Abstract
Background
The opioid epidemic is a growing concern and emerging evidence suggests that morphine use may be associated with sepsis. Enteric glial cells (EGCs) are the most numerous cell type in the enteric nervous system and regulate gastrointestinal function through the production of trophic factors, including Glial Derived Neurotrophic Factor (GDNF). We sought to determine the effect of morphine on enteric glia and hypothesized that morphine contributes to EGC dysfunction and increased gut permeability.
Materials and Methods
Rat Intestinal Epithelial Cells (IECs) and EGC lines were purchased from ATCC. Immunocytochemistry was used to evaluate the impact of EGCs on IEC barrier proteins and detect the μ-opioid receptor. Co-culture assays were used to determine the effect of EGCs, GDNF, and morphine on barrier integrity. QPCR and western blotting was performed to determine the impact of morphine in GDNF production. Trans-epithelial resistance (TER) of IEC-6 cell monolayers was measured in the presence of EGC-conditioned media (EGC-CM) and morphine treated (EGC-CM) using ECIS.
Results
EGC conditioned media (EGC-CM) enhanced tight junction organization in IECs. IEC barrier integrity was enhanced when co-cultured with unstimulated EGCs or with GDNF alone; this barrier protective effect was lost with morphine treated EGCs. GDNF RNA and protein expression were decreased by morphine treatment. TER was decreased in IEC confluent monolayers when exposed to morphine-treated EGC-CM compared to control.
Conclusions
Morphine compromises intestinal epithelial cell barrier function through a mechanism which appears to involve GDNF. Further studies are warranted to delineate the role of enteric glial cell function in opioid signaling and sepsis.
Keywords: Enteric Nervous System, Morphine, GDNF, Enteric Glia, Opioids, Intestinal Barrier Integrity
Introduction
The opioid epidemic in the United States has been a growing source of concern for American physicians. According to recently published data from the Centers for Disease Control, 259 million opioid prescriptions were written in 2012 for chronic pain [1]. This number has increased from 2008, when 205 million opioid prescriptions were written which represents opioids given for all sources of pain [2]. Furthermore, in the emergency department opioid prescriptions continue to increase and in 2011 numbered at 184.1 prescriptions per 100,000 compared to 82.5 per 100,000 in 2004 [3]. Concomitantly to the increase in opioid prescriptions, there has been an alarming increase in opioid related complications including sepsis and death. For example, in 2013, prescription opioids contributed to more deaths than all other illicit drugs combined (>16,200 versus 14,775) [3].
The common side effects of opioids have been well described and include nausea, vomiting, constipation, somnolence, and respiratory depression [4]. There is now a growing interest in elucidating the effects of opioid use on intestinal barrier function. Specifically, recent studies have demonstrated an association between opioid use and sepsis through animal model experiments demonstrating increased gut bacterial translocation in morphine treated mice [5,6].
Enteric Glial Cells (EGCs) are the most numerous cell population within the enteric nervous system and are identified by their staining positivity for GFAP [7]. Previous studies have identified that EGCs play a role in the integrity of the intestinal barrier function through the release of various trophic factors including Glial Derived Neurotrophic Factor (GDNF) [7,8,9,10]. It has been previously shown that in the murine models that have EGCs selectively abolished, there is decreased intestinal epithelial integrity leading to intestinal hemorrhage and necrosis [8,11,12]. Less is known, however, about the impact of opioids on enteric glial-mediated enhancement of intestinal barrier function. The purpose of our experiments was to determine the impact of morphine stimulation on EGC cell function. We hypothesized that EGC-mediated intestinal barrier enhancement would be lost when stimulated with morphine.
Methods
Cell Culture
Disposable culture ware was purchased from Thermo Scientific (Thermo Scientific, Waltham, MA). IEC-6 and EGC/PK060399egrf cell lines were purchased from American Type Culture Collection (Manassas, VA) and the cells were cultured as the supplier recommends. IEC-6 cells are rat small intestinal epithelial cells. EGCs are rat enteric glial cells. Culture media was purchased from Gibco (Waltham, MA) and Sigma-Aldrich (St. Louis MO). Both cell lines were cultured in DMEM supplemented by penicillin/streptomycin (100u/ml, Gibco) and 10% FBS (Gibco). IEC-6 cells were cultured with 0.1U/ml bovine insulin. Cells were cultured in a NAPCO Series 8000DH incubator (ThermoFischer Scientific, Waltham, MA) at 37.0°C at 5% CO2.
