Abstract
Background
Schizophrenia, a neurodevelopmental disorder, involves abnormalities in functional connectivity (FC) across distributed neural networks, which are thought to antedate the emergence of psychosis. In a cohort of adolescents and young adults at clinical high risk (CHR) for psychosis, we applied data-driven approaches to resting-state fMRI data so as to systematically characterize FC abnormalities during this period and determine whether these abnormalities are associated with psychosis risk and severity of psychotic symptoms.
Methods
Fifty-one CHR participants and 47 matched healthy controls (HCs) were included in our analyses. Twelve of these CHR participants developed psychosis within 3.9 years. We estimated one multivariate measure of FC and studied its relationship to CHR status, conversion to psychosis and positive symptom severity.
Results
Multivariate analyses revealed between-group differences in whole-brain connectivity patterns of bilateral temporal areas, mostly affecting their functional connections to the thalamus. Further, more severe positive symptoms were associated with greater connectivity abnormalities in the anterior cingulate and frontal cortex.
Conclusions
Our study demonstrates that the well-established FC abnormalities of the thalamus and temporal areas observed in schizophrenia are also present in the CHR period, with aberrant connectivity of the temporal cortex most associated with psychosis risk.
Keywords: temporal cortex, prodrome, clinical high-risk, connectivity, connectome, thalamus
INTRODUCTION
Schizophrenia is increasingly conceptualized as a brain disorder of abnormal anatomical (1–3) and functional connectivity(4–8). First, studies of functional connectivity have observed hyperconnectivity in the prefrontal cortex (9) and in the default mode network(10), though hypoconnectivity appears to be more frequently reported (4). Second, disruptions in connectivity affect the general architecture of brain networks in schizophrenia. Findings of reduced distal connectivity and enhanced local connectivity between cognitive control networks (11) and disruptions of topological properties with decreased hub dominance (12) both point to impaired coordination among different networks and to an organizational rearrangement within each network. Moreover, available studies in patients with psychotic illness suggest the existence of network-level dysconnectivity (13) involving both the frontoparietal (14) and the frontotemporal (15) networks. Reductions in frontoparietal connectivity have been found in psychotic illness (16), in addition to hypoconnectivity within fronto-thalamic circuits (17).
Though these studies are informative, insights into the period of clinical high risk (CHR) for psychosis are critical in order to understand the pathophysiology of psychosis. Though CHR cohorts are fairly heterogeneous, a significant portion of these individuals transitions to psychosis within a few years (18)(19)(20)(21). Consequently, the investigation of the CHR period could help us disentangle truly pathogenic abnormalities from the effects of chronic illness and its treatments.
Despite these advantages, few studies have investigated functional connectivity during the period of clinical high risk (CHR) for psychosis (22, 23) (24).
Findings similar to those reported in schizophrenia (10) have been shown in the CHR period, with reports of hyperconnectivity within default network regions (23) and of loss of coordination between task positive and default mode networks (25). Moreover, dysconnectivity appears to affect language-related regions as suggested by the observation of reduced connectivity in CHR individuals between the frontal cortex and Broca’s area (22).
Similar to studies in individuals with schizophrenia (26, 27) (28) and in their first-degree relatives (29), CHR individuals also exhibit dysconnectivity within the cortico-striatal-thalamo-cortical networks, specifically hypoconnectivity between the dorsal caudate and the right dorsolateral prefrontal cortex (DLPFC), rostral medial prefrontal cortex and thalamus (30). More recent findings of thalamo-cortical dysconnectivity in CHR individuals, especially in those who later convert to psychosis (24), further corroborate the notion that abnormalities of functional connectivity in cortico-striatal-thalamic cortical loops play an important role in the pathogenesis of psychotic illness.
Taken together, these findings suggest that circuit-level functional dysconnectivity could be a risk phenotype for psychosis. Such network-level dysconnectivity has been hypothesized to be the common final pathway through which diverse causal influences may lead to illness (31).
The majority of the studies in CHR individuals to date have used hypothesis-driven approaches limiting the analyses to preselected regions of interest (ROIs) or seeds. These ROI-based approaches offer several statistical advantages, though they may present problems of interpretability, especially when investigating distributed relationships among brain regions, such as those captured by measures of functional connectivity. Other studies of both schizophrenia (32), the CHR phase (33) (34) and similar cohorts with psychosis spectrum symptoms (35) have applied data-driven approaches to the analysis of structural and functional imaging data. These data-driven methods can complement and extend hypothesis-driven methods because of their potential to assess whole-brain measures of functional connectivity in an unbiased manner, and to allow for data-driven selection of ROIs that can be subsequently investigated with more traditional seed-based analyses.
In this study, we aimed to characterize intrinsic functional connectivity in CHR individuals using well–established data-driven metrics of functional connectivity, in order to capture regional, and whole-brain functional interactions between brain regions. We employed a multivariate approach (multivariate distance matrix regression or MDMR) to evaluate simultaneously the contribution of all the possible functional connections of a given brain region to risk-status and symptoms severity in CHR individuals. This multivariate approach, already successfully applied to the study of psychosis (35), has the advantage of providing a comprehensive and unbiased characterization of the totality of functional connections in the brain, without requiring the high number of statistical tests inherent in the frequently used massive univariate approaches.
Based on previous studies in schizophrenia (17, 26) and in clinical high-risk (CHR) cohorts (24), we predicted that CHR individuals would have abnormal functional connectivity involving subcortical regions, in particular the thalamus, and multimodal association cortical areas, belonging to the frontoparietal cortical network, chiefly the DLPFC, in addition to temporal and parietal regions (32). Specifically, we expected an increase in functional connectivity between temporal areas and the thalamus consistent with previous findings in schizophrenia by Anticevic (26), showing increased thalamic connectivity with a set of lateral cortices (including the superior temporal gyrus) and with more recent findings of hyper- and hypoconnectivity of the thalamus with several cortical areas in individuals with psychotic illness (17). This hypothesis is also in line with reports of disrupted structural thalamo-cortical connectivity in CHR subjects and individuals with first episode psychosis (3). Moreover, recent evidence from animal models of schizophrenia points to a specific alteration of thalamic input to the auditory cortex subsequent to elevation in dopamine receptor levels in the thalamus (36) (37).
Finally we predicted that this dysconnectivity would be correlated with severity of symptoms in independently conducted, data-driven analyses.
METHODS
Participants
Our original cohort included 61 CHR individuals enrolled in the COPE (Center of Prevention and Evaluation) clinic at the New York State Psychiatric Institute. Baseline resting state imaging data and clinical baseline and follow-up data were available. Out of the available resting state scans (55), four CHR individuals (among whom two were converters) were excluded as motion outliers (either mean frame-wise displacement (FD power) outside of three standard deviation or over 50% frames with FD>0.2mm). Our final sample for the analyses consisted of 51 CHR individuals (21.0 ±3.78 years, 37males/14 females) and 47 HCs (21.6 ± 3.84 years, 27 males/20 females). Risk for psychosis was assessed using the Structured Interview for Psychosis-Risk Syndromes (SIPS)(21), CHR participants and HCs were matched for minority status (here defined as being non-Caucasian) and did not differ in age, gender, or in-scanner head motion (all p> 0.05; see also Table 1).
