mechanisms by which SR impacts unhealthy alcohol use and other health related outcomes would be useful.
Despite these limitations, we used a composite measure of alcohol use that included a highly specific alcohol biomarker (PEth) to supplement self-report. Self-report of alcohol use in this population has been shown to be liable to reporting bias (25). In addition, we measured unhealthy alcohol use several times over the one-year follow-up period analyzed in this study.
In conclusion, we found that levels of SR were high among treatment-naïve HIV infected clients in Uganda. We were unable to detect a statistically significant association between overall SR score and unhealthy alcohol use; however, religious behaviors, as well as affiliation with religions that proscribe against alcohol use, appear to be associated with less unhealthy alcohol use. As unhealthy drinking is common in Uganda, churches and religious institutions that facilitate religious behavior may be able to play a useful role in promoting and maintaining reductions in alcohol use.
Acknowledgments
We would like to acknowledge the study participants and the ISS clinic counselors, as well as the study research assistants for their tireless effort in collecting the data.
University of California, San Francisco International Traineeship in Aids Prevention Studies (ITAPS): NIMH, R25MH064712 and CV Starr foundation for the technical help in preparing this manuscript.
We would also like to thank Gail Ironson and Lauren Kaplan for providing guidance on how to interpret the spirituality/religiousness scale.
Footnotes
References
- 1.Hahn JA, Woolf-king SE, Muyindike W. Adding Fuel to the Fire : Alcohol ‘ s Effect on the HIV Epidemic in Sub-Saharan Africa. Curr HIV/AIDS Rep. 2011;8(3):172–80. doi: 10.1007/s11904-011-0088-2. [DOI] [PubMed] [Google Scholar]
- 2.Woolf-King Sarah E, Steinmaus Craig M, Reingold Arthur LJAH. An update on alcohol use and risk of HIV infection in sub-Saharan Africa: Meta-analysis and future research directions. International journal of alcohol and drug research. 2013;2(1):99–110. [Google Scholar]
- 3.Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, Altice FL. The Impact of Alcohol Use and Related Disorders on the HIV Continuum of Care : a Systematic Review Alcohol and the HIV Continuum of Care. Curr HIV/AIDS Rep. 2015:421–36. doi: 10.1007/s11904-015-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: Review and metaanalysis. Journal of acquired immune deficiency syndromes (2009) 2009 Oct;52(2):180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Global status report on alcohol and health 2014. 2014. [Google Scholar]
- 6.Ministry of Health Uganda. AIDS Indicator Survey (AIS) 2011. Kampala: 2011. [Google Scholar]
- 7.Asiki G, Baisley K, Kamali A, Kaleebu P, Seeley J, Newton R. A prospective study of trends in consumption of cigarettes and alcohol among adults in a rural Ugandan population cohort, 1994–2011. Tropical Medicine & International Health. 2015;20(4):527–36. doi: 10.1111/tmi.12451. [DOI] [PubMed] [Google Scholar]
- 8.Hahn JA, Emenyonu NI, Fatch R, Muyindike WR, Kekiibina A, Carrico AW, et al. Declining and rebounding unhealthy alcohol consumption during the first year of HIV care in rural Uganda, using phosphatidylethanol to augment selfreport. Addiction. 2015;111(2):272–9. doi: 10.1111/add.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraemer KL. Can a Behavioral Alcohol Intervention be Delivered CostEffectively to Persons Living with HIV/AIDS in Sub-Saharan Africa. Alcoholism: Clinical and Experimental Research. 2016;40(1):50–1. doi: 10.1111/acer.12934. [DOI] [PubMed] [Google Scholar]