Immunocytochemistry
μ-opioid receptor staining
EGCs were plated in 8-well chamber slides (ThermoScientific, Waltham, MA). Cells were grown x24 hours and fixed with 4% paraformaldehyde. Cells were blocked with 5% FBS, washed with ice cold PBS and permeabilized with 0.1% Triton-x and incubated overnight with primary anti-rabbit anti-μ opioid receptor antibody (Abcam 10275, Cambridge, MA). Cells were washed three times with PBS and stained with goat anti-rabbit FITC conjugated secondary antibody for 1 hour (Jackson Immunoresearch, #111-095-003). Cells were stained with DAPI-prolong gold anti-fade (Invitrogen) and analyzed for fluorescent antibody receptor expression.
IEC-6 tight junction protein staining
IEC-6 cells (ATCC, Manassas, VA) were cultured as recommended by the supplier and plated in the presence of normal medium or the supernatant of rat enteric glial cells for 72 hours. Cells were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature. After washing in PBS and blocking of nonspecific binding sites with 5% BSA, tissues were incubated with polyclonal rabbit anti-occludin (used at 5 mg/ml, Invitrogen) in PBS with 5% bovine serum albumin (BSA) for 120 min at room temperature. After washing, cells were incubated with rhodamine phalloidin (Invitrogen) and DyLightTM 488-conjugated AffiniPure Donkey anti-rabbit IgG (0.075 mg/ml, Jackson Lab, WestGrove, PA) for 60 min. Cells were then washed and mounted under coverslips using ProLong Gold antifade reagent with DAPI (blue) (Invitrogen).
Transwell Co-culture Experiments
IECs (Rat, ATCC IEC-6, Manassas, VA) were plated at a concentration of 2 × 10^5 cells/well in the apical chamber of the transwell plate (0.4μm pore size, Corning, Kennebunk, ME). In the basal chamber of the transwell plate, EGCs (Rat, ATCC EGC/PK060399egfr, Manassas, VA) were plated at a concentration of 8 × 10^4 cells/well. When EGCs were not plated into the well, EGC-conditioned media (EGC-CM, media from untreated or morphine treated confluent EGCs plated in a separate 6 well plate) was added to the well. If no EGCs or EGC-CM was present in the well, 2ml of cell growth media was added (10% FBS). Final volumes were 500μl to the insert and 2ml to the well. In morphine treated EGCs in the well, the cells were treated with 1μM morphine 6 hours after cell plating. GDNF was obtained from Abcam (187229, Cambridge, MA). When GDNF was added to the plate well (containing only 2ml growth media), it was added at a concentration of 100ng/ml immediately after adding media to the plate well.
The plate was incubated without disruption for 72 hours to allow cell growth to confluence. At 72 hours, all culture media was removed from the apical chamber and replaced with a 10 mg/mL 4KDa FITC-Dextran (Sigma, St. Louis, MO) and 2.5 mg/mL 70KDa Rhodamine-Dextran solution (Sigma, St. Louis, MO). After four hours and 24 hrs, the basal chamber media was removed and fluorescence was measured (FITC - 485nm excitation/535nm emission; Rhodamine - 530nm excitation/590nm emission) using a Synergy II Plate Reader (BioTek, Winooski, VT). Data was charted and analyzed using Prism (Graphpad) software (La Jolla, CA) and evaluated for trends and statistical significance using Student’s t-test.
Realtime PCR
Total cellular RNA was extracted using TRIzol (Invitrogen) and isolated utilizing chloroform and centrifugation with isopropyl alcohol. cDNA was synthesized with the M-MLV reverse transcription kit from Promega. Quantitative real-time polymerase chain reaction (PCR) was performed utilizing Roche lightcycler 480 PCR detection system on all samples in triplicate. 18S RNA was used to normalize mRNA expression levels. mRNA analysis was performed by culturing IECs to confluence and treating them for 24 hours with 1 μM morphine. qPCR analysis was performed using primers for Glial-Derived Neurotrophic Factor (GDNF). Primers were purchased from IDT. Primer sequences were as follows: GDNF - Forward 5′-CGAAGATTATCCTGACCAGTTTGA-3′, Reverse 5′ CAGTTCCTCCTTGGTTTCGTAG 3′; 18S 5′-GTAACCCGTTGAACCCCATT-3′.