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Characteristics | CHR participants (n=51) | HCs (n=47) | F/T or Chi-sq | df | p-value |
|---|---|---|---|---|---|
| Gender M/F (n) | 37/14 | 27/20 | 2.462 | 1 | 0.117 |
| Age at MRI (years) | 21.04 (SD 3.78) | 21.64 (SD=3.84) | 0.009/−0.776 | 96 | 0.440 |
| Education (years)* | 13.18 (SD 2.18) | 13.74 (SD 2.51) | 1.059/−1.158 | 91 | 0.250 |
| Head motion (mean FD in mm) | 0.12 (SD 0.06) | 0.10 (SD 0.06) | 0.038/1.00 | 96 | 0.316 |
| Minority/Caucasian (n) | 35/16 | 28/19 | 0.873 | 1 | 0.35 |
Indicates incomplete data: n=50 for CHR, n=43 for HC.
Eight CHR individuals were on antipsychotic medication at the time of scanning (including 3 converters). Functional outcome was measured at baseline and at the last follow-up (mean time to last follow-up = 17.37 ± 15.0 months) using two subscales of the Global Functioning Scale: Social (GSF:S) and Role (GSF:R)(38). Scores ≤6 indicate poor outcome and scores >6 indicate good outcome(39). Further details on this CHR cohort are available in the supplementary material.
MRI Data
Acquisition For details of the MRI data acquisition and preprocessing, refer to the supplementary material. Briefly, we acquired images on a GE Signa 3-T whole-body scanner. For RS acquisition, participants were instructed to remain still with their eyes closed and to let their minds wander freely. The total acquisition time for the resting state sequence was 5 minutes and 21 seconds. Imaging data were preprocessed using Configurable Pipeline For The Analysis Of Connectomes (CPAC version 0.3.3, http://fcp-indi.github.io/docs/user/index.html).
Data Analyses
Multivariate approach MDMR identifies voxels where whole-brain connectivity patterns vary significantly with a phenotypic variable of interest (in our case, CHR status or severity of total positive symptoms)(40). The MDMR approach was conducted using the publicly available R package Connectir (connectir.projects.nitrc.org). In order to reduce computational demands, we restrained our MDMR analyses within a group mask including only voxels present in all participants (3mm3 resolution, in MNI152 standard space) and falling within the 25% gray matter tissue prior mask provided by FSL.
For each voxel, the MDMR analysis included: (1) Estimation of the whole-brain FC pattern of this voxel for each participant by calculating the Pearson’s correlation coefficient between the time series of this voxel and that of all other voxels within the group mask; (2) estimation of the spatial similarity (Pearson’s correlation) of the whole-brain connectivity pattern between pairs of participants; this yields an n × n correlation matrix, where n is the number of participants (n = 98 for categorical analysis and n = 51 for dimensional analyses); (3) conversion of each Pearson’s r into a distance measure using to produce an n × n distance matrix(41); (4) testing of the extent to which diagnostic status or symptom severity explains the distances of whole-brain connectivity between participants by using the following MDMR models(42):
| (1) |
| (2) |
At the group level, we examined the categorical effect of Group (CHR vs. HCs)(model 1) and the dimensional effect of clinical symptoms at baseline within the CHR group (model 2). For model (2), we used the total positive symptoms score from the SOPS (the sum of item P1 through P5 of the positive symptom subscale), as done in previous CHR studies (24). Results were corrected for multiple comparisons using Gaussian Random Field (GRF) theory. We used the Easythresh tool in FSL and the smoothness of the statistical images was estimated automatically through the “smoothest” program in FSL. This effective smoothness is slightly bigger than the applied smoothness kernel (FWHM=8mm) during preprocessing because the pre-smooth image has implicit smoothness(43).
MDMR yields a pseudo-F statistic analogous to the F statistic obtained in ANOVA. The significance of the pseudo-F value for a given voxel is assessed using a permutation test (15,000 permutations) as described in Shehzad(40). We permuted: 1) group membership assignments (CHR, HC) when we compared CHR individuals and controls; and 2) scores of symptom severity when we investigated MDMR-symptom correlations in the CHR group. The resulting p values were converted to Z scores and corrected for multiple comparisons using GRF. The cluster-defining threshold was Z>1.96, which is equivalent to a one-tailed p of 0.025; the corrected cluster-level threshold was p<0.05. Given the exploratory nature of MDMR, previous reports(35) in clinical samples had used more liberal criteria (for instance cluster defining threshold of Z>1.64 and a smaller number of permutations). Here, we chose a higher height threshold in order to be more conservative and improve the interpretability of the results.
MDMR can reveal the presence of a relationship between phenotypic variables and the pattern of whole-brain connectivity. This method, however, does not inform us about the specific connections that drive these brain-phenotype associations. We performed, as follow-up analyses, ROI-based functional connectivity analyses to identify specific connections implicated in the observed MDMR effect by using as seeds regions significant in the MDMR analyses. At the individual level, the average time-series across all voxels of a given seed ROI was computed and Pearson-correlated (Fisher Z-transformed) with all voxels within the brain.
We carried out univariate analyses in order to identify patterns of FC that significantly differ between the CHR and HC groups. At the group level, the General Linear Model (GLM) implemented in the toolbox for Data Processing & Analysis of Brain Imaging(43–45)(DPABI: www.rfmri.org/dpabi) was used to examine the categorical effect of Group (CHR vs. HCs). Age, gender, handedness, and head motion (mean FD), and global mean of functional connectivity (FC) of the given seed (46) were entered as nuisance covariates in the following model:
| (3) |
Results were corrected for multiple comparisons using Gaussian Random Field (GRF) theory. We applied the following thresholds: the cluster-defining threshold was Z > 2.58, which is equivalent to a two-tailed p of 0.01. The corrected cluster-level threshold was p<0.025. The threshold of p < 0.025 was used to account for the number of seed ROIs, which was derived from a threshold value of p = 0.05, Bonferroni-corrected for 2 seeds (0.05/2=0.025).
The minimum number of voxels in a cluster was based on the cluster extent threshold.
Secondary analyses: Medication Effects
In order to rule out the potential confounding contribution of medications, we carried out the same group analyses described above after excluding individuals taking antipsychotic medications at the time of scanning.
Secondary analyses: Conversion Effects
In order to rule out the potential that CHR converters in our sample were driving the group differences observed in the entire CHR group, we carried out the same group analyses described above after excluding the twelve individuals who later developed psychosis (CHR converters).