- 10.Wandera B, Tumwesigye NM, Nankabirwa JI, Kapiga S, Sethi AK. Alcohol Consumption among HIV-Infected Persons in a Large Urban HIV Clinic in Kampala Uganda : A Constellation of Harmful Behaviors. PLos ONe. 2015;38(10(5)):1–16. doi: 10.1371/journal.pone.0126236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kader R, Seedat S, Govender R, Koch JR, Parry CD. Hazardous and Harmful use of Alcohol and/or Other Drugs and Health Status Among South African Patients Attending HIV Clinics. AIDS and Behavior. 2014;18:525–34. doi: 10.1007/s10461-013-0587-9. [DOI] [PubMed] [Google Scholar]
- 12.Francis JM, Weiss HA, Mshana G, Baisley K. The Epidemiology of Alcohol Use and Alcohol Use Disorders among Young People in Northern Tanzania. PLos ONe. 2015;10(10):1–17. doi: 10.1371/journal.pone.0140041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn JA, Fatch R, Wanyenze RK, Baveewo S, Kamya MR, Bangsberg DR, et al. Decreases in self-reported alcohol consumption following HIV counseling and testing at Mulago Hospital, Kampala, Uganda. BMC Infectious diseases. 2014;14(1):403. doi: 10.1186/1471-2334-14-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koenig HG. Religion, Spirituality, and Health : The Research and Clinical Implications. ISRN Psychiatry. 2012;2012 doi: 10.5402/2012/278730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uganda Bureau of statistics. 2002 Uganda Population and Housing Census Main Report. Kampala: 2002. [Google Scholar]
- 16.Kagimu M, Guwatudde D, Rwabukwali C, Kaye S, Walakira Y, Ainomugisha D. Religiosity for HIV prevention in Uganda: A case study among Muslim youth in Wakiso district. African Health Sciences. 2012;12(3):282–90. [PMC free article] [PubMed] [Google Scholar]
- 17.Tumwesigye NM, Atuyambe L, Kibira SPS, Wabwire-Mangen F, Tushemerirwe F, Wagner GJ. Do Religion and Religiosity Have Anything to Do With Alcohol Consumption Patterns? Evidence From Two Fish Landing Sites on Lake Victoria Uganda. Substance Use & Misuse. 2013;48(12):1130–7. doi: 10.3109/10826084.2013.808464. [DOI] [PubMed] [Google Scholar]
- 18.Ironson G, Ph D, Solomon GF, Balbin E, COAG, et al. The Ironson – Woods Spirituality/Religiousness Index Is Associated With Long Survival, Health Behaviors, Less Distress, and Low Cortisol in People With HIV/AIDS. Ann Behav Med. 2002;24(1):34–48. doi: 10.1207/S15324796ABM2401_05. [DOI] [PubMed] [Google Scholar]
- 19.Ironson G, Stuetzle R, Fletcher MA. An increase in religiousness/spirituality occurs after HIV diagnosis and predicts slower disease progression over 4 years in people with HIV. Journal of General Internal Medicine. 2006;21(SUPPL 5):62–8. doi: 10.1111/j.1525-1497.2006.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safiya DG, Marcia HM, Colleen D, Gary L. Spiritual well-being, depressive symptoms, and immune status among women living with HIV/AIDS. Women Health. 2009;49(2–3):119–43. doi: 10.1080/03630240902915036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi MS, Mrus JM, Wade TJ, Ho ML, Hornung RW, Cotton S, et al. Religion, Spirituality, and Depressive Symptoms in Patients with HIV/AIDS. Journal of General Internal Medicine. 21(5):21–7. doi: 10.1111/j.1525-1497.2006.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuria MW, Ndetei DM, Obot IS, Khasakhala LI, Bagaka BM, Mbugua MN, et al. The Association between Alcohol Dependence and Depression before and after Treatment for Alcohol Dependence. ISRN Psychiatry. 2012;2012 doi: 10.5402/2012/482802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotton S, Puchalski CM, Sherman SN, Mrus JM, Peterson AH, Feinberg J, et al. Spirituality and Religion in Patients with HIV/AIDS. Journal of General Internal Medicine. 2009;24(8):994. doi: 10.1111/j.1525-1497.2006.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babor TF, Higgins-biddle JC, Saunders JB, Monteiro MG, Higgins-biddle JC, Saunders JB, et al. The Alcohol Use Disorders Identification Test [Google Scholar]
