Quantitative MR imaging features of functional tumor volume, lesion signal enhancement ratio, and mean normal tissue background parenchymal enhancement show promise for use in the noninvasive assessment of ductal carcinoma in situ.
Abstract
Purpose
To investigate whether specific imaging features on breast magnetic resonance (MR) images are associated with ductal carcinoma in situ (DCIS) recurrence risk after definitive treatment.
Materials and Methods
Patients with DCIS who underwent preoperative dynamic contrast material–enhanced (DCE) MR imaging between 2004 and 2014 with ipsilateral recurrence more than 6 months after definitive surgical treatment were retrospectively identified. For each patient, a control subject with DCIS that did not recur was identified and matched on the basis of clinical, histopathologic, and treatment features known to affect recurrence risk. On DCE MR images, lesion characteristics (longest diameter, functional tumor volume [FTV], peak percentage enhancement [PE], peak signal enhancement ratio [SER], and washout fraction) and normal tissue features (background parenchymal enhancement [BPE] volume, mean BPE) were quantitatively measured. MR imaging features were compared between patients and control subjects by using the Wilcoxon signed-rank test, with adjustment for multiple comparisons.
Results
Of 415 subjects with DCIS who underwent preoperative MR imaging, 14 experienced recurrence and 11 had an identifiable matching control subject (final cohort, 11 patients and 11 control subjects). Median time to recurrence was 14 months, and median follow-up for control subjects was 102 months. When compared with matched control subjects, patients with DCIS recurrence exhibited significantly greater FTV (median, 9.3 cm3 vs 1.3 cm3, P = .01), lesion peak SER (median, 1.7 vs 1.2; P = .03), and mean BPE (median, 58.3% vs 41.1%; P = .02).
Conclusion
Quantitative lesion and normal breast tissue characteristics at preoperative MR imaging in women with newly diagnosed DCIS show promise for association with breast cancer recurrence after treatment.
© RSNA, 2017
Introduction
Ductal carcinoma in situ (DCIS) is a proliferation of abnormal epithelial cells confined within the breast duct, and it accounts for approximately one-quarter of breast cancers detected with screening mammography (1–3). Although modern therapies facilitate DCIS survival rates that approach 100% (4,5), it is estimated that as many as half of the affected women undergo unnecessary surgery or radiation or endocrine therapy for this variably aggressive disease because of a paucity of clinical and pathologic criteria to guide individualized care (6). As a result, the National Institutes of Health outlined a critical need to identify magnetic resonance (MR) imaging features that can improve DCIS risk stratification (2).
While some pathologic and multigene assays have shown promise in the prediction of clinical outcomes (7,8), they are based on limited amounts of tissue and are thus prone to sampling error inherent to core-needle biopsy (CNB) techniques. For example, high-nuclear-grade DCIS lesions with comedonecrosis are more likely to recur after treatment, but these markers cannot be relied on to guide personalized treatments, such as the need for postsurgical radiation therapy. Furthermore, traditional pathologic evaluation of DCIS lesions is prone to sampling error (approximately 50% of DCIS lesions exhibit multiple nuclear grades [7], and up to 20% harbor invasive disease that is occult at CNB but is identified at surgical excision [9]) and wide intra- and interobserver variability (10,11). A multigene assay, Oncotype DX Breast DCIS score (Genomic Health, Redwood City, Calif), has also been shown to correlate with treatment outcome (8), but it does have substantial limitations. Similar to standard pathology tests, this assay is performed in limited tissue specimens and may also be subject to inaccuracy from sampling. In addition, this genetic assay was validated in a patient cohort that included mostly small DCIS lesions with widely negative margins, causing some experts to question its purported broad clinical application (12). Finally, the cost-effectiveness of this expensive proprietary assay is questionable in the setting of current treatment paradigms (13).
Although imaging features have been less explored to date, they have shown potential to assist with DCIS risk assessment. Unlike pathology assays, imaging parameters allow for whole-lesion assessments that may be less prone to sampling error. Several prior studies have identified promising basic MR imaging features that can capture DCIS biology, in general demonstrating that low-grade DCIS lesions are more likely to manifest as small focal areas of enhancement at dynamic contrast material–enhanced (DCE) MR imaging or exhibit high contrast-to-noise ratios and apparent diffusion coefficient values at diffusion-weighted MR imaging (14–16). However, few studies have documented the relationship between specific MR imaging features and meaningful clinical outcomes, such as the risk of recurrence after therapy. The purpose of this study was to investigate whether specific imaging features on breast MR images are associated with DCIS recurrence risk after definitive treatment.
Materials and Methods
Study Population
This retrospective study was approved by the institutional review board, who waived the requirement for informed consent, and was compliant with the Health Insurance Portability and Accountability Act.
Our study cohort was obtained by querying the Consortium Oncology Data Integration (CODI) project, which is a solid tumor clinical research database maintained by the Fred Hutchison Cancer Research Center and the University of Washington. CODI data sources include our institutional pathology database, prospectively recorded breast MR imaging data forms, and the Cancer Surveillance System regional tumor registry for western Washington. The Cancer Surveillance System registry has 95% completeness of ascertainment according to the North American Association of Central Cancer Registries. We queried the CODI database on August 6, 2014, to identify all patients without a previous history of ipsilateral breast cancer who received a diagnosis of DCIS at CNB and who underwent preoperative breast MR imaging between January 1, 2004, and August 31, 2013. Subjects who had breast cancer recurrence in the ipsilateral breast (defined as DCIS or invasive breast cancer) at least 6 months (180 days) after definitive surgical treatment were included as patients with DCIS recurrence.