RESULTS
Clinical Sample
Fourteen participants in this CHR sample eventually developed psychosis within 3.9 years (conversion rate 23%). Functional outcome was measured at baseline and at the last follow-up (mean time to last follow-up = 17.37 ± 15.0 months) using two subscales of the Global Functioning Scale: Social (GSF:S) and Role (GSF:R)(38). Scores ≤6 indicate poor outcome and scores >6 indicate good outcome(39).
At baseline, our cohort was characterized by impairments in role (mean= 5.86 ± 2.2; n= 45; 86.7% of the sample ≤ 6) and social functioning (mean= 5.9 ± 1.4; n= 45; 73.3% of the sample ≤ 6). At last follow-up, impairment in role functioning (mean= 6.28 ± 1.65; n= 39; 48.7% of the sample ≤ 6) and impairment in social functioning (mean = 5.72 ± 3.00; n = 40; 47.5% of the sample ≤ 6) were still observed (Table 2). Our rates of conversion to psychosis, rates of exposure to antipsychotic medications(47–49), and the magnitude of social and role functioning impairments(39) were comparable to those reported in other CHR cohorts (also refer to the supplementary material for further details on our CHR cohort). Four participants converted in less than 1 year, five participants between 1 and 2 years, three participants between 2 and 3 years and two participants between 3 and 4 years. In essence, almost 65% of the converters’ subsample converted within 2 years and almost 86% of the converters’ subsample converted within 3 years, which is a typical pattern for CHR studies (19).
Table 2.
Additional Characteristics of CHR Participants
| Characteristics | CHR Participants (n=51) |
|---|---|
| SIPS Score | |
| Total Positive Symptoms | 12.33 (SD 4.58) |
| Total Negative Symptoms | 14.98 (SD 7.73) |
| CHR Outcome | |
| Converted to Psychosis, n (%) | 12/51 (23.5%) |
| Mean Time to Conversion, months | 17.05 ± 7.5 |
| Baseline GSF:R*, n=45 | 5.86 (SD=2.2) |
| Last GFS:R**, n=39 | 6.28 (SD=1.65) |
| Baseline GSF:S*, n=45 | 5.9 (SD=1.4) |
| Last GFS:S**, n=40 | 5.72 (SD=3.00) |
| Poor Outcome: GFS: ≤ 6, n (%) | 19/39 (48.72%) |
| Poor Outcome: GFS:S ≤ 6, n (%) | 19/40 (47.5%) |
| Medication Type***, n | |
| Atypical/Typical Antipsychotics | 8/0 |
| Mood Stabilizers | 1 |
| Antidepressants | 17 |
| Stimulants | 5 |
| Benzodiazepines | 5 |
Indicates incomplete data;
The mean Follow-Up Period (Time to Last Follow Up) was 17.37 months (n=51; SD= 15.0).
Information about medication was unavailable for 5 participants
Functional connectivity associated with CHR for psychosis
MDMR indicated that abnormal brain-wide connectivity of a left hemispheric cluster centered at the temporal lobe (mostly HG) and at the supramarginal gyrus (SMG) was associated with diagnostic status (CHR). Similarly, abnormal brain-wide connectivity of a right hemispheric cluster centered at the MTG was also associated with CHR status (cluster-defining threshold Z>1.96; corrected cluster-level threshold p<0.05; refer to Table S1). These clusters were used as seeds in the follow-up functional connectivity (FC) analyses and these analyses revealed that, compared with HCs, CHR individuals exhibited higher functional connectivity of the left hemispheric cluster with the thalamus, bilaterally, the right superior frontal gyrus (SFG), including the DLPFC, and the right paracingulate gyrus (Cluster-defining threshold Z > 2.58; corrected cluster-level threshold p<0.025; refer to Table S1). CHR individuals also exhibited higher functional connectivity of the right hemispheric cluster bilaterally with the thalamus, striatum and midbrain. In addition, we also observed, hypo-connectivity of this seed with the middle and superior portions of the posterior left temporal lobe (Cluster-defining threshold Z > 2.58; corrected cluster-level threshold p<0.025; refer to Table S1).
Functional connectivity associated with symptom severity
MDMR identified clusters of regions where patterns of whole-brain functional connectivity were significantly related to the severity of positive symptoms (cluster-defining threshold Z > 1.96; corrected cluster-level threshold p < 0.05)(Fig.3). These clusters included the right and left medial frontal gyri (MFG), the left SFG (including the DLPFC), the left dACC, the right and left amygdala, the right and left the hippocampus and thalamus (bilaterally).
Fig. 3. Intrinsic Functional Connectivity Associated with Positive Symptoms.
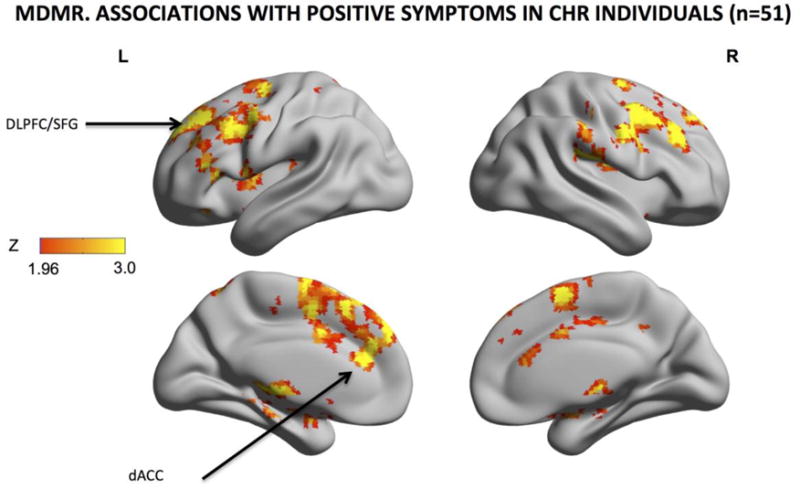
Z scores of areas whose intrinsic functional connectivity were significantly associated with the severity of positive symptoms were shown on the surface map for (multivariate distance matrix regression or MDMR). The warm color indicates the presence and strength of an association between the pattern of whole-brain connections and the severity of total positive symptoms. Progressively more severe symptoms are associated with whole-brain differences in the pattern of functional connectivity of the dACC, midcingulate cortex, supplementary motor area and mesial superior frontal gyrus. Results were GRF-corrected for multiple comparisons. MDMR: cluster-defining threshold Z > 1.96; corrected cluster-level threshold p < 0.05; (estimated smoothness of the statistical images in FSL Easythresh: FWHMx= 13.5mm; FWHMy= 13.4mm; FWHMz=11.8mm).
Secondary Analyses: Medication Effects
Our findings for the comparison of CHR subjects with HCs survived after excluding those eight subjects who were on antipsychotic medications at the time of scanning (Fig. S1).
