Abstract
Singapore experienced its first documented Zika virus outbreak in 2016. We identified clinical and laboratory parameters that increase the probability for Zika or dengue virus infection. Early during the illness, combinations of key parameters obtained through clinical assessment and hematologic tests can help distinguish between these infections.
Keywords: disease outbreaks, dengue, mosquitoes, Aedes, Zika virus, dengue virus, viruses, vector-borne infections, Singapore
Zika virus recently emerged as a clinically important arbovirus that can cause fetal complications in infected pregnant women and Guillain-Barré syndrome in adults (1–3). Since the first reported large Zika outbreak on Yap Island in 2007 (4), widespread community outbreaks have been reported in many other countries (4,5).
Dengue virus (DENV) threatens the 50% of the world’s population who live in at-risk areas and causes ≈390 million infections annually (6). The frequency and magnitude of epidemic dengue have increased exponentially during the past 4 decades because of such factors as population growth and rising temperatures (7). Severe dengue can result in plasma leakage, systemic shock, multiorgan failure, and eventual death (8).
Zika and dengue have similar presenting symptoms (including fever, rash, arthralgia, myalgia, and headache) (4,9); incubation periods; and transmission routes through Aedes mosquitos (5,6). Accurate and early diagnosis is essential to properly manage the unique complications of each disease.
In Singapore, a tropical island city-state in Southeast Asia, DENV is endemic. Dengue epidemics have been recorded every few years since the 1990s and now predominantly affect adults (8). In August 2016, Singapore experienced its first documented Zika outbreak (10). Before that outbreak, national surveillance of community-based patients (ongoing since 2014) had not detected any local Zika cases (11). The Zika outbreak occurred on the background of ongoing DENV circulation, and medical practitioners had to consider concurrent testing for both infections as part of clinical management.
Using the likelihood ratio approach, we identified clinical and laboratory parameters that increase the likelihood of a laboratory-confirmed diagnosis of Zika virus or DENV infection at first presentation to clinical care. The clinical diagnostic process may lack sensitivity and specificity (12). A positive likelihood ratio (LR+) is calculated using the proportion of patients with the disease having a positive clinical or laboratory finding divided by the proportion of patients without the disease having that same finding (13). This information adds value to clinical diagnosis by refining the posttest probability of a disease. In the absence of confirmatory laboratory tests, a thorough assessment of such parameters may help distinguish between the 2 diseases.
The Study
We reviewed 2 prospectively recruited cohorts of patients with suspected Zika virus and DENV infections treated at Tan Tock Seng Hospital (Singapore), an adult tertiary care hospital. This hospital also houses the Communicable Disease Centre, the designated institution for centralized management of emerging infectious diseases in Singapore.
The Zika cohort comprised persons with suspected Zika virus infection recruited during August and September 2016. We followed the case definition used by the Singapore Ministry of Health (i.e., any person living, working, or studying in the outbreak area with fever and maculopapular rash plus >1 additional symptom of arthralgia, myalgia, headache, or conjunctivitis). Patients whose illness partially or fully met the case definition underwent confirmatory laboratory testing through detection of Zika virus RNA in serum and urine samples using real-time reverse transcription PCR (RT-PCR) (14). Confirmed cases were defined as a positive result for a Zika virus serum or urine test. We excluded patients with laboratory-confirmed dengue co-infection, using dengue NS1 antigen (Bio-Rad Laboratories, Marnes-la-Coquette, France) or RT-PCR (15).
The dengue cohort comprised persons with suspected DENV infection recruited during January 2010–September 2012. Persons in this cohort had fever (temperature >37.5°C), with or without additional signs or symptoms, and no alternative diagnosis at the time they sought care. Cases were defined as a serum-positive DENV NS1 antigen or RT-PCR.
To evaluate parameters during the early phase of each illness for both cohorts, we limited participant recruitment to persons who sought care within 5 days after symptom onset. We obtained ethics approval from the National Healthcare Group (NHG Domain Specific Review Board reference no. 2016/01027).
The Zika cohort comprised 281 persons with suspected Zika virus infection (Technical Appendix Table 1): 130 case-patients (without dengue co-infection) and 151 non–case-patients. The median age of case-patients (34 years [interquartile range (IQR) 26–49 years]) was similar to that of non–case-patients (31 years [IQR 24–38 years]). Sex distribution was similar between case-patients (60% male) and non–case-patients.