Matched control subjects were identified from among patients with DCIS in the same CODI database query who did not have recurrence during the study period. For each patient with recurrence, a list of potential matched control subjects aged within 4 years of the respective patient with recurrence was generated. This list was ordered by proximity in age, but otherwise it was random. A radiologist (B.S.J.) then proceeded down a list of potential control subjects for each patient, assessing for the following matching criteria: (a) menopausal status; (b) presence of a high-risk genetic mutation; (c) history of prior chemoprevention for breast cancer; (d) DCIS Van Nuys Pathologic Grade (VNPG) (17), where grade 3 is high nuclear grade, grade 2 is nonhigh nuclear grade with comedonecrosis, and grade 1 is nonhigh nuclear grade without comedonecrosis; (e) estrogen receptor status; (f) final surgical margins scored with the Van Nuys Prognostic Index classification (17), where a score of 1 indicates widely free margins of 10 mm or more, a score of 2 indicates intermediate margins of 1–9 mm, and a score of 3 indicates involved or close margins smaller than 1 mm; (g) postsurgical endocrine therapy; and (h) postsurgical radiation therapy. For each case of recurrence, the first subject who matched all of the criteria and who had a follow-up time that exceeded the patient’s time to recurrence was selected to be the matched control subject to ensure that each matched patient had the closest possible age to his or her respective control subject. If no control subject could be identified with an exact match for pathology or margin status, a control subject with more aggressive features was selected for that category. Time to recurrence was defined as the time from definitive (final) surgical treatment to the date of tissue collection that led to the ipsilateral cancer recurrence diagnosis. Follow-up time for patients and control subjects was defined as the time from definitive surgical treatment to the time of the last clinical visit or tumor registry inquiry (August 6, 2014), whichever was later.
MR Imaging Use and Technique
At the University of Washington, preoperative MR imaging is routinely performed to evaluate the extent of disease in patients with newly diagnosed breast cancer, including DCIS. A prior study showed that approximately 90% of women with DCIS diagnosed at CNB undergo preoperative MR imaging and that patients who undergo preoperative MR imaging do not significantly differ from those who do not in terms of relevant clinical features (mammographic density, disease subtype, age, and menopausal status) (18).
Over the course of the study period, three American College of Radiology–accredited breast MR imaging protocols (19) were used as clinical practice and technology evolved. Patients were examined in the prone position. Imaging examinations included unenhanced and at least two contrast-enhanced T1-weighted fat-suppressed three-dimensional fast spoiled gradient recall sequences.
From January 2004 through November 2006, examinations were performed with a 1.5-T LX imager (GE Healthcare, Waukesha, Wis) using a dedicated seven-channel breast coil (MRI Devices, Waukesha, Wis). Sagittal DCE images were obtained with the following parameters: repetition time msec/echo time msec, 6.7/4.2; flip angle, 10°; field of view, 18–22 cm; section thickness, 2 mm; and matrix size, 256 × 192. From November 2006 through January 2010, imaging was performed with the aforementioned unit using a dedicated eight-channel breast coil (GE Healthcare or Hologic, Bedford, Mass). Axial DCE images were obtained with the following parameters: 5.5/2.7; flip angle, 10°; field of view, 32–38 cm; section thickness, 1.6 mm; and matrix size, 420 × 420. Initial contrast-enhanced acquisitions were centered at 90 seconds after contrast material administration. After January 2010, imaging was performed with a 3-T Achieva TX scanner (Philips Healthcare, Best, the Netherlands) using a 16-channel dedicated breast coil (Philips Healthcare). Axial DCE images were obtained with the following parameters: 5.9/3.0; flip angle, 10°; field of view, 22–33 cm; section thickness, 1.3 mm; and matrix size, 440 × 660. Initial contrast-enhanced acquisitions were centered at 110 seconds after contrast material administration. For all examinations, gadolinium contrast material (Omniscan [GE Healthcare] was used prior to November 2010, ProHance [Bracco Diagnostics, Princeton, NJ] was used from November 2010 on) was power injected (0.1 mmol per kilogram of body weight at a rate of 2 mL/sec) followed by a 20-mL saline flush, and images were processed by using a commercially available computer-aided evaluation system (CADstream; Merge Healthcare, Chicago, Ill).
MR Imaging Measurements
Clinically reported longest diameter at MR imaging for each DCIS lesion was obtained from the prospectively recorded MR imaging database for each patient and control subject. All other MR imaging features were measured retrospectively, with the observers blinded to outcomes. Prior to obtaining these MR imaging measurements, a research scientist (A.E.K., 7 years of image processing experience, 3 years of breast MR image processing experience) and a radiologist (B.S.J., 4 years of breast imaging experience, 1 year of breast MR imaging experience) evaluated pre- and postcontrast DCE images to confirm the absence of substantial misregistration artifacts. DCE MR imaging volumes were analyzed to quantify lesion parameters using customized semiautomated software developed in ImageJ (National Institutes of Health, Bethesda, Md). Contrast enhancement kinetic features were characterized by two parameters: initial phase percentage enhancement (PE) and delayed-phase signal enhancement ratio (SER). PE reflects the initial degree of signal enhancement in the lesion and is calculated as follows:
where S0 is MR imaging signal intensity prior to administration of contrast material and S1 is MR imaging signal intensity 90 or 110 seconds after contrast material delivery (depending on MR imaging protocol, as described previously). SER is used to measure the rate of contrast material washout in the tumor and was calculated as follows:
where S2 is the MR imaging signal intensity 270 seconds after contrast material delivery. Two radiologists (B.S.J., H.R.) supervised definition of tumor extent in three dimensions on orthogonal maximum intensity projection images. The MR imaging postprocessing pipeline is summarized in Figure 1. PE and SER were calculated on a voxel-by-voxel basis within the tumor, with SER calculated for only those voxels with PE of 50% or more. This threshold was selected based on the American College of Radiology Breast Imaging Reporting and Data System atlas definition of medium initial phase of enhancement (20). FTV was calculated by adding the volumes of all voxels with PE of 50% or more, similar to that described previously (21). “Hot spot” regions of peak PE and peak SER within the three-dimensional tumor, defined as at least eight contiguous voxels producing the highest mean PE and SER values, respectively, were automatically identified by the software. The washout fraction or percentage of voxels exhibiting washout (defined as SER >1.1) was also determined.