Secondary Analyses: Converters Effects
Our findings for the comparison of CHR subjects with HCs survived after excluding CHR-converters (Fig. S2).
DISCUSSION
We examined functional connectivity (FC) in 51 CHR and 47 HC individuals and found that abnormalities in connectivity within the temporal cortex, thalamus, striatum and midbrain were consistently associated with risk for psychosis.
We observed abnormal patterns of whole-brain connectivity of a left perisylvian and of a right temporal cortical cluster. These clusters included the temporal cortex, bilaterally, (encompassing the primary auditory cortex) and the left supramarginal gyrus, though the differential contributions of these regions to the group effect cannot be determined (50).
Findings from a large number of studies converge on the superior and middle temporal cortex as critical loci of anatomical and functional pathology in schizophrenia (51) and, to a lesser extent, in the CHR period (52). Volumetric reductions of the STG(53) and morphological abnormalities of the planum temporale(54) have long been observed in different phases of the illness, and these same temporal regions have been implicated in the genesis of psychotic symptoms(55).
The posterior STG in particular is part of a network that supports language-related processing in the left hemisphere, in addition to emotion recognition (56)and musicality(57) in the right hemisphere. Aberrant functional connectivity in these critical areas is associated with semantic incoherence in schizophrenia (58) and thought disorder in the CHR period(59). Furthermore, the Heschl’s and supramarginal gyri are also components of the perisylvian cortices that lie along the course of the arcuate fasciculus, a key substrate of human language capacity, also affected in schizophrenia (60). The connectivity abnormalities that we observed in these gyri are in line with prior findings further supporting the involvement of language-related circuits in the clinical high-risk phase and with the observation that specific features of speech complexity may predict conversion to psychosis (61).
By conducting seed-based analyses in those cortical regions showing abnormal whole-brain patterns of functional connectivity (Fig1,2), we determined that left perisylvian areas were hyperconnected to the thalamus, the midbrain, the striatum, the anterior cingulate gyrus and the superior frontal cortex. The right middle temporal gyrus was hyperconnected with the thalamus, midbrain and striatum and hypoconnected with the left superior and middle temporal gyri and with the left angular gyrus.
Fig.1. Intrinsic Functional Connectivity Associated with Clinical High Risk (CHR) for Psychosis. MDMR and MDMR-guided functional connectivity analyses.
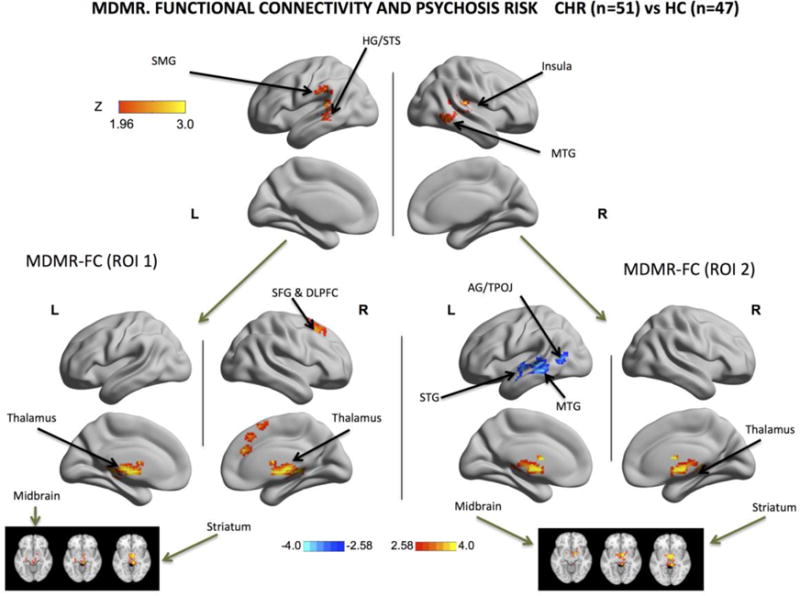
Using Brainnetview, the Z scores of areas exhibiting significant MDMR-related group effects (CHR vs. HCs) were plotted on the surface map. Results for the MDMR (Multivariate Distance Matrix Regression, Upper Panel) were GRF-corrected for multiple comparisons: cluster-defining threshold Z > 1.96; corrected cluster-level threshold p < 0.05 (estimated smoothness of the statistical images in FSL Easythresh: FWHMx=9.9.mm; FWHMy=10.2mm; FWHMz=8.9 mm). MDMR-guided functional connectivity (FC) analyses were conducted using the MDMR-significant regions in the left hemisphere (Lower Left Panel) and in the right hemisphere (Lower Right Panel) with correction for multiple comparisons. MDMR univariate FC analyses, left cluster: cluster-defining threshold Z > 2.58; corrected cluster-level threshold p<0.025. The threshold of 0.025 is used to account for the number of seed ROIs, which is derived from a threshold value of p = 0.05 Bonferroni-corrected for 2 seeds (0.05/2=0.025); (estimated smoothness of the statistical images in FSL Easythresh: FWHMx= 14.9.mm; FWHMy= 15.2mm; FWHMz=13.8mm). MDMR univariate FC analyses, right cluster: cluster-defining threshold Z > 2.58; corrected cluster-level threshold p<0.025. The threshold of 0.025 is used to account for the number of seed ROIs, which is derived from a threshold value of p = 0.05 Bonferroni-corrected for 2 seeds (0.05/2=0.025);(estimated smoothness of the statistical images in FSL Easythresh: FWHMx=14.5mm; FWHMy= 13.9mm; FWHMz=13.1mm). For MDMR, the warm color indicates the presence and strength of an association between the pattern of whole-brain connections and the diagnostic group membership. For MDMR-guided functional connectivity, warm colors indicate that the CHR individuals had higher FC than controls and cold colors indicate that CHR individuals had lower FC than controls. These group comparisons show that CHR individuals have different patterns of whole-brain connectivity in the following cortical regions: left superior temporal cortex (including Heschl’s gyrus or HG), left superior temporal sulcus, left supramarginal gyrus and left middle temporal gyrus and on the right side mostly right middle temporal gyrus. Seed-based FC analyses of these regions of abnormal whole-brain connectivity in the left hemisphere (Lower Left Panel) showed increased connectivity with prefrontal regions, the cingulate cortex, the thalamus and the midbrain. Regions of abnormal brain connectivity in the right hemisphere exhibited, on the other hand, decreased connectivity with the temporal lobe and increased connectivity with the thalamus, the striatum and the midbrain (this is also shown in 3 axial slices at the bottom right and bottom left corners). HG: Heschl’s Gyrus; MTG: Middle Temporal Gyrus; SMG: Supramarginal Gyrus; STG: Superior Temporal Gyrus. STS: Superior Temporal Sulcus. ROI 1: Region Of Interest 1 identified by MDMR in the Left Hemisphere and displayed in the upper left half of the figure. ROI 2: Region Of Interest 2 identified by MDMR in the Right Hemisphere and displayed in the upper right half of the figure.