Table 1. Diagnostic accuracy of clinical and laboratory parameters for Zika virus infection cohort. Singapore*.
| Parameters | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | LRP (95% CI) | LRN (95% CI) |
|---|---|---|---|---|---|---|
| Clinical | ||||||
| Documented fever >37.5°C | 0.35 (0.26–0.43) | 0.58 (0.50–0.66) | 0.42 (0.32–0.51) | 0.51 (0.43–0.58) | 0.83 (0.61–1.12) | 1.12 (0.93–1.35) |
| Headache | 0.23 (0.16–0.30) | 0.62 (0.54–0.69) | 0.34 (0.24–0.44) | 0.48 (0.41–0.55) | 0.60 (0.41–0.87) | 1.25 (1.07–1.46) |
| Myalgia | 0.41 (0.33–0.49) | 0.41 (0.34–0.47) | 0.32 (0.25–0.39) | 0.5 (0.42–0.57) | 0.66 (0.52–0.84) | 1.54 (1.20–1.97) |
| Arthralgia | 0.21 (0.14–0.28) | 0.79 (0.72–0.85) | 0.46 (0.33–0.58) | 0.54 (0.47–0.60) | 0.98 (0.62–1.54) | 1.01 (0.90–1.13) |
| Rash | 0.94 (0.90–0.98) | 0.59 (0.51–0.67) | 0.67 (0.59–0.73) | 0.92 (0.86–0.97) | 2.29 (1.88–2.78) | 0.10 (0.05–0.21) |
| Conjunctivitis | 0.23 (0.16–0.3) | 0.88 (0.83–0.93) | 0.63 (0.49–0.76) | 0.57 (0.51–0.63) | 1.93 (1.13–3.31) | 0.87 (0.78–0.98) |
| Nausea | 0.04 (0.01–0.07) | 0.95 (0.91–0.98) | 0.38 (0.12–0.65) | 0.53 (0.47–0.59) | 0.73 (0.24–2.16) | 1.02 (0.96–1.07) |
| Vomiting | 0.02 (0–0.04) | 0.94 (0.90–0.98) | 0.18 (0–0.41) | 0.53 (0.47–0.58) | 0.26 (0.06–1.17) | 1.04 (1.00–1.10) |
| Diarrhea | 0.03 (0.001–0.06) | 0.97 (0.94–1.00) | 0.44 (0.12–0.77) | 0.54 (0.48–0.60) | 0.93 (0.25–3.39) | 1.00 (0.96–1.05) |
| Abdominal pain | 0.02 (0–0.04) | 0.98 (0.96–1.00) | 0.40 (0–0.82) | 0.54 (0.48–0.60) | 0.77 (0.13–.57) | 1.00 (0.97–1.04) |
| Runny nose | 0.02 (0–0.05) | 0.92 (0.88–0.96) | 0.20 (0–0.40) | 0.52 (0.46–0.58) | 0.29 (0.08–1.01) | 1.06 (1.006–1.12) |
| Sore throat | 0.18 (0.12–0.25) | 0.83 (0.77–0.89) | 0.48 (0.34–0.62) | 0.54 (0.48–0.61) | 1.07 (0.65–1.77) | 0.98 (0.88–1.10) |
| Cough | 0.12 (0.06–0.17) | 0.72 (0.64–0.79) | 0.26 (0.15–0.37) | 0.49 (0.42–0.55) | 0.41 (0.24–0.69) | 1.24 (1.10–1.39) |
| Breathlessness | 0.01 (0–0.02) | 0.97 (0.95–1.00) | 0.20 (0–0.55) | 0.53 (0.47–0.59) | 0.29 (0.03–2.56) | 1.02 (0.99–1.05) |
|
|
|
|
|
|
|
|
| Laboratory† | ||||||
| Leukocytes <3.6 × 109 cells/L | 0.17 (0.11–0.24) | 0.9 (0.85–0.94) | 0.54 (0.4–0.69) | 0.6 (0.55–0.66) | 2.00 (1.06–3.79) | 0.90 (0.82–0.99) |
| Hemoglobin <13 g/dL | 0.22(0.15–0.29) | 0.80 (0.74–0.87) | 0.50 (0.37–0.63) | 0.54 (0.47–0.60) | 1.13 (0.72–1.79) | 0.97 (0.86–1.09) |
| Platelets <100 × 109/L | 0.01 (0–0.02) | 0.99 (0.97–1.00) | 0.33 (0–0.87) | 0.53 (0.47–0.59) | 0.57 (0.05–6.16) | 1.01 (0.98–1.03) |
| Neutrophils <1.4 × 109 cells/L | 0.06 (0.02–0.10) | 0.99 (0.97–1.00) | 0.80 (0.55–1.00) | 0.53 (0.47–0.59) | 4.27 (0.93–19.78) | 0.95 (0.91–1.00) |
| Neutrophils >5.9 × 109 cells/L | 0.04 (0.01–0.08) | 0.66 (0.58–0.74) | 0.1 (0.02–0.18) | 0.42 (0.36–0.49) | 0.11 (0.05–0.28) | 1.45 (1.28–1.64) |