Western Blot
Enteric Glial cells were lysed using radioimmunoprecipitation assay (RIPA) buffer (Sigma). Cell lysates (60μg protein per lane) from control and 1μM morphine treatment x72hrs (+MS) were resolved in a 12% SDS-PAGE gel and transferred onto a nitrocellulose membrane. The membrane was blocked in 5% milk in TBS buffer and the blot was probed with antibodies against GDNF (Abcam 18956, Cambridge, MA) and b-actin (Abcam 8226, Cambridge, MA).
Densitometric analysis of GDNF level in control and morphine treatment was determined using ImageJ (NIH). GDNF protein level was normalized to the level of b-actin in each sample. The normalized control was considered as 100%, +MS = morphine treated EGCs. Paired Student’s t-test was performed and graph was plotted using Prism 4.0 (Graphpad).
Measurement of trans-epithelial resistance
ECIS 1600R (Applied BioPhysics, Troy, NY) was used to measure trans-epithelial resistance (TER) of epithelial monolayers as described previously [13]. Epithelial cells (IEC-6) were seeded in the wells of the electrode array and grown to confluence over 48 hours. The medium was exchanged, and cells were treated with either un-stimulated EGC-conditioned media (EGC-CM) or morphine-stimulated (1μM) EGC-CM. The ECIS measurements were continuously obtained for a minimum of another 48 hours.
Statistical Analysis
Data was plotted and analyzed by Student’s t-test using Prism 4.0 software (Graphpad). Quantitative data points are expressed as mean values with the standard deviation.
Results
Enteric glia possess the μ-opioid receptor
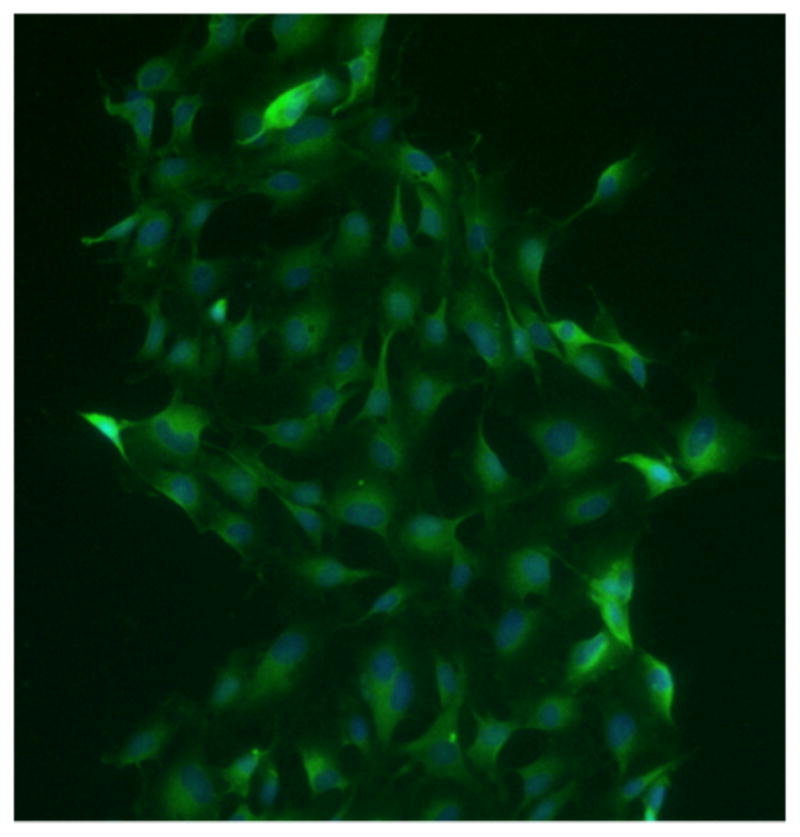
Our initial step was to ensure EGCs had the appropriate receptors to respond to Morphine Sulfate. Stimulation of our confluent EGCs with anti μ-Opioid Receptor antibody demonstrated a positive staining pattern when compared with negative control (Figure 1).
Figure 1. EGCs possess the μ-opioid receptor.
EGCs were stained with rabbit anti-μ opioid receptor primary antibody and FITC-conjugated goat anti-rabbit secondary antibody.