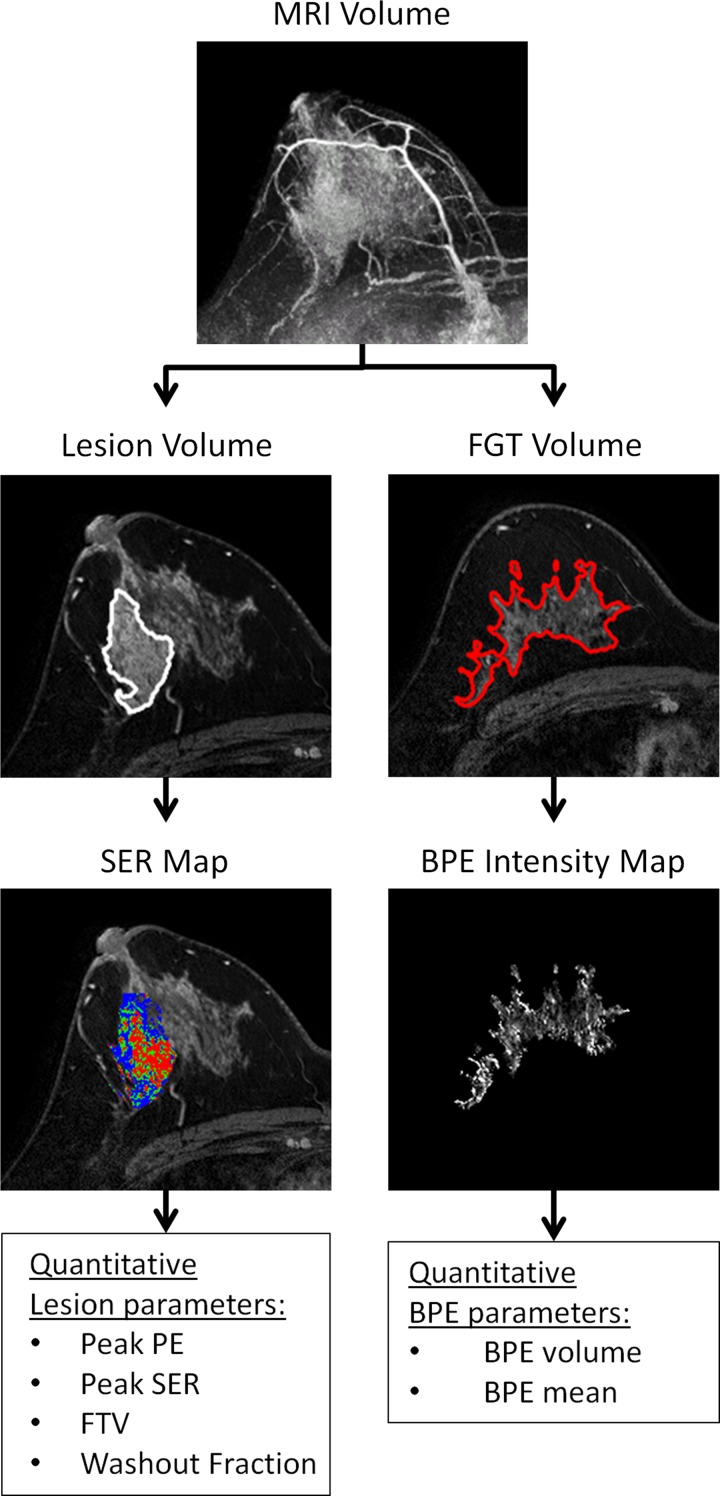
Figure 1:
Quantitative postprocessing pipeline for lesion and normal tissue parameters at DCE MR imaging in a 38-year-old woman with biopsy-proven DCIS. Representative images from each step of the pipeline are shown. Three-dimensional tumor extent is specified by manually drawn regions of interest on two orthogonal maximum intensity projection images (MRI Volume). Custom software uses a PE threshold greater than 50% to automatically segment the tumor (Lesion Volume). The software calculates PE on a voxel-by-voxel basis within the tumor and calculates whole lesion parameters (peak PE, peak SER, functional tumor volume [FTV], and washout fraction). The SER map is depicted as a color overlay: blue indicates persistent enhancement (SER <0.9); green, plateau enhancement (SER, 0.9–1.1); and red, washout (SER >1.1). To quantify normal tissue features, a signal intensity threshold is applied to the initial MR volume to segment the fibroglandular tissue (FGT ) volume, and it can be manually refined on each MR section to exclude adipose tissue, lesion, and blood vessels. A BPE intensity map is then generated by excluding voxels with PE less than 10%, and quantitative BPE parameters are calculated (BPE volume and BPE mean).
MR imaging quantification of background parenchymal enhancement (BPE) in the ipsilateral breast was performed by using custom software developed in MatLab (Mathworks, Natick, Mass). A signal intensity threshold was applied to the precontrast S0 MR imaging volume to select fibroglandular tissue. The resulting segmentation was manually refined by comparing it with postcontrast S1 images to ensure the accuracy of boundaries and to exclude the lesion, nipple, and large vessels. A BPE intensity map was created for the three-dimensional fibroglandular tissue region by calculating enhancement for each voxel, as defined in Equation 1. BPE maps were generated at varying PE thresholds ranging from 5% to 100%, and a 10% PE threshold was applied to distinguish BPE from unenhancing fibroglandular tissue and low-level image noise based on ongoing work at our institution evaluating quantitative measurement of BPE (22). BPE volume and BPE mean were calculated by adding the volumes and by averaging the PE values of each voxel of the BPE map, respectively.
Statistical Analyses
Differences in seven DCE MR imaging parameters (longest diameter, lesion peak PE, lesion peak SER, lesion washout fraction, FTV, BPE volume, and BPE mean) between patients and matched control subjects were assessed with the Wilcoxon signed rank test. P values were adjusted for multiple comparisons with the Holm method, which is a more powerful alternative to the Bonferroni correction (23). Adjusted P values less than .05 were indicative of a significant difference. The correlation between MR imaging parameters found to be significantly associated with recurrence at univariate analysis was evaluated by using Spearman correlation statistics. Conditional logistic regression was used to estimate an odds ratio for each DCE MR imaging parameter as a measure of effect size. Prior to inclusion in the conditional logistic regression model, notably right-skewed variables were log transformed to reduce skewness. All variables (transformed or not) were divided by their standard deviation, pooled across the patients and control subjects, to standardize the odds ratios to make them more comparable. All statistical calculations were performed by using the statistical computing language R (version 3.2.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Cohorts
A total of 415 women with a diagnosis of DCIS at CNB in one breast and without prior history of ipsilateral breast cancer who underwent preoperative MR imaging were identified in our database. Fourteen (3.4%) of these 415 women were identified as having ipsilateral breast cancer recurrence (eight with invasive breast cancer, six with DCIS). A control subject matched on the basis of clinical, histopathologic, and treatment features could not be found for three patients (two with invasive ductal carcinoma, one with DCIS); therefore, these patients were excluded. Thus, the final cohort included 11 patients who had ipsilateral breast cancer recurrence and 11 matched control subjects who did not have ipsilateral breast cancer recurrence after treatment.