| Lymphocytes <0.9 × 109 cells/L | 0.18 (0.12–0.25) | 0.78 (0.71–0.85) | 0.44 (0.31–0.57) | 0.50 (0.44–0.58) | 0.83 (0.51–1.33) | 1.04 (0.93–1.18) |
| Creatinine >105 μmol/L | 0.08 (0.03–0.13) | 0.94 (0.91–0.98) | 0.56 (0.33–0.79) | 0.54 (0.47–0.60) | 1.41 (0.57–3.46) | 0.98 (0.91–1.04) |
| Urea >9.3 mmol/L | 0 | 0.99 (0.98–1.00) | 0 | 0.54 (0.48–0.59) | 0 | 1.01 (0.99–1.02) |
| Alanine aminotransferase >54 U/L | 0.08 (0.03–0.13) | 0.94 (0.91–0.98) | 0.56 (0.33–0.79) | 0.56 (0.5–0.61) | 0.72 (0.37–1.39) | 1.05 (0.96–1.14) |
| Aspartate aminotransferase >41 U/L | 0.07 (0.03–0.11) | 0.83 (0.77–0.89) | 0.27 (0.12–0.42) | 0.50 (0.43–0.56) | 0.42 (0.20–0.86) | 1.12 (1.02–1.22) |
|
|
|
|
|
|
|
|
| Combinations | ||||||
| Any GI symptom (nausea, vomiting, diarrhea, abdominal pain) | 0.06 (0.02–0.10) | 0.89 (0.84–0.94) | 0.33 (0.14–0.52) | 0.53 (0.46–0.59) | 0.58 (0.26–1.31) | 1.04 (0.98–1.13) |
| Any GI symptom + lymphopenia | 0.01 (0–0.03) | 0.96 (0.93–0.99) | 0.20 (0–0.45) | 0.56 (0.5–0.61) | 0.53 (0.10–2.87) | 1.01 (0.98–1.05) |
| Rash + conjunctivitis | 0.22 (0.15–0.29) | 0.97 (0.94–0.99) | 0.85 (0.73–0.97) | 0.59 (0.53–0.65) | 6.73 (2.68–16.90) | 0.80 (0.73–0.89) |
| Documented fever + rash | 0.31 (0.23–0.39) | 0.86 (0.81–0.92) | 0.66 (0.54–0.77) | 0.59 (0.53–0.66) | 2.21 (1.38–3.55) | 0.80 (0.71–0.92) |
| Documented fever + rash + any GI symptom | 0.02 (0–0.04) | 0.99 (0.98–1.00) | 0.75 (0.33–1.17) | 0.54 (0.48–0.60) | 3.48 (0.37–33.10) | 0.98 (0.95–1.01) |
| Documented fever + rash + lymphopenia | 0.07 (0.02–0.11) | 0.94 (0.90–0.98) | 0.50 (0.27–0.73) | 0.53 (0.47–0.60) | 1.14 (0.47–2.78) | 0.99 (0.93–1.05) |
| Documented fever + lymphopenia | 0.10 (0.05–0.15) | 0.86 (0.80–0.91) | 0.38 (0.22–0.55) | 0.52 (0.46–0.58) | 0.70 (0.37–1.34) | 1.05 (0.96–1.15) |
| Documented fever + thrombocytopenia | 0 | 0.98 (0.97–1.00) | 0 | 0.53 (0.48–0.59) | 0 | 1.01 (0.99–1.03) |
| Documented fever + lymphopenia + thrombocytopenia | 0 | 0.99 (0.97–1.00) | 0 | 0.53 (0.48–0.59) | 0 | 1.01 (0.99–1.03) |
| Documented fever + rash, with >1 of arthralgia, myalgia, headache, conjunctivitis | 0.21 (0.14–0.28) | 0.89 (0.84–0.94) | 0.63 (0.48–0.77) | 0.57 (0.50–0.63) | 1.96 (1.11–3.47) | 0.90 (0.80–0.98) |
*GI, gastrointestinal; LRN, negative likelihood ratio; LRP, positive likelihood ratio; NPV, negative predictive value; PPV, positive predictive value. †Reference values: leukocytes, 3.6–9.3 × 109 cells/L; hemoglobin, 11.0–15.0 g/dL; platelets, 170–420 × 109/L; neutrophils, 1.40–5.90 × 109 cells/L; lymphocytes, 0.90–3.30 × 109 cells/L; creatinine, 40–75 μmol/L; urea, 2.9–9.3 mmol/L; alanine aminotransferase, 14–54 U/L; aspartate aminotransferase, 15–41 U/L.