Fig. 2. Intrinsic Functional Connectivity Associated with Clinical High Risk (CHR) for Psychosis.
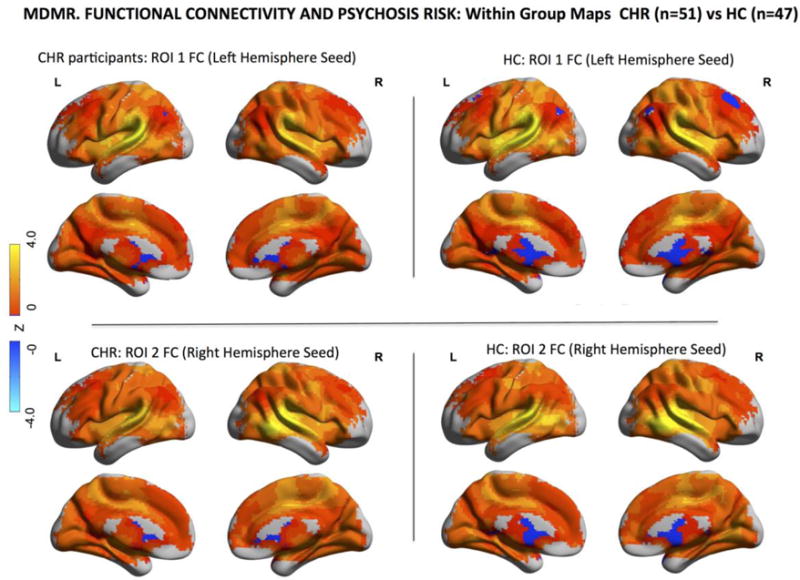
Within-group maps of brain-wide functional connectivity for healthy controls (HCs) and CHR participants for the two ROIs identified by MDMR and displayed in Fig.1. The comparison of these connectivity maps, here displayed separately, for CHR individuals and healthy controls yields the between-group maps displayed in the lower half of Fig. 1 also referred to as MDMR-follow up analyses. For these maps of MDMR-guided functional connectivity, warm colors indicate positive values of functional connectivity and cold colors indicate negative values of functional connectivity between the seed regions (ROI 1 and 2) and other brain areas.
Thalamo-cortical dysconnectivity has been previously reported in schizophrenia (26) and in the clinical high-risk phase (24) and in patients with schizophrenia the thalamus was found to be more strongly connected to a large band of lateral cortex including temporal areas and sensorimotor areas and hypo-connected to frontal areas. Our results are mostly consistent with these findings, because the cortical areas described in these studies include the temporal areas found to be abnormal in our report (Fig.1,2). Our study is however different because it narrows the locus of abnormalities to thalamo-temporal connections. Furthermore, instead of probing the functional connections of the thalamus chosen as an a priori ROI (26), we examined in a more unbiased way the functional connections of those brain regions which demonstrated abnormal brain-wide connectivity profiles, thus relying on a data-driven rather than a–priori ROI selection. These temporal areas were also more strongly connected to the midbrain and striatum consistent with a large body of literature that also points to the midbrain and the striatum as critical loci of pathology in schizophrenia (62–64) and in the phase of clinical high risk for psychosis (65, 66) (67). Our MDMR-based seed analyses indicated that the right temporal regions had lower connectivity with the left temporal cortices, consistent with findings of interhemispheric functional and anatomical dysconnectivity in schizophrenia (68) (69, 70) and in clinical high risk cohorts (71).
Whole-brain analyses using MDMR have been used in different cohorts including pediatric samples (72), individuals with Attention Deficit/Hyperactivity Disorder (40, 73), cohorts with psychosis-spectrum symptoms (35) as well as depression and post-traumatic stress disorder (74). Compared to classic seed-based analyses, MDMR has the advantage of using information from all voxels in order to identify “hot spots” of dysconnectivity. More recently, this data-driven approach has been used to explore brain-behavior correlations in effort to identify phenotypic domains (75) consistent with more the dimensional diagnostic framework advocated by the Research Domain Criteria (RDoC) initiative(76)
The severity of positive symptoms appeared to be more associated with dysconnectivity in a different cluster of cortical areas than is risk for psychosis, centering predominantly on the cingulate gyrus and frontal cortex rather than on the lateral temporal cortex associated with CHR status.
Though, the differential contributions of the different regions cannot be determined (50), more severe positive symptoms were associated with disrupted brain-wide functional connectivity in the dACC, vACC, and DLPFC in addition to the hippocampus and the amygdala.
This observation is consistent with the presence of anatomical abnormalities of the cingulate gyrus and frontal lobe in the CHR phase of psychotic illness (77) (78) and schizophrenia (79, 80) (81). Our results suggest that in the period of clinical high risk for psychotic illness lateral temporal cortices may play a different role than frontal and cingulate areas, with the former associated with psychosis-risk and the latter with symptom burden. We speculate that this difference could be reflected in different patterns of dysconnectivity. For instance, symptoms could be related to failure of top-down cognitive control on subcortical nodes, in particular the amygdala (82) and hippocampus (83) and in fact, dysconnectivity in cognitive control networks has been shown in a variety of psychotic illnesses (84) (32).
Several limitations need to be considered. First, there is a relatively small absolute number of CHR converters in our sample. Our conversion rate for our sample (23%) however is considered typical of CHR research (47, 49, 85, 86) and similar to the rates observed in a recent meta-analysis (18) and in various consortia, such as the European Prediction Of Psychosis Study (87). Second, the heterogeneous nature of CHR cohorts and their typically small sample sizes carry a risk of incurring in false negatives rather than false positives, thus our choice of a threshold that was not too stringent (yet more conservative than previous studies) for the initial data-driven identification of ROI whose patterns of functional connectivity was associated with CHR status. We acknowledge that as the sample sizes for such cohorts grow, more stringent corrections can be used. Furthermore, one must keep in mind only liberal thresholds that yield larger clusters can detect significant effects in cases where the true activity pattern is not localized to a discrete anatomical region but it is, on the contrary, more distributed.
Third, our CHR participants were taking medications at the time of scan. However, our rate of exposure to antipsychotic medications and stimulant medications was similar to other CHR cohorts(24). More importantly, our findings remained in analyses that excluded participants taking antipsychotic medications (Fig. S1), suggesting that abnormal connectivity patterns in CHR individuals cannot be accounted for by exposure to antipsychotic medications.
Fourth, a unified correction for the different data-driven measures of functional connectivity, though desirable, is only feasible for much larger sample sizes. We also note that such unified correction may pose problems such as overcorrection because MDMR, Degree Centrality and Voxel Mirrored Homotopic Connectivity are not entirely independent measures.
