Findings suggest that 13C-pyruvate–to-lactate conversion can serve as an early indicator of response to multityrosine kinase inhibitor pazopanib in an ovarian cancer model; findings also suggest that evaluation of late events in glycolysis, such as by hyperpolarized 13C-pyruvate MR spectroscopy, may be predictive of response even when imaging of early events, such as by 18F-FDG PET, is not predictive.
Abstract
Purpose
To assess in a mouse model whether early or late components of glucose metabolism, exemplified by fluorine 18 (18F) fluorodeoxyglucose (FDG) positron emission tomography (PET) and hyperpolarized carbon 13 (13C)–pyruvate magnetic resonance (MR) spectroscopy, can serve as indicators of response in ovarian cancer to multityrosine kinase inhibitor pazopanib.
Materials and Methods
In this Animal Care and Use Committee approved study, 17 days after the injection of 2 × 106 human ovarian SKOV3 tumors cells into 14 female nude mice, treatment with vehicle or pazopanib (2.5 mg per mouse peroral every other day) was initiated. Longitudinal T2-weighted MR imaging, dynamic MR spectroscopy of hyperpolarized pyruvate, and 18F-FDG PET/computed tomographic (CT) imaging were performed before treatment, 2 days after treatment, and 2 weeks after treatment.
Results
Pazopanib inhibited ovarian tumor growth compared with control (0.054 g ± 0.041 vs 0.223 g ± 0.112, respectively; six mice were treated with pazopanib and seven were control mice; P < .05). Significantly higher pyruvate-to-lactate conversion (lactate/pyruvate + lactate ratio) was found 2 days after treatment with pazopanib than before treatment (0.46 ± 0.07 vs 0.31 ± 0.14, respectively; P < .05; six tumors after treatment, seven tumors before treatment). This was not observed with the control group or with 18F-FDG PET/CT imaging.
Conclusion
The findings suggest that hyperpolarized 13C-pyruvate MR spectroscopy may serve as an early indicator of response to tyrosine kinase (angiogenesis) inhibitors such as pazopanib in ovarian cancer even when 18F-FDG PET/CT does not indicate a response.
© RSNA, 2017
Introduction
Ovarian cancer is the leading cause of death from gynecologic cancers (1). Therapies that include tyrosine kinase inhibitors such as pazopanib can alter both tumor cell signaling and angiogenesis, and these can alter cell growth and metabolism. Presently, clinical response to therapeutics is generally based on decreased tumor size, but this can take months and delay decisions of whether to continue with effective therapy or stop ineffective therapy. Additional early functional information regarding response could allow early treatment modification and avoid unnecessary toxicity. Malignant lesions exhibit altered metabolism compared with normal tissues. Upstream metabolic events can be imaged by positron emission tomography (PET) with the glucose analog fluorine 18 (18F) fluorodeoxyglucose (FDG). It recently became possible to image the downstream molecule pyruvate and its metabolites by using hyperpolarized magnetic resonance (MR) imaging. Whether early or late metabolic events can serve as early predictors of response to tyrosine kinase inhibitors such as pazopanib in ovarian cancer needs further evaluation.
18F-FDG PET imaging is clinically used to help detect and stage ovarian cancer. It has been used to monitor efficacy of carboplatin and paclitaxel therapy (2) and as an early indicator of response to a vascular disrupting agent (3) in mouse ovarian cancer models. 18F-FDG enters the cell via glucose transporters and is phosphorylated by hexokinase, but this phosphorylated form is not a good substrate for phosphoglucose isomerase and its charge prevents escape from the cell. Therefore, 18F-FDG accumulation reflects early events in glycolysis. Downstream, normal glycolysis produces pyruvate, a key molecule at the branching point of entering aerobic versus anaerobic respiration. Pyruvate can be produced by other pathways such as by way of amino acid metabolism (4). Hyperpolarization can increase signal 10 000–100 000-fold (5) and, in conjunction with MR imaging, has made it possible to image exogenous hyperpolarized pyruvate and its metabolites such as lactate. Most cancers are hyperproliferative and therefore tend to have high metabolic demand.