Median age of the 11 patients with recurrence was 46 years (range, 33–78 years). Median time to recurrence was 14 months (range, 6–60 months). Median follow-up time in control subjects was 102 months (range, 42–144 months), and median follow-up time in patients with recurrence was 86 months (range, 59–149 months) (Table 1). Seven women who experienced recurrence after treatment were premenopausal, while four were postmenopausal. All included women who had ipsilateral breast cancer recurrence had estrogen receptor–positive DCIS. Of the 11 women who experienced recurrence, nine had primary VNPG 3 lesions (high nuclear grade present), one had a VNPG 2 lesion (intermediate nuclear grade with comedonecrosis), and one had a VNPG 1 lesion (intermediate nuclear grade without comedonecrosis). No subjects had a record of BRCA or other known high-risk genetic mutation, and no subjects had a documented history of prior chemoprevention.
Table 1.
Clinical, Histopathologic, and Treatment Features in Patients and Control Subjects
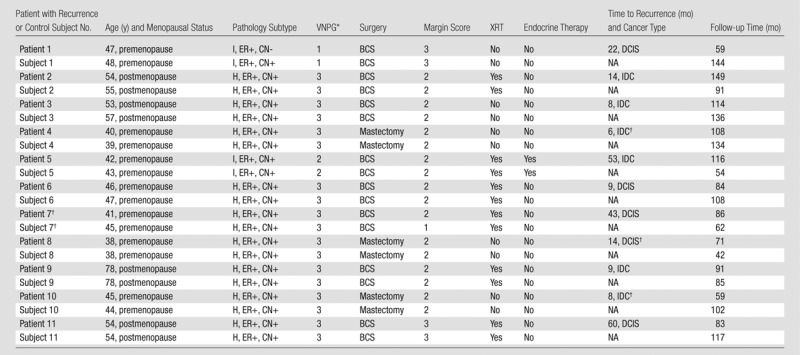
Note.—BCS = breast conserving therapy, CN = comedonecrosis, ER = estrogen receptor, H = high nuclear grade, I = intermediate nuclear grade, IDC = invasive ductal carcinoma, XRT = external beam radiation therapy.
*3 = widely clear margin, 10 mm or more; 2 = 1–9-mm margin; 1 = close or positive margins, <1 mm.
†Recurrence occurred in the soft tissue and/or skin in the mastectomy bed. For the one case of DCIS recurrence, the presentation was Paget disease of the skin.
‡All criteria matched except margin status.
All underwent primary surgical treatment of DCIS: Eight (73%) of 11 patients with recurrence underwent breast-conserving surgery as their definitive surgical procedure, while the other three underwent mastectomy. Of the 11 women who experienced recurrence, five (45%) did not undergo radiation therapy (all underwent breast-conserving surgery), and one (9%) did not undergo endocrine therapy; however, all women underwent either radiation therapy or endocrine therapy. Control subjects were matched on all specified factors, with the exception of margin status in patient 7. In this instance, the patient with recurrence had intermediate margins (Van Nuys margin score = 2, 1–9 mm), and the matched control subject had a worse margin (Van Nuys margin score = 1, <1 mm); all other criteria matched.
Associations of MR Imaging Features of DCIS Lesions and Normal Fibroglandular Tissue with Recurrence
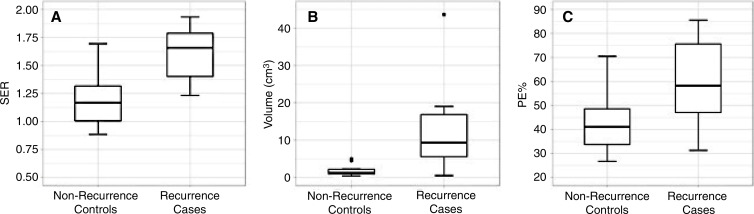
In our cohort, women with recurrence exhibited larger FTVs at preoperative MR imaging than did patients who did not experience recurrence (median, 9.3 cm3 vs 1.3 cm3; adjusted P = .01) (Table 2, Fig 2). Every woman with recurrence had a higher FTV than did her matched control subject, leading to an infinite estimated conditional odds ratio. DCIS lesions in women with recurrence demonstrated higher peak SER than did lesions in women who did not experience recurrence (median, 1.7 vs 1.2; conditional odds ratio, 4.3 per 1 standard deviation increase; adjusted P = .03). Mean BPE of the ipsilateral normal breast tissue was higher in women with recurrence than in women without recurrence (median, 58.3% vs 41.0%; conditional odds ratio, 20.6 per 1 standard deviation increase; adjusted P = .02). There was no significant difference between patients with recurrence and control subjects for the MR imaging parameters of longest diameter (adjusted P = .07), BPE volume (adjusted P = .16), lesion peak PE (adjusted P = .58), and lesion washout fraction (adjusted P = .20). An example of a woman who experienced recurrence after treatment and her matched control subject is shown in Figure 3.
Table 2.
Comparison of Preoperative MR Imaging Biomarkers in Patients and Control Subjects
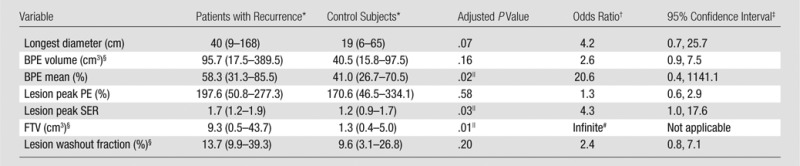
*Data are the median, and data in parentheses are the range.
†Univariate conditional odds ratio from conditional logistic regression per 1 standard deviation increase in the variable after log transformation, if applicable.
‡Confidence intervals are approximate and may conflict slightly with the P values, which are exact.
§Variable was log transformed to reduce right skewness before being included in the conditional logistic regression model.
||Statistically significant, defined as adjusted P < .05.
#Odds ratio estimate was infinite because FTV in every patient with recurrence was greater than that in the matched control subject.