- 25.Bajunirwe F, Haberer JE, YB, Hunt P. Comparison of Self-Reported Alcohol Consumption to Phosphatidylethanol Measurement among HIV-Infected Patients Initiating Antiretroviral Treatment in Southwestern Uganda. PLos ONe. 2014;9(12):e113152. doi: 10.1371/journal.pone.0113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley AK, Bush RK, Amee Epler R, Dobie D, Sporleder JL, Maynard C, et al. Two Brief Alcohol-Screening Tests From the Alcohol Use Disorders Identification Test (AUDIT) Archives of Internal Medicine. 2003;163(7):821–9. doi: 10.1001/archinte.163.7.821. [DOI] [PubMed] [Google Scholar]
- 27.Bush K, Kivlahan D, McDonell BM, Fihn DS. The AUDIT Alcohol Consumption Questions (AUDIT-C) An effective brief screening test for problem drinking. Archives of Internal Medicine. 1998;158(16):1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 28.Stewart SH, Koch DG, Willner IR, Anton RF, Reuben A. Validation of Blood Phosphatidylethanol as an Alcohol Consumption Biomarker in Patients with Chronic Liver Disease. Alcoholism: Clinical and Experimental Research. 2014;38(6):1706–11. doi: 10.1111/acer.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrico AW, Hunt PW, Emenyonu NI, Muyindike W, Ngabirano C, Cheng DM, et al. Unhealthy Alcohol Use is Associated with Monocyte Activation Prior to Starting Antiretroviral Therapy. Alcohol Clin Exp Res. 2015;39(12):2422–6. doi: 10.1111/acer.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radloff S. The-CES-D-Scale: A Self-report Depression Scale for Research in the General Population.pdf. Appl Psychol Measur. 1977:385–401. [Google Scholar]
- 31.Justice AC, Chang CH, Rabeneck L, Zackin R. Clinical importance of provider-reported HIV symptoms compared with patient-report. Medical care. 2001;39(4):397–408. doi: 10.1097/00005650-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Broadhead WE, Gehlbach SH, De Gruy FV, Kaplan BH. The Duke UNC Functional Social Support Questionnaire. Medical Care. 1988;26:709–23. doi: 10.1097/00005650-198807000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Mast TC, Kigozi G, Wabwire-Mangen F, Black R, Sewankambo N, Serwadda D, et al. Measuring quality of life among HIV-infected women using a culturally adapted questionnaire in Rakai district, Uganda. AIDS care. 2004;16(October 2015):81–94. doi: 10.1080/09540120310001633994. [DOI] [PubMed] [Google Scholar]
- 34.Wu AW. MOS-HIV Health Survey Users Manual. 1999 [Google Scholar]
- 35.Jones J, Jones M, Plate C, Lewis D. The detection of 1-palmitoyl-2-oleoyl-snglycero-3-phosphoethanol in human dried blood spots. Analytical Methods. 2011;3(5):1101. [Google Scholar]
- 36.Santos GM, Emenyonu NI, Bajunirwe F, Rain Mocello a, Martin JN, Vittinghoff E, et al. Self-reported alcohol abstinence associated with ART initiation among HIV-infected persons in rural Uganda. Drug and Alcohol Dependence. 2014;134(1):151–7. doi: 10.1016/j.drugalcdep.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. Journal of acquired immunedeficiency syndromes (1999) 2007;46(2):194–9. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hout M, Fischer S. Explaining Why More Americans Have No Religious Preference : Political Backlash and Generational Succession,1987–2012. sociological science. 2014;1(October):423–47. [Google Scholar]
- 39.Drerup ML, Johnson TJ, Bindl S. Mediators of the relationship between religiousness/spirituality and alcohol problems in an adult community sample. Addictive Behaviors. 2011;36(12):1317–20. doi: 10.1016/j.addbeh.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Johnson TJ, Sheets VL, Kristeller JL. Identifying mediators of the relationship between religiousness/spirituality and alcohol use. Journal of studies on alcohol and drugs. 2008;69(1):160–70. doi: 10.15288/jsad.2008.69.160. [DOI] [PubMed] [Google Scholar]
- 41.Sundararajan R, Wyatt MA, Hahn JA, Ware NC. Qualitative Study of Changes in Alcohol Use Among HIV-Infected Adults Entering Care and Treatment for HIV/AIDS in Rural Southwest Uganda. AIDS and Behavior. 2015;19(4):732–41. doi: 10.1007/s10461-014-0918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]