Pazopanib inhibits multiple tyrosine kinase receptors by way of adenosine triphosphate competition for receptor phosphorylation, including c-kit, c-fms, fibroblast growth factor receptor, platelet-derived growth factor receptor, and vascular endothelial growth factor receptor (6–8) (ie, receptors involved in regulating tumor cell growth and metabolism, and receptors that regulate angiogenesis, a process critical for obtaining oxygen and nutrients for metabolism). Pazopanib showed promise as a monotherapy in patients with ovarian cancer in a phase II study (9) and extended median progression-free survival by 5.6 months in a phase III study. Metabolism can be regulated by tumor cell receptor activation and nutrient supply. Our purpose was to assess in a mouse model whether early or late components of glucose metabolism, exemplified by 18F-FDG PET and hyperpolarized carbon 13 (13C)–pyruvate MR spectroscopy, can serve as indicators of response in ovarian cancer to multityrosine kinase inhibitor pazopanib.
Materials and Methods
Hyperpolarized 13C-Pyruvate
Twenty-six milligrams of [1-13C]-pyruvic acid (Sigma-Aldrich) with 15 mmol/L OX063 (GE Healthcare, Fairfield, Conn) and 1.5 mmol/L Prohance (Bracco Diagnostics, Monroe Township, NJ) were hyperpolarized in a dynamic nuclear polarization system (HyperSense DNP Polarizer; Oxford Instruments, Austin, Tex) as previously described (5–10). The sample was dissolved in 4 mL of buffer, which consisted of 40 mmol/L Trizma preset crystals (pH, 7.6), 80 mmol/L sodium hydroxide, 50 mmol/L sodium chloride, and 0.1 mg/L ethylenediaminetetraacetic acid. Once dissolution was complete, 0.2 mL of 80 mmol/L hyperpolarized [1-13C] pyruvate (nominally 30% polarization) was drawn into a syringe for injection into the animals.
Cell Lines and Culture
The human ovarian cancer cell line SKOV3 (11) (a kind gift from Dr Anil K. Sood, Department of Gynecologic Oncology, MD Anderson Cancer Center, Houston, Tex) was maintained in Roswell Park Memorial Institute medium 1640 supplemented with 15% fetal bovine serum and 50 μg/mL gentamicin sulfate (Gemini Bioproducts, West Sacramento, Calif). Cell lines were validated by short tandem repeat DNA fingerprinting by using a short tandem repeat analysis kit (16 HS System; Promega, Madison, Wis). The short tandem repeat profiles were compared with online search databases (Deutsche Sammlung von Mikroorganismen und Zellkulturen, American Type Culture Collection, Japanese Cancer Research Resources Bank, and Rikagaku Kenkyusho) of 2455 known profiles, along with the MD Anderson–characterized cell line core (CCLC database of 2556 known profiles). The short tandem repeat profiles matched known DNA fingerprints or were unique. For subcutaneous injection, cells were dissociated by using trypsin, centrifuged at 1000 rotations per minute × 7 minutes at 4°C, washed twice, and suspended again in serum-free buffered salt solution (HBSS; Life Technologies, Rockville, Md).
In Vivo Mouse Model
Fourteen female athymic nude mice (6–8 weeks old) were purchased from the experimental radiation oncology department of the MD Anderson Cancer Center and housed in specific pathogen-free conditions. They were cared for in accordance with guidelines set forth by the American Association for Accreditation of Laboratory Animal Care and all studies were approved and supervised by the MD Anderson Cancer Center Animal Care and Use Committee.
For tumor implantation, mice were anesthetized by using isoflurane. The skin over the injection site was cleaned with 70% ethanol. We subcutaneously injected 2 × 106 SKOV3 human ovarian cancer cells into the right hind leg of mice by using a syringe with a 28-gauge needle. At weekly intervals after injection, the mice were monitored for tumor growth. After 2 weeks, the mice were imaged. Then, mice were randomly assigned into two groups (seven mice per group), treated with pazopanib (2.5 mg per mouse orally every alternative day for 2 weeks) or with vehicle control. One mouse in the treated group died of unknown cause after pretherapy imaging. Tumors were measured by calipers every 2 days. PET and MR imaging was performed 1–2 days before therapy, 2 days after the initiation of therapy, and at 2 weeks after initiation of therapy. Mice were killed after final imaging and tumors were weighed.