The DENV cohort comprised 310 persons with suspected DENV infection: 175 case-patients and 135 non–case-patients. Age groups were similar for case-patients (median age 36 years [IQR 29–43 years]) and non–case-patients (median age 32 years [IQR 27–42 years]), and both groups consisted primarily of male patients.
Zika virus infection case-patients most commonly had rash (94%), myalgia (41%), and documented fever (35%) (Figure 1); non–case-patients mainly had myalgia (62%), documented fever (42%), and rash (41%). Low proportions of both groups had marked thrombocytopenia (platelets <100 × 109/L [reference 170–420 × 109/L]), leukopenia, or lymphopenia.
Figure 1.
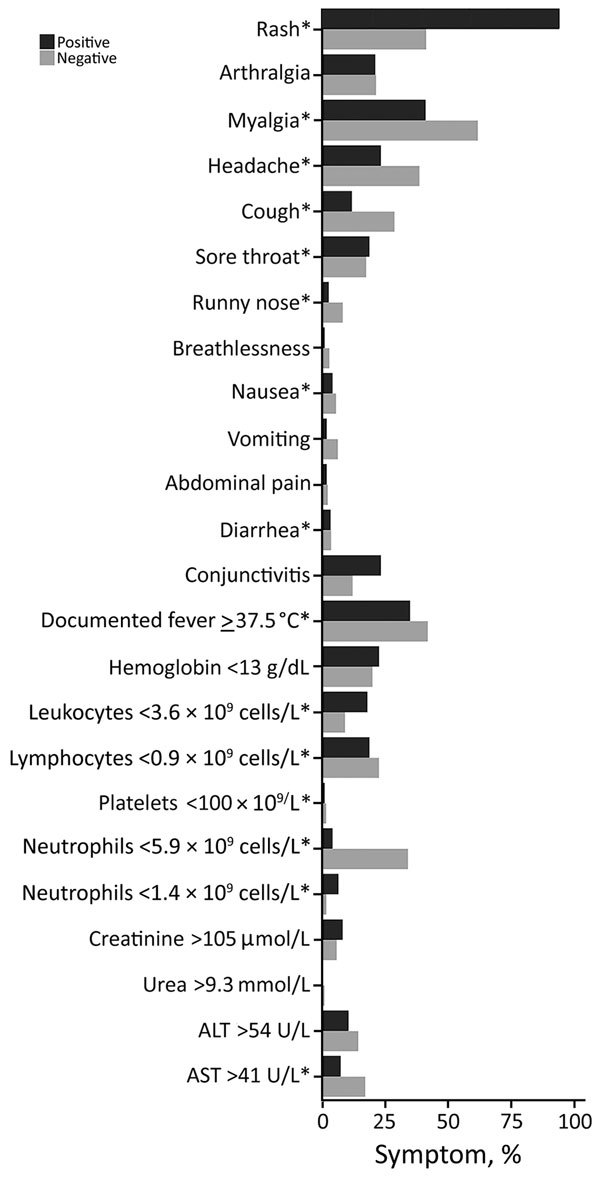
Clinical and laboratory parameters of Zika virus cohort, Singapore. *Statistically significant differences (p<0.05).
DENV case-patients and non–case-patients most commonly had headache, myalgia, and nausea (Figure 2). Eighty-two percent of case-patients reported gastrointestinal symptoms and, compared with non–case-patients, much higher proportions of leukopenia (89% vs. 39%), lymphopenia (81% vs. 37%), and marked thrombocytopenia (53% vs. 31%).
Figure 2.