We also need to emphasize the need for replication of our results, in light of the post hoc nature of the MDMR-follow up analyses of functional connectivity (50). Such follow up analyses are not independent from MDMR because they simply interrogate the MDMR-identified voxels in order to understand in what particular way the connectivity pattern of these voxels differs between CHR participants and HCs.
Finally, our analyses do not allow us to specify which area or areas - among those that compose a given cluster- is driving the findings in that cluster or to what proportion such areas contribute to the observed effects relative to one another. Therefore no conclusions can be drawn about any of the specific locations within a given cluster (50). Moreover, we remind the reader that the MDMR follow-up analyses describe the connectivity patterns of the entire cluster rather than that of the individual regions included in the cluster.
In conclusion, our data-driven approach identifies cortical areas such as the temporal cortex and the thalamus as key regions associated with risk for psychosis, whereas symptom severity appears correlated with functional dysconnectivity in cognitive control regions. These findings suggest that temporal dysconnectivity may be a trait-related risk marker for psychotic illness, whereas functional disturbances in the anterior cingulate cortex and in the dorsolateral prefrontal cortex may constitute a more general non-specific state-related marker of concurrent positive symptom severity.
Supplementary Material
Acknowledgments
Funding Statement This work was supported by NIMH grants 5T32MH15144, K23 MH85063, R21MH086125 and R01MH04933423, by the National Center for Advancing Translational Research (NIHUL1 TR000040) and the New York State Office of Mental Health, and by funding from the Brain and Behavior Research Foundation (NARSAD), the Sackler Institute, the Herbert Irving Scholar Award, the Bodini Fellowship at Columbia University and the Hundred Talents Program of the Chinese Academy of Sciences (Y5CX072006). The funders had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, the preparation, review, or approval of the manuscript and the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bradley S. Peterson and Michael P. Milham equally contributed as senior authors in this study.
Financial Disclosures Dr Girgis has received research support from Otsuka and Genentech. All other authors reported no biomedical financial interests and no potential conflicts of interest.
BIBLIOGRAPHY
- 1.Sun Y, Chen Y, Lee R, Bezerianos A, Collinson SL, Sim K. Disruption of brain anatomical networks in schizophrenia: A longitudinal, diffusion tensor imaging based study. Schizophrenia research. 2016;171(1–3):149–57. doi: 10.1016/j.schres.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 2.Cocchi L, Harding IH, Lord A, Pantelis C, Yucel M, Zalesky A. Disruption of structure-function coupling in the schizophrenia connectome. NeuroImage Clinical. 2014;4:779–87. doi: 10.1016/j.nicl.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho KI, Shenton ME, Kubicki M, Jung WH, Lee TY, Yun JY, et al. Altered Thalamo-Cortical White Matter Connectivity: Probabilistic Tractography Study in Clinical-High Risk for Psychosis and First-Episode Psychosis. Schizophrenia bulletin. 2016;42(3):723–31. doi: 10.1093/schbul/sbv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neuroscience and biobehavioral reviews. 2011;35(5):1110–24. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophrenia bulletin. 2009;35(3):509–27. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016) Schizophrenia research. 2016;176(2–3):83–94. doi: 10.1016/j.schres.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RC, Cahn W, et al. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA psychiatry. 2013;70(8):783–92. doi: 10.1001/jamapsychiatry.2013.1328. [DOI] [PubMed] [Google Scholar]
- 8.van den Heuvel MP, Fornito A. Brain networks in schizophrenia. Neuropsychology review. 2014;24(1):32–48. doi: 10.1007/s11065-014-9248-7. [DOI] [PubMed] [Google Scholar]
- 9.Anticevic A, Hu X, Xiao Y, Hu J, Li F, Bi F, et al. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35(1):267–86. doi: 10.1523/JNEUROSCI.2310-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(4):1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biological psychiatry. 2011;69(10):967–73. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, et al. Functional connectivity and brain networks in schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(28):9477–87. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong Q, Hu X, Pettersson-Yeo W, Xu X, Lui S, Crossley N, et al. Network-Level Dysconnectivity in Drug-Naive First-Episode Psychosis: Dissociating Transdiagnostic and Diagnosis-Specific Alterations. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt A, Diwadkar VA, Smieskova R, Harrisberger F, Lang UE, McGuire P, et al. Approaching a network connectivity-driven classification of the psychosis continuum: a selective review and suggestions for future research. Frontiers in human neuroscience. 2014;8:1047. doi: 10.3389/fnhum.2014.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khadka S, Meda SA, Stevens MC, Glahn DC, Calhoun VD, Sweeney JA, et al. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biological psychiatry. 2013;74(6):458–66. doi: 10.1016/j.biopsych.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker ST, Yucel M, Fornito A, Allen NB, Lubman DI. A systematic review of diffusion weighted MRI studies of white matter microstructure in adolescent substance users. Neuroscience and biobehavioral reviews. 2013;37(8):1713–23. doi: 10.1016/j.neubiorev.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Woodward ND, Heckers S. Mapping Thalamocortical Functional Connectivity in Chronic and Early Stages of Psychotic Disorders. Biological psychiatry. 2016;79(12):1016–25. doi: 10.1016/j.biopsych.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Archives of general psychiatry. 2012;69(3):220–9. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 19.Ruhrmann S, Schultze-Lutter F, Schmidt SJ, Kaiser N, Klosterkotter J. Prediction and prevention of psychosis: current progress and future tasks. European archives of psychiatry and clinical neuroscience. 2014;264(Suppl 1):S9–16. doi: 10.1007/s00406-014-0541-5. [DOI] [PubMed] [Google Scholar]