In Vivo MR Spectroscopy
Experiments were performed by using a 7-T and 30-cm (bore size) system (BioSpec System, Bruker Biospin, Billerica, Mass) with B-GA12 gradients (120-mm inner diameter). A 13C-surface coil (15-mm inner diameter) was placed directly over the tumor, and the animal holder was then placed inside a dual-tuned 13C/hydrogen 1 (1H)–volume coil with a 72-mm inner diameter (Bruker Biospin). The 1H channel of this volume coil was used for 1H MR imaging signal excitation and reception, and the 13C channel of the volume coil was used for 13C signal excitation, whereas the 13C surface coil was used to help detect the 13C signal with high sensitivity to the tumor at the center of the coil. Transverse 1H scout images and T2-weighted images were acquired by using a rapid acquisition with relaxation enhancement spin-echo pulse sequence (repetition time msec/echo time msec, 2500/50; field of view, 50 × 50 mm; 256 × 192 matrix; two averages per increment; echo train, eight; section thickness, 1.5 mm; imaging time, 2 minutes). Dynamic 13C spectra were acquired by using a section-localized pulse-acquired sequence (bandwidth, 5 kHz; 2048 spectral points; excitation pulse, 10°; repetition time, 2 seconds; section thickness, 12 mm; 96 repetitions). 13C data collection was triggered by the Hypersense system (Oxford Instruments) to dynamically monitor the fate of hyperpolarized pyruvate after 200-μL injection via tail vein catheter. The catheter was made of 2-F silicone tubing, trimmed to reach from isocenter to the end of the magnet bore, with a total dead volume of approximately 100 μL. Injections were administered over approximately 11 seconds and were completed by approximately 21 seconds after dissolution.
Spectra were acquired by using a nominal excitation angle of 10° at intervals of 2 seconds. The half-height full-width area under spectral peaks for hyperpolarized [1-13C]-pyruvate and hyperpolarized [1-13C]-lactate were calculated to quantify their relative concentration at each time. Dynamic signal curves for hyperpolarized pyruvate and lactate were then integrated in time to calculate the total signal measured over the observable lifetime of each metabolite. The hyperpolarized lactate signal was normalized to the total 13C signal (hyperpolarized lactate and pyruvate) to correct for delivery and quantify the fraction of metabolically active hyperpolarized signal (precursor and product) observed in tumor that arises from the product lactate; this is referred to as the normalized lactate ratio [lactate/(lactate + pyruvate)] (J.L., a postdoctoral fellow with >2 years of experience, and J.A.B., associate professor in imaging physics with >15 years of experience). For anatomic tumor measurement, with software (Image J; National Institutes of Health, Bethesda, Md) the periphery of the tumor on T2-weighted MR images was manually traced on axial images to define a region of interest on each section (M.K.R., research scientist with >12 years of experience), and the area of the enclosed region was calculated. The area was then multiplied by the section thickness to obtain the volume of the object of interest within a section. To avoid overestimation of tumor size, half of the volume from the most dorsal and most ventral images that contained tumor was used in the volume calculations. Assuming a tumor density of 1 g/mL, tumor volumes (in cubic millimeters) were converted to weight (in grams) for analysis, as previously described (12).
PET/CT Imaging
For PET/CT imaging, anesthetized mice were injected intravenously with 150 μCi of 18F-FDG. Thirty minutes later, animals anesthetized with 2% isofluorane were imaged for 15 minutes by using a preclinical multimodal PET/CT scanner (Inveon; Siemens Healthcare, Erlangen, Germany). Images were reconstructed by using software provided by the manufacturer (Osem 3D; Siemens Healthcare). Regions of interest were placed in three planes (by M.K.R.) of each tumor to measure 18F-FDG uptake in percent injected dose per gram for each mouse by using software provided by the manufacturer.
Statistical Analysis
The primary comparison for the normalized lactate ratio was at 2 days. A linear mixed model (39 observations, three observations per mouse) was used to estimate and compare mean normalized lactate ratios between control and treatment group over time. Group and time nested within group were modeled as fixed effects and mouse as a random effect. The correlations between observations from the same mouse were accounted for by incorporating compound symmetric covariance structure into the modeling. Normalized lactate ratios were estimated and compared by using model estimates. All tests were two sided, and P values of .05 or less were considered to indicate statistical significance. Statistical analysis was performed by using statistical software (SAS version 9.4; SAS Institute, Cary, NC).