Enteric glia enhance barrier protein organization
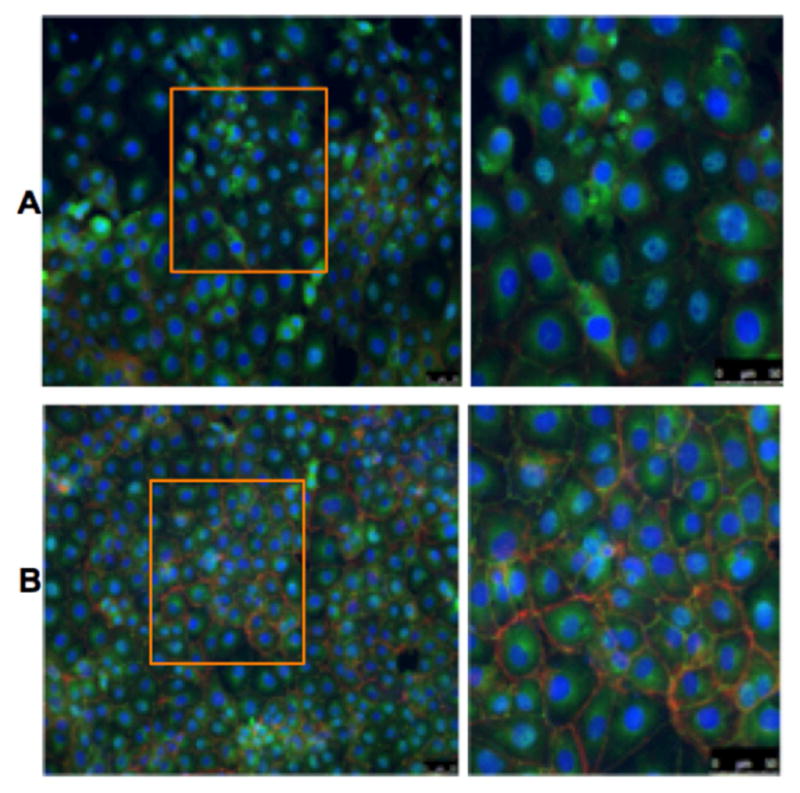
Using immunocytochemistry, confluent IECs were treated with unstimulated EGC-CM to determine the effects on IEC barrier protein expression. IEC Stimulation with EGC-CM, compared to control, resulted in increased organization of occludin as well as f-actin (figure 2).
Figure 2. Enteric glial cell products facilitate tight junction formation in cultured intestinal epithelial cells.
IEC-6 cells treated with control media (A) compared with conditioned media from rat enteric glial cells (B). Note organized formation of Occludin staining (green) in panel B. Red represents F-actin staining.
Enteric glia enhance intestinal barrier function
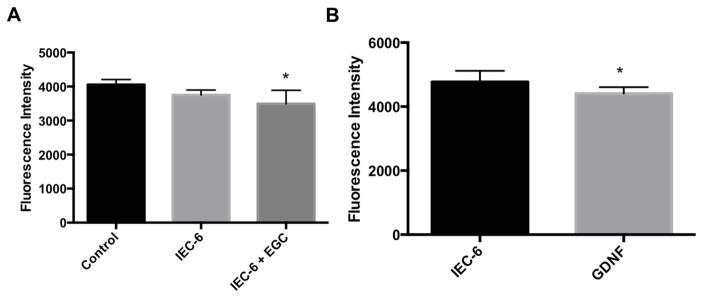
To determine the effects of our in EGCs upon confluent IECs, we used an in vitro co-culture model. EGCs enhanced the barrier function of IECs in this system. There was significant decrease in fluorescence intensity in the wells of the co-culture plate containing both IECs and EGCs compared to IECs alone (Figure 3A, p < 0.05). The decreased fluorescence intensity in the lower well correlates with enhancement of intercellular barrier integrity between IECs when co-cultured with EGCs. Our results additionally showed that GDNF treatment to the wells in culture with IECs resulted in similar barrier enhancement (p < 0.05, figure 3B).
Figure 3 (A,B). EGC-mediated barrier enhancement.
A. Control = no cells in either chamber. IEC6 cells alone (plated in transwell insert) or IEC6 + EGCs (EGCs in plate well) were grown to confluence in the apical chamber or plate well, respectively. B. GDNF (100ng/ml) was stimulated to the well of the transwell plate, fluorescence was measured 24 hours after stimulation. * = p < 0.05, n>3 for all experimental groups.