Figure 2:
Box plots show differences between patients with recurrence after DCIS treatment and their matched control subjects with respect to the three quantitative breast MR imaging parameters that were significant. Patients with recurrence had significantly greater, A, lesion peak SER (P = .03), B, FTV (P = .01), and, C, mean BPE (P = .02).
Figure 3a:
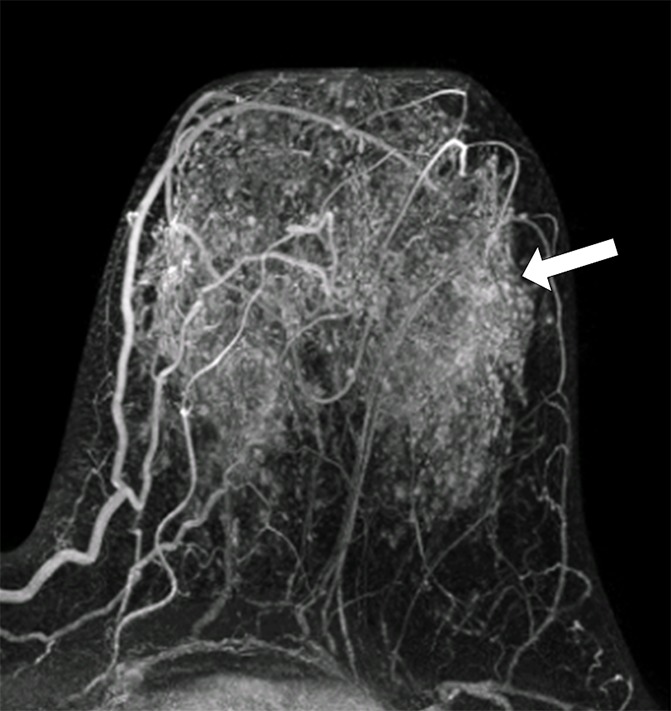
MR images in (a, b) a 41-year-old woman with recurrence after definitive surgical treatment for DCIS and (c, d) the matched 45-year-old control subject. (a) Subtracted maximum intensity projection shows marked BPE, which was quantitatively measured as mean BPE of 86%. The BPE measurement excludes the known DCIS lesion in the ipsilateral breast (arrow). (b) SER map with color overlay shows a peak SER of 1.57 and an FTV of 8.69 cm3 were calculated from kinetics data. Blue indicates persistent delayed enhancement (SER <0.9); green, plateau enhancement (SER, 0.9–1.1); and red, washout (SER >1.1). (c) Subtracted maximum intensity projection for the matched control subject shows minimal BPE (arrow), which was quantitatively measured as mean BPE of 71%. (d) SER map with color overlay shows a peak SER of 1.17 and an FTV of 1.27 cm3 were calculated from kinetics data.
Figure 3b:
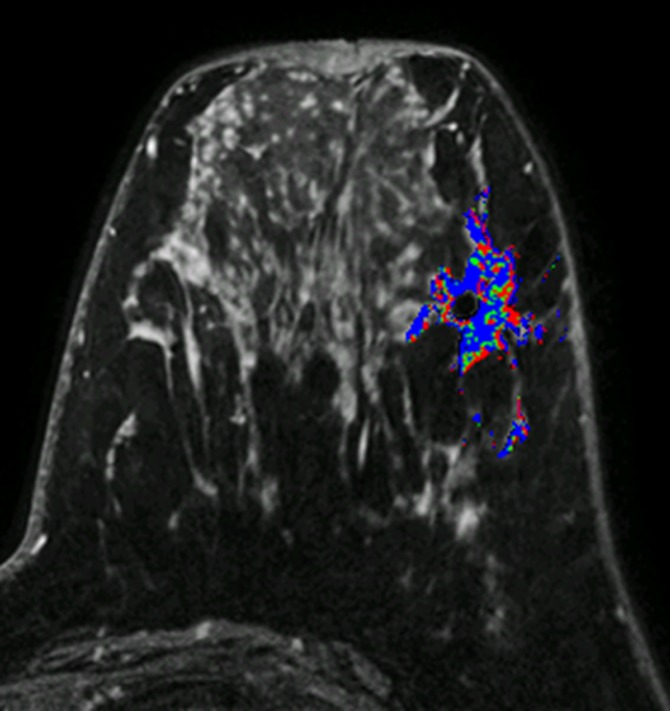
MR images in (a, b) a 41-year-old woman with recurrence after definitive surgical treatment for DCIS and (c, d) the matched 45-year-old control subject. (a) Subtracted maximum intensity projection shows marked BPE, which was quantitatively measured as mean BPE of 86%. The BPE measurement excludes the known DCIS lesion in the ipsilateral breast (arrow). (b) SER map with color overlay shows a peak SER of 1.57 and an FTV of 8.69 cm3 were calculated from kinetics data. Blue indicates persistent delayed enhancement (SER <0.9); green, plateau enhancement (SER, 0.9–1.1); and red, washout (SER >1.1). (c) Subtracted maximum intensity projection for the matched control subject shows minimal BPE (arrow), which was quantitatively measured as mean BPE of 71%. (d) SER map with color overlay shows a peak SER of 1.17 and an FTV of 1.27 cm3 were calculated from kinetics data.
Figure 3c:
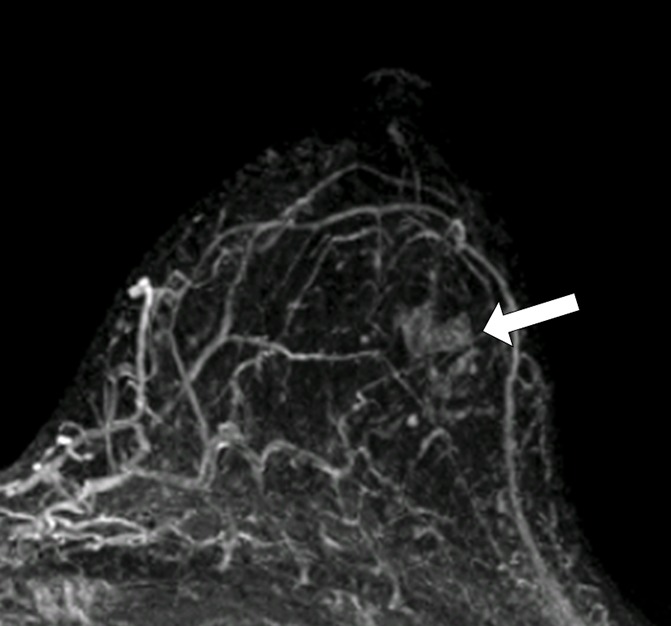
MR images in (a, b) a 41-year-old woman with recurrence after definitive surgical treatment for DCIS and (c, d) the matched 45-year-old control subject. (a) Subtracted maximum intensity projection shows marked BPE, which was quantitatively measured as mean BPE of 86%. The BPE measurement excludes the known DCIS lesion in the ipsilateral breast (arrow). (b) SER map with color overlay shows a peak SER of 1.57 and an FTV of 8.69 cm3 were calculated from kinetics data. Blue indicates persistent delayed enhancement (SER <0.9); green, plateau enhancement (SER, 0.9–1.1); and red, washout (SER >1.1). (c) Subtracted maximum intensity projection for the matched control subject shows minimal BPE (arrow), which was quantitatively measured as mean BPE of 71%. (d) SER map with color overlay shows a peak SER of 1.17 and an FTV of 1.27 cm3 were calculated from kinetics data.