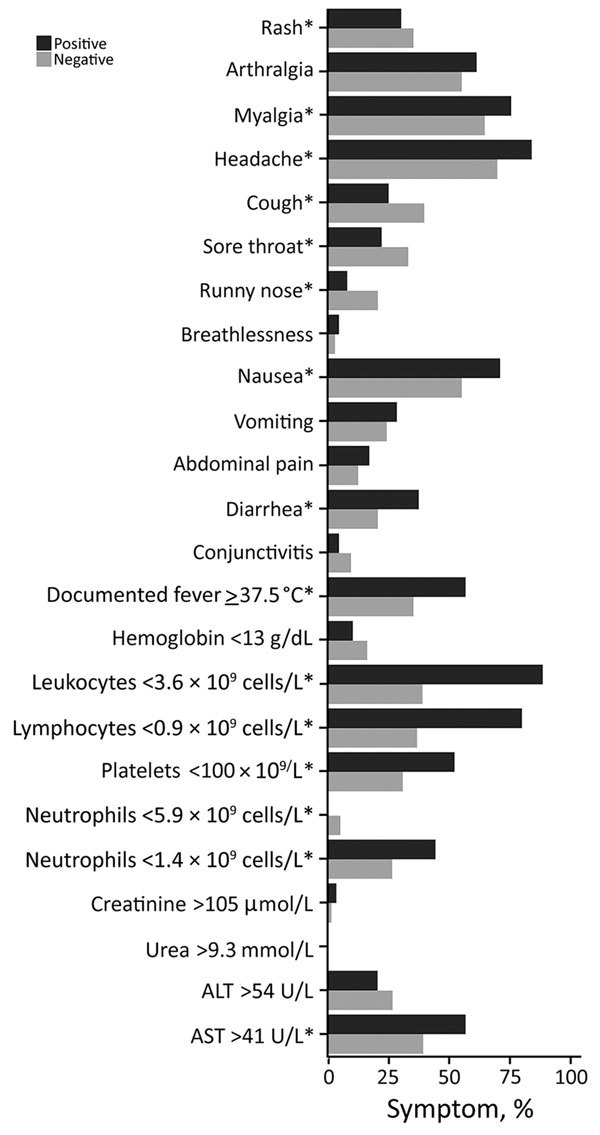
Clinical and laboratory parameters of dengue cohort, Singapore. *Statistically significant differences (p<0.05).
For assessment of Zika virus infection, among individual parameters evaluated, presence of rash gave the highest LR+, sensitivity and negative predictive value, and lowest negative likelihood ratio (Table 1). We obtained the highest LR+ using a combination of rash and conjunctivitis (LR+ = 6.73, 95% CI 2.68–16.90).
For assessment of DENV infection, documented fever gave the highest individual LR+ of 3.13 (95% CI 2.48–3.94) (Table 2). We obtained the highest LR+ using a combination of documented fever, lymphopenia, and thrombocytopenia (LR+ = 5.11, 95% CI 2.51–10.38).
Table 2. Diagnostic accuracy of clinical and laboratory parameters for dengue virus infection cohort, Singapore*.
| Parameter | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | LRP (95% CI) | LRN (95% CI) |
|---|---|---|---|---|---|---|
| Clinical | ||||||
| Documented fever >37.5°C | 0.57 (0.50–0.64) | 0.64 (0.56–0.73) | 0.68 (0.60–0.75) | 0.54 (0.46–0.61) | 3.13 (2.48–3.94) | 0.61 (0.54–0.69) |
| Headache | 0.84 (0.79–0.90) | 0.30 (0.22–0.37) | 0.61 (0.55–0.67) | 0.60 (0.48–0.71) | 1.20 (1.06–1.36) | 0.52 (0.34–0.80) |
| Myalgia | 0.76 (0.70–0.82) | 0.35 (0.27–0.43) | 0.6 (0.54–0.67) | 0.53 (0.42–0.63) | 1.17 (1.005–1.35) | 0.69 (0.49–0.98) |
| Arthralgia | 0.62 (0.55–0.69) | 0.44 (0.36–0.53) | 0.59 (0.52–0.66) | 0.47 (0.36–0.56) | 1.11 (0.92–1.34) | 0.86 (0.66–1.12) |
| Rash | 0.30 (0.23–0.37) | 0.64 (0.56–0.73) | 0.52 (0.43–0.62) | 0.42 (0.35–0.48) | 0.85 (0.62–1.17) | 1.08 (0.92- 1.27) |
| Conjunctivitis | 0.05 (0.01–0.08) | 0.90 (0.85–0.95) | 0.38 (0.17–0.59) | 0.42 (0.37–0.48) | 0.47 (0.20–1.11) | 1.06 (0.99–1.13) |
| Nausea | 0.71 (0.65–0.78) | 0.44 (0.36–0.53) | 0.63 (0.56–0.69) | 0.54 (0.45–0.64) | 1.29 (1.08–1.54) | 0.64 (0.48–0.87) |
| Vomiting | 0.29 (0.22–0.35) | 0.76 (0.68–0.83) | 0.60 (0.50–0.71) | 0.45 (0.38–0.51) | 1.17 (0.80–1.71) | 0.94 (0.83–1.08) |
| Diarrhea | 0.38 (0.31–0.45) | 0.79 (0.72–0.86) | 0.70 (0.61–0.79) | 0.50 (0.43–0.56) | 1.82 (1.24–2.66) | 0.79 (0.68–0.91) |
| Abdominal pain | 0.17 (0.12–0.23) | 0.87 (0.82–0.93) | 0.64 (0.50–0.78) | 0.45 (0.39–0.51) | 1.36 (0.78–2.36) | 0.95 (0.86–1.04) |
| Runny nose | 0.08 (0.04–0.12) | 0.79 (0.72–0.86) | 0.33 (0.19- 0.48) | 0.40 (0.34- 0.46) | 0.39 (0.21–0.70) | 1.16 (1.05–1.28) |
| Sore throat | 0.22 (0.16–0.28) | 0.67 (0.59–0.75) | 0.46 (0.36- 0.57) | 0.40 (0.33–0.46) | 0.67 (0.46–0.96) | 1.17 (1.01–1.35) |