Morphine is disruptive to EGC-mediated barrier enhancement
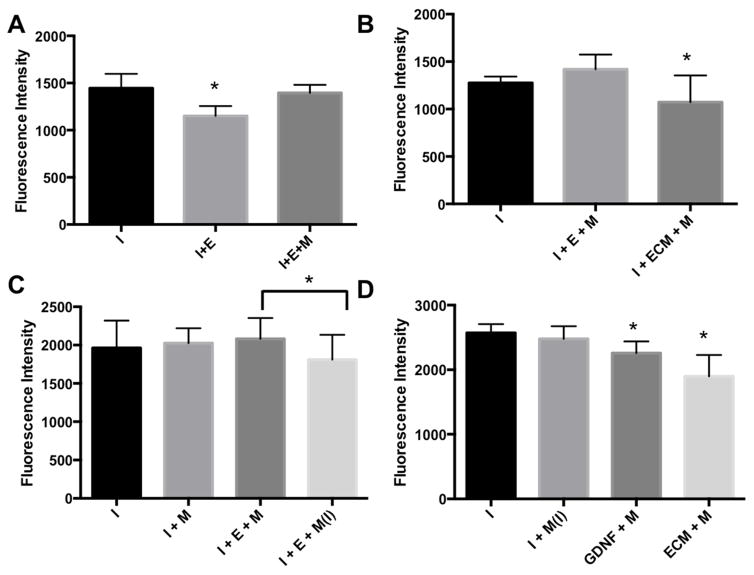
EGCs in the basal compartment of transwell plate were stimulated with Morphine Sulfate (1 μM) to determine the effect of morphine on EGC barrier enhancing function. Interestingly, when EGCs were stimulated with morphine, there was increased permeability of FITC-Dextran which indicates loss of the normal barrier enhancement which was seen without morphine (Figure 4A,3A). When morphine (1μM) was added to the EGC-CM or GDNF treatment groups, barrier enhancement was preserved which suggests that the disruptive effect of morphine is due to its action directly onto EGCs (figure 4B,C).
Figure 4 (A,B,C,D). Morphine and EGCs.
A. Control = unstimulated IEC6 cells. I+E = IEC6 cultured in plate insert and EGCs cultured in plate well. B. I = unstimulated IEC6 cells. M= morphine (1μM) stimulated in well onto EGCs. ECM = EGC conditioned media added to well of transwell plate without enteric glial cells. C. Morphine (1μM) was always added to the EGCs or empty plate well unless it was treated to only the IEC6 cells shown as M(I). * = p<0.05 (0.0447). D. GDNF = glial derived neurotrophic factor (100ng/ml). In all columns, IECs were placed in the apical chamber of the transwell plate and EGCs were placed in the basal chamber. A= Morphine treatment to IEC-6 cells in the apical chamber. Morphine was always treated to EGCs the well unless designated by Ins. * = p < 0.05, n>3 for all experimental groups.
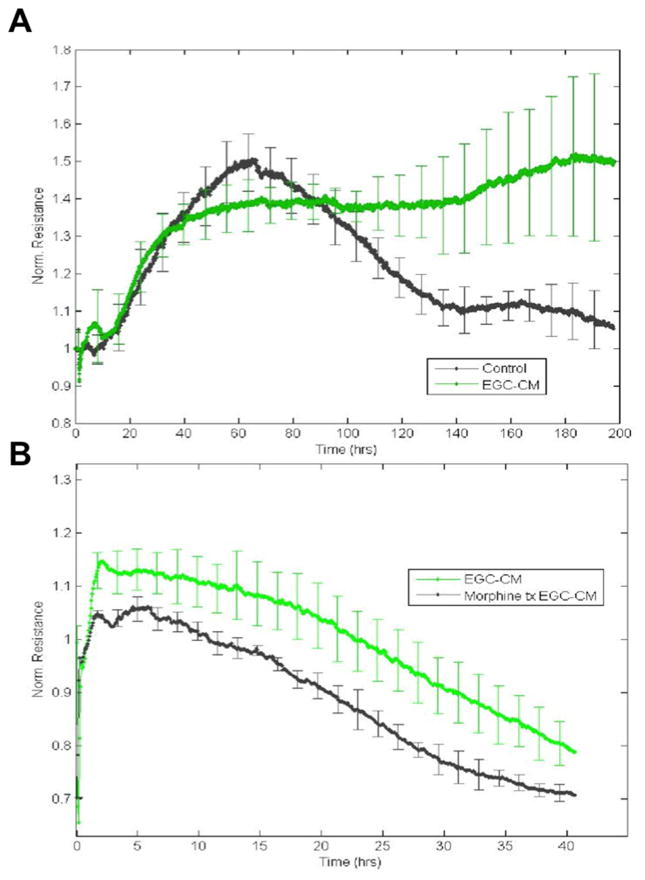
To further clarify the impact of morphine treatment of EGCs upon the of IEC monolayers, we used electrical cell impedance sensing (ECIS) arrays. IECs, when grown to confluence and stimulated with EGC-CM, have increased TER, which indicates enhanced barrier function (figure 5A). This barrier enhancement begins within the first 5 hours after stimulation and continues throughout the duration of the experiment as barrier function is gradually lost as the cells age and media nutrients are consumed. When EGC-CM from morphine treated EGCs is used to treat IECs, TER is significantly reduced in comparison to untreated EGC-CM (figure 5B). This loss of barrier enhancement is also lasting throughout the duration of the experiment.