- 20.Cannon TD, Cornblatt B, McGorry P. The empirical status of the ultra high-risk (prodromal) research paradigm. Schizophrenia bulletin. 2007;33(3):661–4. doi: 10.1093/schbul/sbm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophrenia bulletin. 2003;29(4):703–15. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 22.Jung WH, Jang JH, Shin NY, Kim SN, Choi CH, An SK, et al. Regional brain atrophy and functional disconnection in Broca’s area in individuals at ultra-high risk for psychosis and schizophrenia. PloS one. 2012;7(12):e51975. doi: 10.1371/journal.pone.0051975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shim G, Oh JS, Jung WH, Jang JH, Choi CH, Kim E, et al. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behavioral and brain functions : BBF. 2010;6:58. doi: 10.1186/1744-9081-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. JAMA psychiatry. 2015;72(9):882–91. doi: 10.1001/jamapsychiatry.2015.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wotruba D, Michels L, Buechler R, Metzler S, Theodoridou A, Gerstenberg M, et al. Aberrant coupling within and across the default mode, task-positive, and salience network in subjects at risk for psychosis. Schizophrenia bulletin. 2014;40(5):1095–104. doi: 10.1093/schbul/sbt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, et al. Characterizing Thalamo-Cortical Disturbances in Schizophrenia and Bipolar Illness. Cerebral cortex. 2013 doi: 10.1093/cercor/bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anticevic A, Yang G, Savic A, Murray JD, Cole MW, Repovs G, et al. Mediodorsal and Visual Thalamic Connectivity Differ in Schizophrenia and Bipolar Disorder With and Without Psychosis History. Schizophrenia bulletin. 2014 doi: 10.1093/schbul/sbu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. The American journal of psychiatry. 2012;169(10):1092–9. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fornito A, Harrison BJ, Goodby E, Dean A, Ooi C, Nathan PJ, et al. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA psychiatry. 2013;70(11):1143–51. doi: 10.1001/jamapsychiatry.2013.1976. [DOI] [PubMed] [Google Scholar]
- 30.Dandash O, Fornito A, Lee J, Keefe RS, Chee MW, Adcock RA, et al. Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophrenia bulletin. 2014;40(4):904–13. doi: 10.1093/schbul/sbt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon TD. Network dysconnectivity: a psychosis-triggering mechanism? Biological psychiatry. 2015;77(11):927–8. doi: 10.1016/j.biopsych.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA psychiatry. 2014;71(2):109–18. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt A, Crossley NA, Harrisberger F, Smieskova R, Lenz C, Riecher-Rossler A, et al. Structural Network Disorganization in Subjects at Clinical High Risk for Psychosis. Schizophrenia bulletin. 2016 doi: 10.1093/schbul/sbw110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinze K, Reniers RL, Nelson B, Yung AR, Lin A, Harrison BJ, et al. Discrete alterations of brain network structural covariance in individuals at ultra-high risk for psychosis. Biological psychiatry. 2015;77(11):989–96. doi: 10.1016/j.biopsych.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Satterthwaite TD, Vandekar SN, Wolf DH, Bassett DS, Ruparel K, Shehzad Z, et al. Connectome-wide network analysis of youth with Psychosis-Spectrum symptoms. Molecular psychiatry. 2015;20(12):1508–15. doi: 10.1038/mp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chun S, Westmoreland JJ, Bayazitov IT, Eddins D, Pani AK, Smeyne RJ, et al. Specific disruption of thalamic inputs to the auditory cortex in schizophrenia models. Science. 2014;344(6188):1178–82. doi: 10.1126/science.1253895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chun S, Du F, Westmoreland JJ, Han SB, Wang YD, Eddins D, et al. Thalamic miR-338-3p mediates auditory thalamocortical disruption and its late onset in models of 22q11.2 microdeletion. Nature medicine. 2016 doi: 10.1038/nm.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophrenia bulletin. 2007;33(3):688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrion RE, McLaughlin D, Goldberg TE, Auther AM, Olsen RH, Olvet DM, et al. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA psychiatry. 2013;70(11):1133–42. doi: 10.1001/jamapsychiatry.2013.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shehzad Z, Kelly C, Reiss PT, Cameron Craddock R, Emerson JW, McMahon K, et al. A multivariate distance-based analytic framework for connectome-wide association studies. NeuroImage. 2014;93(Pt 1):74–94. doi: 10.1016/j.neuroimage.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gower J, WJ K. Analysis of distance for structured multivariate data and extensions to multivariate analysis of varinace J R Stat Soc. Ser C Applied Statistics. 1999;48:505–19. [Google Scholar]
- 42.Zapala MA, Schork NJ. Statistical properties of multivariate distance matrix regression for high-dimensional data analysis. Frontiers in genetics. 2012;3:190. doi: 10.3389/fgene.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016;14(3):339–51. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- 44.Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Frontiers in systems neuroscience. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PloS one. 2011;6(9):e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niendam TA, Lesh TA, Yoon J, Westphal AJ, Hutchison N, Daniel Ragland J, et al. Impaired context processing as a potential marker of psychosis risk state. Psychiatry research. 2013 doi: 10.1016/j.pscychresns.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dandash O, Fornito A, Lee J, Keefe RS, Chee MW, Adcock RA, et al. Altered Striatal Functional Connectivity in Subjects With an At-Risk Mental State for Psychosis. Schizophrenia bulletin. 2013 doi: 10.1093/schbul/sbt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yaakub SN, Dorairaj K, Poh JS, Asplund CL, Krishnan R, Lee J, et al. Preserved working memory and altered brain activation in persons at risk for psychosis. The American journal of psychiatry. 2013;170(11):1297–307. doi: 10.1176/appi.ajp.2013.12081135. [DOI] [PubMed] [Google Scholar]
- 50.Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage. 2014;91:412–9. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Zheng J, Fan X, Guo X, Guo W, Yang G, et al. Dysfunctional resting-state connectivities of brain regions with structural deficits in drug-naive first-episode schizophrenia adolescents. Schizophrenia research. 2015;168(1–2):353–9. doi: 10.1016/j.schres.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 52.Yoon YB, Yun JY, Jung WH, Cho KI, Kim SN, Lee TY, et al. Altered Fronto-Temporal Functional Connectivity in Individuals at Ultra-High-Risk of Developing Psychosis. PloS one. 2015;10(8):e0135347. doi: 10.1371/journal.pone.0135347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi T, Wood SJ, Soulsby B, Kawasaki Y, McGorry PD, Suzuki M, et al. An MRI study of the superior temporal subregions in first-episode patients with various psychotic disorders. Schizophrenia research. 2009;113(2–3):158–66. doi: 10.1016/j.schres.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 54.Ratnanather JT, Cebron S, Ceyhan E, Postell E, Pisano DV, Poynton CB, et al. Morphometric differences in planum temporale in schizophrenia and bipolar disorder revealed by statistical analysis of labeled cortical depth maps. Frontiers in psychiatry. 2014;5:94. doi: 10.3389/fpsyt.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horga G, Schatz KC, Abi-Dargham A, Peterson BS. Deficits in predictive coding underlie hallucinations in schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(24):8072–82. doi: 10.1523/JNEUROSCI.0200-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watson R, Latinus M, Noguchi T, Garrod O, Crabbe F, Belin P. Crossmodal adaptation in right posterior superior temporal sulcus during face-voice emotional integration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(20):6813–21. doi: 10.1523/JNEUROSCI.4478-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Albouy P, Mattout J, Bouet R, Maby E, Sanchez G, Aguera PE, et al. Impaired pitch perception and memory in congenital amusia: the deficit starts in the auditory cortex. Brain : a journal of neurology. 2013;136(Pt 5):1639–61. doi: 10.1093/brain/awt082. [DOI] [PubMed] [Google Scholar]