Figure 3d:
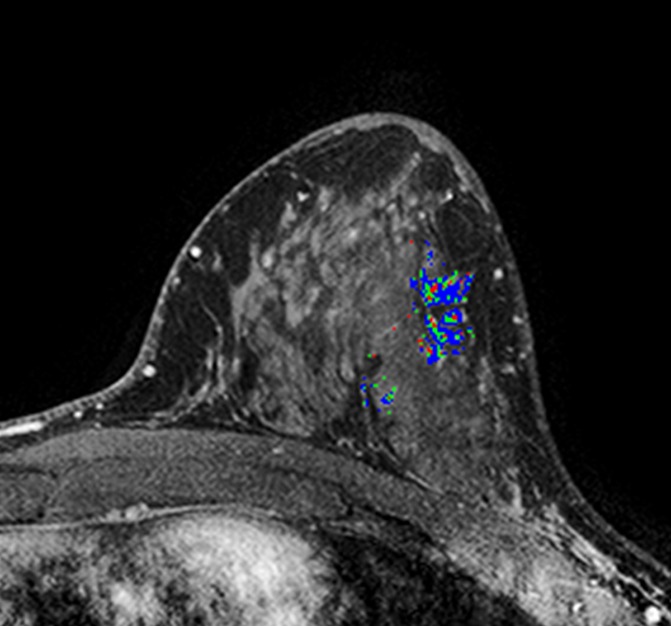
MR images in (a, b) a 41-year-old woman with recurrence after definitive surgical treatment for DCIS and (c, d) the matched 45-year-old control subject. (a) Subtracted maximum intensity projection shows marked BPE, which was quantitatively measured as mean BPE of 86%. The BPE measurement excludes the known DCIS lesion in the ipsilateral breast (arrow). (b) SER map with color overlay shows a peak SER of 1.57 and an FTV of 8.69 cm3 were calculated from kinetics data. Blue indicates persistent delayed enhancement (SER <0.9); green, plateau enhancement (SER, 0.9–1.1); and red, washout (SER >1.1). (c) Subtracted maximum intensity projection for the matched control subject shows minimal BPE (arrow), which was quantitatively measured as mean BPE of 71%. (d) SER map with color overlay shows a peak SER of 1.17 and an FTV of 1.27 cm3 were calculated from kinetics data.
Spearman rank correlation coefficients between the three significant imaging markers—FTV, lesion peak SER, and BPE mean—were calculated to explore whether the MR imaging markers may be independent from each other, as the sample size was insufficient to support multivariate regression analysis. FTV and mean BPE had a relatively low correlation (r = 0.24) while peak SER was moderately correlated with FTV (r = 0.42) and mean BPE (r = 0.40).
Discussion
In this single-institution study of women with recently diagnosed DCIS, we examined the association of MR imaging features with ipsilateral recurrence, and we found that subjects who exhibited higher lesion FTV and peak SER were significantly more likely to have had breast cancer recurrence after therapy. We also found that higher normal tissue enhancement (mean BPE) in the ipsilateral breast was associated with a greater likelihood of breast cancer recurrence. Our findings suggest that these quantitative preoperative MR imaging features may be useful in tailoring therapeutic approaches of DCIS to match risk of recurrence.
The use of MR imaging features to predict clinical outcomes of DCIS treatment has several important advantages. Although newer pathologic and genomic assays have shown promise for improved DCIS risk stratification, they remain prone to sampling error and have not been validated across a wide spectrum of DCIS subtypes. In contrast, imaging markers allow for whole-lesion evaluation with in vivo functional assessment of biology of both the DCIS lesion and the surrounding normal breast tissue. Such normal tissue features, which are not readily assessed at pathology, may provide insight into a tissue microenvironment that is prone to recurrence or future cancer development. Finally, MR imaging markers are noninvasive and can be obtained at routine preoperative MR imaging used for local staging and surgical planning. Thus, when compared with genomic assays that cost over $3000 per lesion (13), these imaging markers may be a cost-effective alternative to individualize therapy.
Among the MR imaging markers identified as potentially useful for determining risk of recurrence, FTV had the highest association. Previous work on invasive breast cancer has shown pretreatment FTV to be a stronger predictor of recurrence-free survival than either clinical examination or tumor size at pathology (24). Decrease in FTV after neoadjuvant therapy for invasive disease was predictive of pathologic response and recurrence-free survival in a large multicenter trial (25,26). Our findings suggest that FTV is also associated with prognosis in patients with DCIS. It should be noted that we could not match pathologic size in this case control study because of the desire to assess the association of FTV with DCIS recurrence, the relatively small number of recurrences, and the difficulty in confirming the true size of DCIS lesions at pathology. Thus, since final surgical pathology size is associated with recurrence, it is unclear whether FTV provides independent prognostic information apart from that obtained at surgery.
Increased lesion peak SER was associated with increased risk of recurrence in our study. Vascular permeability was previously established as the pathophysiologic basis for breast tumor enhancement, measured as the pharmacokinetic rate constant, kep (27). Because direct measurement of kep is labor intensive and sacrifices spatial resolution for temporal resolution at MR imaging, quantification of SER has emerged as a practical and accurate marker of vascularity (28). Prior studies have shown that higher SER of invasive breast cancer enables prediction of recurrence (29,30), and our study suggests that this metric may also provide prognostic information for preinvasive cancers.