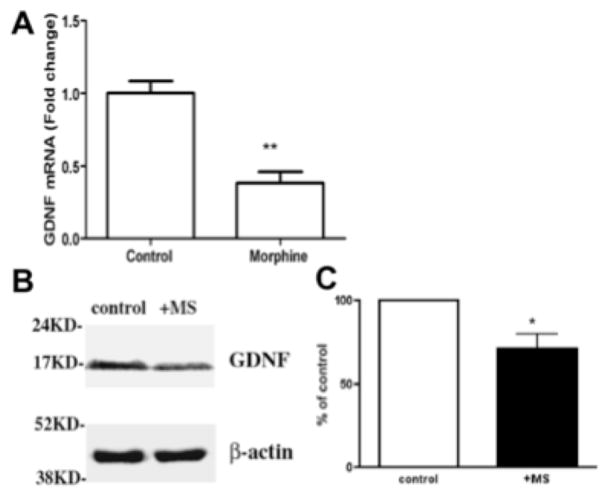
Figure 5 (A,B,C). Morphine decreases GDNF production.
A. Real-time PCR analysis of mRNA levels of GDNF in enteric epithelial cells after 30-hour morphine treatment. **p < 0.01, n=3. B. Cell lysates from control and morphine treatment (+MS) were resolved in SDS-PAGE gel and the blot was probed with antibodies against GDNF (upper panel) and b-actin (lower panel, serving as internal control for equal sample loading). C. Densitometric analysis of GDNF level in control and morphine treatment. The density of each band was determined using ImageJ (NIH). GDNF protein level was normalized to the level of b-actin in each sample. The normalized control was considered as 100%, +MS = morphine treated EGCs (mean = 71.67 +/− 7.71 (SE)). Paired Student’s t-test was performed and graph was plotted using Prizm 4.0 (Graphpad), p = 0.033, n=3, * = p<0.05.
Morphine decreases GDNF production
In our co-culture model we found that morphine mitigated the protective effect of EGCs. Our RNA experiments demonstrated that EGCs stimulated with morphine (1 μM) resulted in an approximately 50% reduction in GDNF production by EGCs - a trophic factor well known to mediate EGC barrier enhancing functions (figure 6A). These findings were also demonstrated through western blot experiments, which identified that morphine treatment results in decreased GDNF production (mean = 71.67 +/− 7.71 (SE)) compared to control (100%, p = 0.033, n=3, * = p<0.05) (figure 6B,C).
Figure 6 (A,B). Morphine Disrupts EGC-mediated Barrier Enhancement. ECIS.
A. Normalized resistance in confluent IECs were stimulated with unstimulated conditioned media (EGC-CM) from confluent EGCs (green) or left unstimulated (black). B. Normalized resistance in confluent IECs cultured in non-Morphine treated EGC-CM (green) or in Morphine treated EGC-CM (black) n=3 in all experimental groups.
Discussion
The rate of adverse effects from opioids experienced by patients taking the medication may be as high as 65% [4]. The most commonly reported adverse effects include constipation (16%), nausea (15%), and dizziness or vertigo (8%) [14]. More specifically, constipation may be seen in up to 65% of patients treated with opioid analgesics in the elderly [15]. For patients to receive analgesia, morphine and other opioids must bind to cell receptors in the peripheral tissue, spinal cord, and ventral caudal thalamus and cortex [16]. Many cell types in their various organ systems systems possess the mu-opioid receptor, including the gastrointestinal system - this is a possible explanation for the gastrointestinal side effect profile of opioids [17]. Furthermore, it is already known that enteric neurons possess the receptor for morphine [18].