| Cough | 0.25 (0.19–0.32) | 0.60 (0.52–0.68) | 0.45 (0.35–0.55) | 0.38 (0.32–0.45) | 0.63 (0.45–0.87) | 1.25 (1.06–1.47) |
| Breathlessness |
0.05 (0.01–0.08) |
0.97 (0.94–1.00) |
0.67 (0.40–0.93) |
0.44 (0.38–0.50) |
1.54 (0.47–5.02) |
0.98 (0.94–1.03) |
| Laboratory† | ||||||
| Leukocytes <3.6 × 109 cells/L | 0.89 (0.85–0.94) | 0.61 (0.53–0.69) | 0.75 (0.69–0.81) | 0.82 (0.74–0.89) | 2.27 (1.83–2.82) | 0.18 (0.11–0.28) |
| Hemoglobin <13 g/dL | 0.10 (0.06–0.15) | 0.84 (0.77–0.90) | 0.45 (0.30–0.60) | 0.42 (0.36–0.48) | 0.63 (0.35–1.12) | 1.07 (0.98–1.17) |
| Platelets <100 × 109/L | 0.53 (0.45–0.60) | 0.67 (0.59–0.75) | 0.67 (0.59–0.75) | 0.52 (0.45–0.59) | 1.58 (1.20–2.08) | 0.71 (0.58–0.87) |
| Neutrophils <1.4 × 109 cells/L | 0.45 (0.37–0.52) | 0.73 (0.66–0.81) | 0.68 (0.60–0.77) | 0.51 (0.44–0.58) | 1.67 (1.21–2.31) | 0.76 (0.64–0.89) |
| Neutrophils >5.9 × 109 cells/L | 0 | 0.95 (0.91–0.99) | 0 | 0.42 (0.37–0.48) | 0 | 1.05 (1.01–1.10) |
| Lymphocytes <0.9 × 109 cells/L | 0.81 (0.75–0.86) | 0.63 (0.55–0.71) | 0.74 (0.68–0.80) | 0.71 (0.63–0.80) | 2.17 (1.73–2.74) | 0.31 (0.22–0.43) |
| Creatinine >105 μmol/L | 0.03 (0.01–0.06) | 0.98 (0.97–1.00) | 0.75 (0.45–1.00) | 0.44 (0.38–0.50) | 2.31 (0.47–11.39) | 0.98 (0.95–1.01) |
| Alanine aminotransferase >54 U/L | 0.21 (0.15–0.27) | 0.73 (0.66–0.81) | 0.50 (0.38–0.62) | 0.41 (0.35–0.47) | 0.77 (0.51–1.15) | 1.09 (0.96–1.23) |
| Aspartate aminotransferase >41 U/L |
0.57 (0.50–0.64) |
0.60 (0.52–0.69) |
0.65 (0.57- 0.73) |
0.52 (0.44–0.60) |
1.44 (1.13–1.85) |
0.71 (0.57–0.88) |
| Combinations | ||||||
| Any GI symptom (nausea, vomiting, diarrhea, abdominal pain) | 0.82 (0.77–0.88) | 0.37 (0.29–0.45) | 0.63 (0.57–0.69) | 0.62 (0.51–0.72) | 1.31 (1.13–1.51) | 0.48 (0.32–0.70) |
| Any GI symptom + lymphopenia | 0.66 (0.59–0.73) | 0.76 (0.68–0.83) | 0.78 (0.71–0.85) | 0.63 (0.56–0.71) | 2.71 (1.98–3.72) | 0.45 (0.35–0.56) |
| Rash + conjunctivitis | 0.02 (0.01–0.04) | 0.93 (0.89–0.98) | 0.31 (0.06–0.56) | 0.42 (0.37–0.48) | 0.34 (0.11–1.09) | 1.04 (0.99–1.10) |
| Documented fever + rash | 0.15 (0.10–0.20) | 0.92 (0.87–0.96) | 0.70 (0.56–0.85) | 0.45 (0.40–0.51) | 1.82 (0.93–3.55) | 0.93 (0.86–1.00) |
| Documented fever + rash + any GI symptom + lymphopenia | 0.01 (0.06–0.15) | 0.96 (0.92–0.99) | 0.76 (0.59–0.93) | 0.45 (0.39–0.51) | 2.44 (1.003–5.95) | 0.93 (0.88–0.99) |
| Documented fever + rash + any GI symptom | 0.14 (0.09–0.19) | 0.96 (0.92–0.99) | 0.80 (0.66–0.94) | 0.46 (0.40–0.52) | 3.09 (1.30–7.34) | 0.90 (0.84–0.97) |