Finally, quantitative measurements of BPE intensity were higher in women with DCIS who experienced recurrence than in those who did not. Two previous studies have shown that qualitative BPE measurements may help identify women with higher risk for breast cancer, including DCIS (31,32). Furthermore, several recent studies on breast MR imaging performed to evaluate newly diagnosed invasive breast cancer with BPE measurements have revealed that increased BPE is an independent predictor of recurrence after neoadjuvant therapy (33–35). Kim and colleagues demonstrated an association of higher quantitative BPE measurements by using circular regions of interest surrounding new DCIS lesions with recurrence (36). Our study differs from that important study in that we used postprocessing techniques to assess the entirety of background parenchyma as opposed to radiologist-selected regions of interest, thereby potentially decreasing interobserver variability and increasing the robustness of our calculations. Our findings lend further support to the belief that parenchymal milieu plays a strong role in breast cancer tumorigenesis and recurrence.
There were several important limitations in our study. This was a single-institution study, and the sample size was small, underscoring the inherent difficulty of performing a DCIS recurrence study because of very low recurrence rates. While the small sample size was mitigated by the rigorous selection of control subjects matched according to age and clinical and pathologic features, these criteria prevented us from identifying a match for three patients with recurrence, leading to their exclusion from this study. In addition, the small sample size limited our ability to conclude that MR imaging features that were not significant, particularly longest diameter, are not associated with recurrence, as these could be false-negative results due to insufficient statistical power. Our study also was limited by an overrepresentation of high VNPG cases, which may limit applicability of these markers in the general population of patients with DCIS. As described previously, we were unable to match participants on the basis of lesion size; as a result, the independence of these markers’ associations with recurrence from lesion size is unclear. From an imaging standpoint, our study included three different MR imaging protocols, which may have affected the consistency of quantitative measurements. Furthermore, imaging was not scheduled according to the patients’ menstrual cycles, and this may have led to a general over- or underestimation of BPE measurements; however, it is unclear whether menstrual cycle timing can assist with normalizing BPE across patients.
In conclusion, quantitative preoperative MR imaging features show promise in the noninvasive assessment of clinical prognosis of DCIS. If these features are further validated as reproducible biomarkers in larger multicenter cohorts, MR imaging features may be integrated with clinical risk assessment tools to better distinguish aggressive forms of DCIS from those that are less likely to recur. The resulting improved risk stratification of DCIS could allow clinicians to better tailor DCIS treatments and decrease overtreatment of less aggressive disease.
Advances in Knowledge
■ Women with ductal carcinoma in situ (DCIS) who experienced recurrence after treatment exhibited greater functional tumor volume (median, 9.3 cm3 vs 1.3 cm3; P = .01) and higher lesion peak signal enhancement ratio (median, 1.7 vs 1.2; P = .03) at preoperative MR imaging than did matched control subjects.
■ Higher background parenchymal enhancement of ipsilateral normal breast tissue (median, 58.3% vs 41.0%; P = .02) was observed in women with DCIS who experienced recurrence when compared with matched control subjects.
Implications for Patient Care
■ Quantitative MR imaging features of DCIS lesions and normal breast tissue show promise in the noninvasive assessment of DCIS.
■ Improved risk stratification of DCIS with preoperative MR imaging could enable clinicians to better tailor DCIS treatment and decrease overtreatment of less aggressive disease.
Received March 13, 2017; revision requested May 2; revision received May 22; accepted June 16; final version accepted June 22.
H.R. supported by a 2014–2016 RSNA Research Scholar Grant.
H.R. supported by the Radiological Society of North America (2014–2016 Research Scholar Grant) and the National Institutes of Health (R01CA203883).
Disclosures of Conflicts of Interest: J.L. disclosed no relevant relationships. B.S.J. disclosed no relevant relationships. A.E.K. disclosed no relevant relationships. D.S.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received grants from Philips Healthcare, GE Healthcare, and Toshiba America Medical Systems. Other relationships: disclosed no relevant relationships. L.A.K. disclosed no relevant relationships. S.J. disclosed no relevant relationships. J.M.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received a grant from GE Healthcare. Other relationships: disclosed no relevant relationships. S.P. disclosed no relevant relationships. C.D.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received grants from GE Healthcare and is a member of the GE Healthcare advisory board. Other relationships: disclosed no relevant relationships. S.C.P. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has a research agreement with Philips Healthcare to access work-in-progress tools for advanced MR imaging. Other relationships: disclosed no relevant relationships. H.R. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received a grant from GE Healthcare. Other relationships: disclosed no relevant relationships.