We sought to determine whether enteric glial cells, which outnumber enteric neurons by 4:1, possess the morphine receptor and what the effects are on the glial cells [19]. It has been well established that EGCs are an essential component of the enteric nervous system (ENS) [7]. In addition, it has been demonstrated in previous studies that complete ablation of these cells leads to fulminant colitis in mice [11]. EGCs are a vital component in maintaining intestinal homeostasis; however, less is known regarding the influence of morphine on EGC function.
In our search to elucidate the impact of morphine on EGCs we first confirmed that our EGC cell line possess the receptor for morphine (figure 1). We next found that EGCs significantly enhance epithelial barrier function in the intestine (figure 3A,4A,6A). Our results demonstrate that this barrier enhancement is elicited through both co-culture of EGCs and IECs or through IEC stimulation with EGC-CM or even with stimulation by GDNF alone. Interestingly, our results demonstrate that this EGC-mediated barrier enhancement is lost when EGCs are stimulated with morphine (figure 4A,B,C). When morphine was treated directly onto the EGCs in the transwell model or when media from morphine treated EGCs is added to IECs, the barrier enhancing effect of EGCs is lost. In effect, morphine mitigates the barrier inducing effects of enteric glia on the intestinal epithelium. EGC-mediated barrier enhancement is preserved when morphine is added directly to the intestinal epithelial cell (i.e. to the apical chamber in the transwell system) which shows that morphine mediated disruption of intestinal barrier integrity occurs in an EGC-dysfunction dependent pathway. We have shown this in both the transwell model as well as though electrical impedance studies using the ECIS model (Figure 6B).
The barrier enhancing activity of EGCs has previously been shown to be the result of secretion of a variety of trophic hormones, including GDNF, which has been shown to enhance production of ZO-1 tight junction protein and thus enhance epithelial barrier integrity [10]. To test our hypothesis that morphine disrupts EGC barrier enhancing activities, we analyzed GDNF production by EGCs with and without morphine. Interestingly, we found that morphine results in a consistent decrease in GDNF RNA production by over 2 fold with a corresponding decrease in GDNF protein production (Figure 5B,C). This morphine induced decrease in GDNF may explain the preservation of EGC barrier enhancing activity when IECs cells are treated with morphine and EGC-CM from untreated EGCs. Morphine does not inhibit the effect of GDNF, and other trophic hormones, themselves but rather inhibits their production (Figure 4D).
Conclusion
Collectively, our results demonstrate the barrier enhancing effects of enteric glial cells upon intestinal epithelial cells. This supports the existing body of literature demonstrating the crucial role played by EGCs in the maintenance of intestinal epithelial barrier function. Our results also demonstrate that morphine stimulated EGCs have decreased production of GDNF. Furthermore, EGCs lose their barrier enhancing function when stimulated with morphine. It is likely that this loss of barrier enhancing activity is the result of decreased production of EGC trophic factors such as GDNF, which influence intestinal epithelial barrier protein expression. Further studies are needed to determine the subcellular mechanisms of morphine upon EGCs and to find a potential therapeutic treatment to enhance intestinal barrier function in patients taking opioid medications.
Acknowledgments
We are grateful to the University of Minnesota Department of Surgery, the University of Minnesota Medical School. Our research was made possible through the NIH1K08GM113055-01A1 and American Pediatric Surgical Association Foundation Scholar Grants (Dr. Segura), and 1 R01 DA043252-01, R01 DA037843, R01 DA034582 and K05 DA033881 grants (Dr. Roy).
Footnotes
Brent Bauman was the lead author and contributed to all dimensions of this project including writing and performing all experiments. Jingjing Meng performed key immunocytochemical experiments and contributed to the writing and editing of the manuscript. Lei Zhang participated in planning experiments for revised manuscript and performed western blotting experiments as well as writing the revised manuscript. Amanda Louiselle contributed to the writing of the manuscript and helped perform cell culture and analyzing results of co-culture experiments. Eugene Zheng contributed to the writing of the manuscript and helped perform key ECIS experiments. Santanu Banerjee aided with the writing and editing of the manuscript and aided with experimental design and data analysis. Sabita Roy helped write and edit the manuscript and also helped with experimental design and data analysis. Bradley Segura was the principal investigator for this project, he was involved and contributed to every aspect of this work.