| Documented fever + rash + lymphopenia | 0.12 (0.07–0.17) | 0.94 (0.90–0.98) | 0.72 (0.56–0.89) | 0.45 (0.39–0.51) | 2.03 (0.93–4.40) | 0.94 (0.87–1.00) |
| Documented fever + lymphopenia | 0.51 (0.44–0.59) | 0.84 (0.77–0.90) | 0.80 (0.73–0.88) | 0.57 (0.50–0.64) | 3.16 (2.10–4.75) | 0.58 (0.50–0.69) |
| Documented fever + thrombocytopenia | 0.33 (0.26–0.40) | 0.88 (0.83–0.94) | 0.78 (0.69–0.88) | 0.50 (0.44–0.57) | 2.74 (1.66–4.56) | 0.76 (0.68–0.86) |
| Documented fever + lymphopenia + thrombocytopenia | 0.30 (0.23–0.37) | 0.94 (0.90–0.98) | 0.87 (0.78–0.95) | 0.51 (0.45–0.57) | 5.11 (2.51–10.38) | 0.74 (0.67–0.82) |
| Documented fever + rash, with >1 of arthralgia, myalgia, headache, conjunctivitis | 0.14 (0.09–0.19) | 0.93 (0.89–0.98) | 0.74 (0.59–0.88) | 0.73 (0.40–0.52) | 2.14 (1.03–4.43) | 0.92 (0.85–0.99) |
*GI, gastrointestinal; LRN, negative likelihood ratio; LRP, positive likelihood ratio; NPV, negative predictive value; PPV, positive predictive value. †Reference values: leukocytes, 3.6–9.3 × 109/L; hemoglobin, 11.0–15.0 g/dL; platelets, 170–420 ×109/L); neutrophils, 1.40–5.90 × 109/L; lymphocytes, 0.90–3.30 × 109/L; creatinine, 40–75 μmol/L; urea, 2.9–9.3 mmol/L;alanine aminotransferase, 14–54 U/L; aspartate aminotransferase, 15–41 U/L.
Conclusions
Our study demonstrated some key differences between the 2 diseases. Presence of rash featured much more prominently in Zika virus infection than DENV infection during the first 5 days of illness. For dengue patients, rashes usually appear during the critical or recovery phases (typically around the fifth day of illness or thereafter) (9). Also, in contrast with dengue patients, relatively few Zika patients had hematologic abnormalities.
Regardless of the patient’s pretest probability for a disease, the change in posttest probability is approximated by a constant (13). In our study, presence of rash with conjunctivitis gave the highest increase in probability (≈40%) of Zika virus infection, whereas a combination of documented fever, lymphopenia, and thrombocytopenia increased the probability for DENV infection by ≈30% (Technical Appendix Table 2). In contrast, absence of rash in a patient with suspected Zika or absence of lymphopenia in a patient with suspected dengue reduced the probability of the respective disease by 30%–45%. In countries where these viruses co-circulate, and where access to confirmatory laboratory testing is limited, these may be simple methods to help medical practitioners assess a patient suspected to have Zika or dengue.