- 58.Tagamets MA, Cortes CR, Griego JA, Elvevag B. Neural correlates of the relationship between discourse coherence and sensory monitoring in schizophrenia. Cortex; a journal devoted to the study of the nervous system and behavior. 2014;55:77–87. doi: 10.1016/j.cortex.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sabb FW, van Erp TG, Hardt ME, Dapretto M, Caplan R, Cannon TD, et al. Language network dysfunction as a predictor of outcome in youth at clinical high risk for psychosis. Schizophrenia research. 2010;116(2–3):173–83. doi: 10.1016/j.schres.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Catani M, Craig MC, Forkel SJ, Kanaan R, Picchioni M, Toulopoulou T, et al. Altered integrity of perisylvian language pathways in schizophrenia: relationship to auditory hallucinations. Biological psychiatry. 2011;70(12):1143–50. doi: 10.1016/j.biopsych.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 61.Gillinder Bedi FC, Cecchi Guillermo A, Fernánde Slezak Diego, Sigman Mariano, Mota Natália B, Sidarta Ribeiro, Javitt Daniel C, Mauro Copelli, Corcoran Cheryl M. Automated analysis of free speech predicts psychosis onset in high-risk youths. NPJ Schizophrenia. 2015 doi: 10.1038/npjschz.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hadley JA, Nenert R, Kraguljac NV, Bolding MS, White DM, Skidmore FM, et al. Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39(4):1020–30. doi: 10.1038/npp.2013.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA psychiatry. 2015;72(4):316–24. doi: 10.1001/jamapsychiatry.2014.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Archives of general psychiatry. 2010;67(3):231–9. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 65.Allen P, Luigjes J, Howes OD, Egerton A, Hirao K, Valli I, et al. Transition to psychosis associated with prefrontal and subcortical dysfunction in ultra high-risk individuals. Schizophrenia bulletin. 2012;38(6):1268–76. doi: 10.1093/schbul/sbr194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Howes OD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. The American journal of psychiatry. 2011;168(12):1311–7. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Archives of general psychiatry. 2009;66(1):13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 68.Hoptman MJ, Zuo XN, D’Angelo D, Mauro CJ, Butler PD, Milham MP, et al. Decreased interhemispheric coordination in schizophrenia: a resting state fMRI study. Schizophrenia research. 2012;141(1):1–7. doi: 10.1016/j.schres.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li HJ, Xu Y, Zhang KR, Hoptman MJ, Zuo XN. Homotopic connectivity in drug-naive, first-episode, early-onset schizophrenia. Journal of child psychology and psychiatry, and allied disciplines. 2014 doi: 10.1111/jcpp.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knochel C, Oertel-Knochel V, Schonmeyer R, Rotarska-Jagiela A, van de Ven V, Prvulovic D, et al. Interhemispheric hypoconnectivity in schizophrenia: fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. NeuroImage. 2012;59(2):926–34. doi: 10.1016/j.neuroimage.2011.07.088. [DOI] [PubMed] [Google Scholar]
- 71.Katagiri N, Pantelis C, Nemoto T, Zalesky A, Hori M, Shimoji K, et al. A longitudinal study investigating sub-threshold symptoms and white matter changes in individuals with an ‘at risk mental state’ (ARMS) Schizophrenia research. 2015;162(1–3):7–13. doi: 10.1016/j.schres.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Yang Z, Jutagir DR, Koyama MS, Craddock RC, Yan CG, Shehzad Z, et al. Intrinsic brain indices of verbal working memory capacity in children and adolescents. Developmental cognitive neuroscience. 2015;15:67–82. doi: 10.1016/j.dcn.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang Z, Kelly C, Castellanos FX, Leon T, Milham MP, Adler LA. Neural Correlates of Symptom Improvement Following Stimulant Treatment in Adults with Attention-Deficit/Hyperactivity Disorder. Journal of child and adolescent psychopharmacology. 2016;26(6):527–36. doi: 10.1089/cap.2015.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Satterthwaite TD, Cook PA, Bruce SE, Conway C, Mikkelsen E, Satchell E, et al. Dimensional depression severity in women with major depression and post-traumatic stress disorder correlates with fronto-amygdalar hypoconnectivty. Molecular psychiatry. 2016;21(7):894–902. doi: 10.1038/mp.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Dam NT, O’Connor D, Marcelle ET, Ho EJ, Cameron Craddock R, Tobe RH, et al. Data-Driven Phenotypic Categorization for Neurobiological Analyses: Beyond DSM-5 Labels. Biological psychiatry. 2016 doi: 10.1016/j.biopsych.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American journal of psychiatry. 2010;167(7):748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 77.Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophrenia research. 2009;108(1–3):85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fornito A, Yung AR, Wood SJ, Phillips LJ, Nelson B, Cotton S, et al. Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biological psychiatry. 2008;64(9):758–65. doi: 10.1016/j.biopsych.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 79.Sun D, Stuart GW, Jenkinson M, Wood SJ, McGorry PD, Velakoulis D, et al. Brain surface contraction mapped in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Molecular psychiatry. 2009;14(10):976–86. doi: 10.1038/mp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fornito A, Yucel M, Wood SJ, Proffitt T, McGorry PD, Velakoulis D, et al. Morphology of the paracingulate sulcus and executive cognition in schizophrenia. Schizophrenia research. 2006;88(1–3):192–7. doi: 10.1016/j.schres.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 81.Fornito A, Yucel M, Wood SJ, Adamson C, Velakoulis D, Saling MM, et al. Surface-based morphometry of the anterior cingulate cortex in first episode schizophrenia. Human brain mapping. 2008;29(4):478–89. doi: 10.1002/hbm.20412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anticevic A, Tang Y, Cho YT, Repovs G, Cole MW, Savic A, et al. Amygdala connectivity differs among chronic, early course, and individuals at risk for developing schizophrenia. Schizophrenia bulletin. 2014;40(5):1105–16. doi: 10.1093/schbul/sbt165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Archives of general psychiatry. 2009;66(9):938–46. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biological psychiatry. 2011;70(1):64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Katsura M, Ohmuro N, Obara C, Kikuchi T, Ito F, Miyakoshi T, et al. A naturalistic longitudinal study of at-risk mental state with a 2.4 year follow-up at a specialized clinic setting in Japan. Schizophrenia research. 2014;158(1–3):32–8. doi: 10.1016/j.schres.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 86.Crossley NA, Constante M, Fusar-Poli P, Bramon E. Neurophysiological alterations in the prepsychotic phases. Current pharmaceutical design. 2012;18(4):479–85. doi: 10.2174/138161212799316307. [DOI] [PubMed] [Google Scholar]
- 87.Salokangas RK, Ruhrmann S, von Reventlow HG, Heinimaa M, Svirskis T, From T, et al. Axis I diagnoses and transition to psychosis in clinical high-risk patients EPOS project: prospective follow-up of 245 clinical high-risk outpatients in four countries. Schizophr Res. 138(2–3):192–7. doi: 10.1016/j.schres.2012.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