Disclosures: The authors have no conflicts of interest or funding to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm Rep. 2016;65(RR-1):1–49. doi: 10.15585/mmwr.rr6501e1. DOI: http://dx.doi.org/10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 2.Mcdonald DC, Carlson K, Izrael D. Geographic Variation in Opioid Prescribing in the U.S. The Journal of Pain. 2012;13(10):988–996. doi: 10.1016/j.jpain.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han B, Compton WM, Jones CM, Cai R. Nonmedical Prescription Opioid Use and Use Disorders Among Adults Aged 18 Through 64 Years in the United States, 2003–2013. Jama. 2015;314(14):1468. doi: 10.1001/jama.2015.11859. [DOI] [PubMed] [Google Scholar]
- 4.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, … Vallejo R. Opioid Complications and Side Effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- 5.Schwacha MG, Mcgwin G, Hutchinson CB, Cross JM, Maclennan PA, Rue LW. The contribution of opiate analgesics to the development of infectious complications in burn patients. The American Journal of Surgery. 2006;192(1):82–86. doi: 10.1016/j.amjsurg.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Meng J, Banerjee S, Li D, Sindberg GM, Wang F, Ma J, Roy S. Opioid Exacerbation of Gram-positive sepsis, induced by Gut Microbial Modulation, is Rescued by IL-17A Neutralization. Sci Rep Scientific Reports. 2015;5:10918. doi: 10.1038/srep10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheadle GA, Costantini TW, Bansal V, Eliceiri BP, Coimbra R. Cholinergic Signaling in the Gut: A Novel Mechanism of Barrier Protection through Activation of Enteric Glia Cells. Surgical Infections. 2014;15(4):387–393. doi: 10.1089/sur.2013.103. [DOI] [PubMed] [Google Scholar]
- 8.Cabarrocas J, Savidge TC, Liblau RS. Role of enteric glial cells in inflammatory bowel disease. Glia. 2002;41(1):81–93. doi: 10.1002/glia.10169. [DOI] [PubMed] [Google Scholar]
- 9.Bar K, Facer P, Williams N, Tam P, Anand P. Glial-derived neurotrophic factor in human adult and fetal intestine and in Hirschsprung’s disease. Gastroenterology. 1997;112(4):1381–1385. doi: 10.1016/s0016-5085(97)70154-9. [DOI] [PubMed] [Google Scholar]
- 10.Zhang DK, He FQ, Li TK, Pang XH, Cui DJ, Xie Q, Huang XL, Gan HT. Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J Pathol The Journal of Pathology. 2010;222(2):213–222. doi: 10.1002/path.2749. [DOI] [PubMed] [Google Scholar]
- 11.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA. Fulminant jejuno-ileitis induced by ablation of enteric glia in adult transgenic mice. Gastroenterology. 2001;120(5) doi: 10.1016/s0016-5085(08)80924-9. [DOI] [PubMed] [Google Scholar]
- 12.Cornet A, Savidge TC, Cabarrocas J, Deng W, Colombel J, Lassmann H, … Liblau RS. Enterocolitis induced by autoimmune targeting of enteric glial cells: A possible mechanism in Crohn’s disease? Proceedings of the National Academy of Sciences. 2001;98(23):13306–13311. doi: 10.1073/pnas.231474098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlegel N, Meir M, Heupel WM, Holthofer B, Leube RE, Waschke J. Desmoglein 2-mediated adhesion is required for intestinal epithelial barrier integrity. AJP: Gastrointestinal and Liver Physiology. 2010;298(5) doi: 10.1152/ajpgi.00239.2009. [DOI] [PubMed] [Google Scholar]
- 14.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: A meta-analysis of effectiveness and side effects. Canadian Medical Association Journal. 2006;174(11):1589–1594. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chau DL, Walker V, Pai L, Cho LM. Opiates and elderly: Use and side effects. Clinical Interventions in Aging. 2008;3(2):273–278. doi: 10.2147/cia.s1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Hasani R, Bruchas MR. Molecular Mechanisms of Opioid Receptor-dependent Signaling and Behavior. Anesthesiology. 2011:1. doi: 10.1097/aln.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holzer P. Opioid receptors in the gastrointestinal tract. Regulatory Peptides. 2009;155(1–3):11–17. doi: 10.1016/j.regpep.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minnis J, Patierno S, Kohlmeier S, Brecha N, Tonini M, Sternini C. Ligand-induced μ opioid receptor endocytosis and recycling in enteric neurons. Neuroscience. 2003;119(1):33–42. doi: 10.1016/s0306-4522(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 19.Ruhl A, Nasser Y, Sharkey KA. Enteric Glia. Neurogastroenterology and motility. 2004;16(1):44–49. doi: 10.1111/j.1743-3150.2004.00474.x. [DOI] [PubMed] [Google Scholar]