The strengths of our study include the analysis of common parameters, standardized to ensure comparability across the 2 cohorts. Our analysis presents likelihood ratios, which are easy to interpret and aid clinical diagnosis by refining the posttest probability of a disease. The main study limitation is that our analysis compared 2 cohorts recruited at separate times and hence does not directly distinguish between the 2 infections. However, hospital workflows and clinical assessment methods for patients largely did not change between these periods. In both cohorts, cases could have been misclassified as noncases because RT-PCR might have missed patients who sought care late. We reduced this risk by restricting recruitment to patients seeking care within 5 days after symptom onset. Our study demonstrates that a thorough assessment of clinical and hematologic parameters can aid the clinical diagnosis of Zika virus and DENV infections in the early stages.
Baseline demographic variables for patients in cohorts suspected to have Zika virus infection or dengue virus infection, Singapore; changes in posttest probability of Zika and dengue virus infections based on presence or absence of selected clinical and laboratory parameters within the first 5 days after symptom onset.
Acknowledgments
We thank the staff of the Department of Clinical Epidemiology, Tan Tock Seng Hospital, who helped extract and collate data from the hospital databases for the study.
This work was supported by the STOP Dengue Translational Clinical Research program, funded by the National Research Foundation through the National Medical Research Council, Singapore (grant no. NMRC/TCR/005/2008).
Biography
Dr. Ho is a public health physician working in infectious disease epidemiology. His research interests include surveillance and control of emerging infectious diseases; implementation of preventive measures, such as adult vaccination programs; and outbreak investigation and management.
Footnotes
Suggested citation for this article: Ho HJ, Wong JGX, Kyaw WM, Lye DC, Leo YS, Chow A. Diagnostic accuracy of parameters for Zika and dengue virus infections, Singapore. Emerg Infect Dis. 2017 Dec [date cited]. https://doi.org/10.3201/eid2312.171224
References
- 1.Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT, do Carmo GM, Henriques CM, Coelho GE, et al. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:242–7. 10.15585/mmwr.mm6509e2 [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374:1981–7. 10.1056/NEJMsr1604338 [DOI] [PubMed] [Google Scholar]
- 3.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–9. 10.1016/S0140-6736(16)00562-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–43. 10.1056/NEJMoa0805715 [DOI] [PubMed] [Google Scholar]
- 5.Paixão ES, Barreto F, Teixeira MG, Costa MC, Rodrigues LC. History, epidemiology, and clinical manifestations of Zika: a systematic review. Am J Public Health. 2016;106:606–12. 10.2105/AJPH.2016.303112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Struchiner CJ, Rocklöv J, Wilder-Smith A, Massad E. Increasing dengue incidence in Singapore over the past 40 years: population growth, climate and mobility. PLoS One. 2015;10:e0136286. 10.1371/journal.pone.0136286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hapuarachchi HC, Koo C, Rajarethinam J, Chong CS, Lin C, Yap G, et al. Epidemic resurgence of dengue fever in Singapore in 2013-2014: A virological and entomological perspective. BMC Infect Dis. 2016;16:300. 10.1186/s12879-016-1606-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Dengue guidelines for diagnosis, treatment, prevention and control: new edition 2009. 2017. [cited 2017 Jul 25]. http://www.who.int/rpc/guidelines/9789241547871/en/ [PubMed]
- 10.Leo YS, Chow A. Zika virus has arrived in Singapore. Lancet Infect Dis. 2016;16:1317–9. 10.1016/S1473-3099(16)30448-0 [DOI] [PubMed] [Google Scholar]
- 11.The Straits Times. Singapore. Parliament: Singapore's Zika efforts started two years ago. 2016. Sep 14 [cited 2017 Jul 25]. http://www.straitstimes.com/singapore/singapores-zika-efforts-started-two-years-ago
- 12.Berner ES, Graber ML. Overconfidence as a cause of diagnostic error in medicine. Am J Med. 2008;121(Suppl):S2–23. 10.1016/j.amjmed.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 13.McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17:646–9. 10.1046/j.1525-1497.2002.10750.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–9. 10.3201/eid1408.080287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai YL, Chung YK, Tan HC, Yap HF, Yap G, Ooi EE, et al. Cost-effective real-time reverse transcriptase PCR (RT-PCR) to screen for Dengue virus followed by rapid single-tube multiplex RT-PCR for serotyping of the virus. J Clin Microbiol. 2007;45:935–41. 10.1128/JCM.01258-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline demographic variables for patients in cohorts suspected to have Zika virus infection or dengue virus infection, Singapore; changes in posttest probability of Zika and dengue virus infections based on presence or absence of selected clinical and laboratory parameters within the first 5 days after symptom onset.