Abbreviations:
- BPE
- background parenchymal enhancement
- CNB
- core-needle biopsy
- CODI
- Consortium Oncology Data Integration
- DCE
- dynamic contrast material enhanced
- DCIS
- ductal carcinoma in situ
- FTV
- functional tumor volume
- PE
- percentage enhancement
- SER
- signal enhancement ratio
- VNPG
- Van Nuys Pathologic Grade
References
- 1.Yamada T, Mori N, Watanabe M, et al. Radiologic-pathologic correlation of ductal carcinoma in situ. RadioGraphics 2010;30(5):1183–1198. [DOI] [PubMed] [Google Scholar]
- 2.Allegra CJ, Aberle DR, Ganschow P, et al. National Institutes of Health State-of-the-Science Conference statement: Diagnosis and Management of Ductal Carcinoma In Situ September 22-24, 2009. J Natl Cancer Inst 2010;102(3):161–169. [DOI] [PubMed] [Google Scholar]
- 3.Sumner WE, 3rd, Koniaris LG, Snell SE, et al. Results of 23,810 cases of ductal carcinoma-in-situ. Ann Surg Oncol 2007;14(5):1638–1643. [DOI] [PubMed] [Google Scholar]
- 4.Ries LAG, Krapcho M, Mariotto A, et al., eds. SEER Cancer Statistics Review, 1975-2004. National Cancer Institute, Bethesda, MD. Based on November 2006 SEER data submission, posted to the SEER web site, 2007. http://seer.cancer.gov/csr/1975_2004/. Accessed September 28, 2016. [Google Scholar]
- 5.Morrow M, Strom EA, Bassett LW, et al. Standard for the management of ductal carcinoma in situ of the breast (DCIS). CA Cancer J Clin 2002;52(5):256–276. [DOI] [PubMed] [Google Scholar]
- 6.Solin LJ. Selecting individualized treatment for patients with ductal carcinoma in situ of the breast: the search continues. J Clin Oncol 2012;30(6):577–579. [DOI] [PubMed] [Google Scholar]
- 7.Miller NA, Chapman JA, Fish EB, et al. In situ duct carcinoma of the breast: clinical and histopathologic factors and association with recurrent carcinoma. Breast J 2001;7(5):292–302. [DOI] [PubMed] [Google Scholar]
- 8.Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst 2013;105(10):701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst 2010;102(3):170–178. [DOI] [PubMed] [Google Scholar]
- 10.Elmore JG, Longton GM, Carney PA, et al. Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA 2015;313(11):1122–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson SL, Frederick PD, Pepe MS, et al. Diagnostic reproducibility: what happens when the same pathologist interprets the same breast biopsy specimen at two points in time? Ann Surg Oncol 2017;24(5):1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagios MD, Silverstein MJ. Risk of recurrence of ductal carcinoma in situ by oncotype dx technology: some concerns. Cancer 2014;120(7):1085. [DOI] [PubMed] [Google Scholar]
- 13.Raldow AC, Sher D, Chen AB, Recht A, Punglia RS. Cost effectiveness of the oncotype dx dcis score for guiding treatment of patients with ductal carcinoma in situ. J Clin Oncol 2016 Sep 12. [Epub ahead of print] [DOI] [PubMed]
- 14.Esserman LJ, Kumar AS, Herrera AF, et al. Magnetic resonance imaging captures the biology of ductal carcinoma in situ. J Clin Oncol 2006;24(28):4603–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iima M, Yano K, Kataoka M, et al. Quantitative non-Gaussian diffusion and intravoxel incoherent motion magnetic resonance imaging: differentiation of malignant and benign breast lesions. Invest Radiol 2015;50(4):205–211. [DOI] [PubMed] [Google Scholar]
- 16.Rahbar H, Partridge SC, Demartini WB, et al. In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters. Radiology 2012;263(2):374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverstein MJ, Lagios MD, Craig PH, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer 1996;77(11):2267–2274. [DOI] [PubMed] [Google Scholar]
- 18.Smith J, Partridge SC, Kim AE, et al. The impact of preoperative breast MRI on surgical management of women with newly diagnosed DCIS. Accepted for presentation at Society of Surgical Oncology Annual Meeting 2017, Seattle, Wash, March 16, 2017. Abstract ID: 2600413.
- 19.ACR Breast MRI Accreditation Program . American College of Radiology. http://www.acr.org/Quality-Safety/Accreditation/BreastMRI. Published 2014. Accessed September 28, 2016.
- 20.Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS Magnetic Resonance Imaging. In: ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, Va: American College of Radiology, 2013. [Google Scholar]
- 21.Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Sudilovsky D, Hylton NM. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol 2002;179(5):1193–1199. [DOI] [PubMed] [Google Scholar]
- 22.Liu CL, Partridge SC, Lam DL, Lehman CD, Rahbar H. Optimization of quantitative mri background parenchymal enhancement metrics to predict breast cancer risk [abstr]. In: Proceedings of the Twenty-Third Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2015. [Google Scholar]
- 23.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6(2):65–70. [Google Scholar]
- 24.Partridge SC, Gibbs JE, Lu Y, et al. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol 2005;184(6):1774–1781. [DOI] [PubMed] [Google Scholar]
- 25.Hylton NM, Blume JD, Bernreuter WK, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy—results from ACRIN 6657/I-SPY TRIAL. Radiology 2012;263(3):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hylton NM, Gatsonis CA, Rosen MA, et al. Neoadjuvant chemotherapy for breast cancer: functional tumor volume by MR imaging predicts recurrence-free survival—results from the ACRIN 6657/CALGB 150007 I-SPY 1 TRIAL. Radiology 2016;279(1):44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knopp MV, Weiss E, Sinn HP, et al. Pathophysiologic basis of contrast enhancement in breast tumors. J Magn Reson Imaging 1999;10(3):260–266. [DOI] [PubMed] [Google Scholar]
- 28.Li KL, Henry RG, Wilmes LJ, et al. Kinetic assessment of breast tumors using high spatial resolution signal enhancement ratio (SER) imaging. Magn Reson Med 2007;58(3):572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li SP, Makris A, Beresford MJ, et al. Use of dynamic contrast-enhanced MR imaging to predict survival in patients with primary breast cancer undergoing neoadjuvant chemotherapy. Radiology 2011;260(1):68–78. [DOI] [PubMed] [Google Scholar]
- 30.Li KL, Partridge SC, Joe BN, et al. Invasive breast cancer: predicting disease recurrence by using high-spatial-resolution signal enhancement ratio imaging. Radiology 2008;248(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011;260(1):50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dontchos BN, Rahbar H, Partridge SC, et al. Are qualitative assessments of background parenchymal enhancement, amount of fibroglandular tissue on MR images, and mammographic density associated with breast cancer risk? Radiology 2015;276(2):371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones EF, Sinha SP, Newitt DC, et al. MRI enhancement in stromal tissue surrounding breast tumors: association with recurrence free survival following neoadjuvant chemotherapy. PLoS One 2013;8(5):e61969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim MY, Cho N, Koo HR, et al. Predicting local recurrence following breast-conserving treatment: parenchymal signal enhancement ratio (SER) around the tumor on preoperative MRI. Acta Radiol 2013;54(7):731–738. [DOI] [PubMed] [Google Scholar]
- 35.Choi JS, Ko ES, Ko EY, Han BK, Nam SJ. Background parenchymal enhancement on preoperative magnetic resonance imaging: association with recurrence-free survival in breast cancer patients treated with neoadjuvant chemotherapy. Medicine (Baltimore) 2016;95(9):e3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SA, Cho N, Ryu EB, et al. Background parenchymal signal enhancement ratio at preoperative MR imaging: association with subsequent local recurrence in patients with ductal carcinoma in situ after breast conservation surgery. Radiology 2014;270(3):699–707. [DOI] [PubMed] [Google Scholar]