Results
Anatomic Evaluation of Efficacy
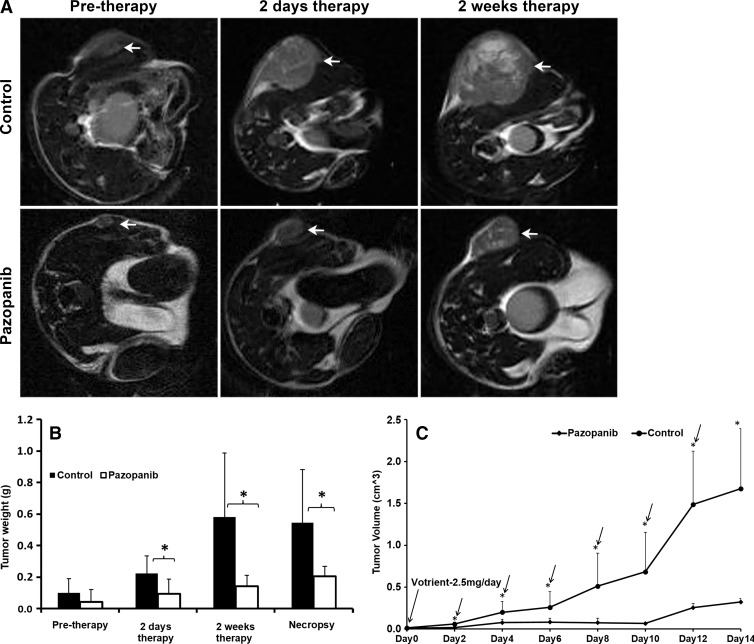
Longitudinal T2-weighted MR imaging revealed that treatment with pazopanib inhibited tumor growth in mice compared with the vehicle control (Fig 1, A), which was consistent with efficacy. Before therapy, there was no statistically significant difference in tumor weight between pazopanib-treated and vehicle control groups. Two days and 2 weeks after initiating treatment with pazopanib (n = 6), mice had significantly smaller tumors compared with control mice (n = 7; 0.054 g ± 0.041 [standard deviation] vs 0.223 g ± 0.112 and 0.142 g ± 0.063 vs 0.607 g ± 0.398, respectively; P < .05; Fig 1, B, Table E1 [online]). Tumor weight differences by necropsy confirmed these findings (0.205 g ± 0.058 vs 0.563 g ± 0.332; P < .05; Fig 1, B, Table E1 [online]). By comparing tumor size with calipers over time (Fig 1, C, Table E2 [online]), the size of tumors treated with pazopanib significantly increased at day 4 versus at day 2 (0.076 cm3 ± 0.040 vs 0.016 cm3 ± 0.005, respectively; P < .01; n = 6), then stabilized, and then increased in size on day 12 versus day 10 (0.255 cm3 ± 0.050 vs 0.067 cm3 ± 0.010 cm3, respectively; P < .001; n = 6), and at day 14 versus day 12 (0.322 cm3 ± 0.040 vs 0.255 cm3 ± 0.050, respectively; P < .05; n = 6). The data show that after day 10, pazopanib-treated tumors began to grow, which suggested less efficacy.
Figure 1:
In vivo MR imaging showed that pazopanib reduced the growth of established SKOV3 tumors. A, Representative axial T2-weighted 1H anatomic MR images of mice before therapy (pre-therapy) or after 2 days and 2 weeks of therapy with vehicle (control mice) or pazopanib. B, Box plot shows tumor weight derived from serial MR images of the mice described in A before therapy or after 2 days and 2 weeks of therapy, or at necropsy (at 2 weeks after therapy; * P < .05). C, Graph of longitudinal caliper measurements. Pazopanib inhibited tumor growth compared with control mice (* P < .05). By comparing growth versus the day before in tumors treated with pazopanib, tumors grew on day 4 versus day 2, stabilized, and then grew on day 12 versus day 10 and day 14 versus day 12 (arrow represents P < .05). The error bars are standard deviation.
Hyperpolarized 13C MR Imaging
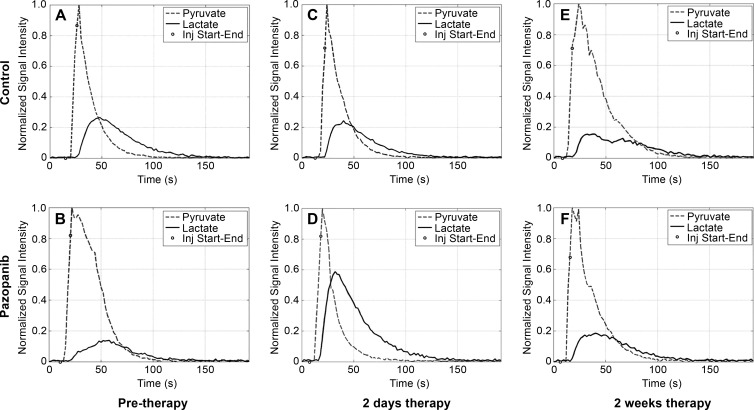
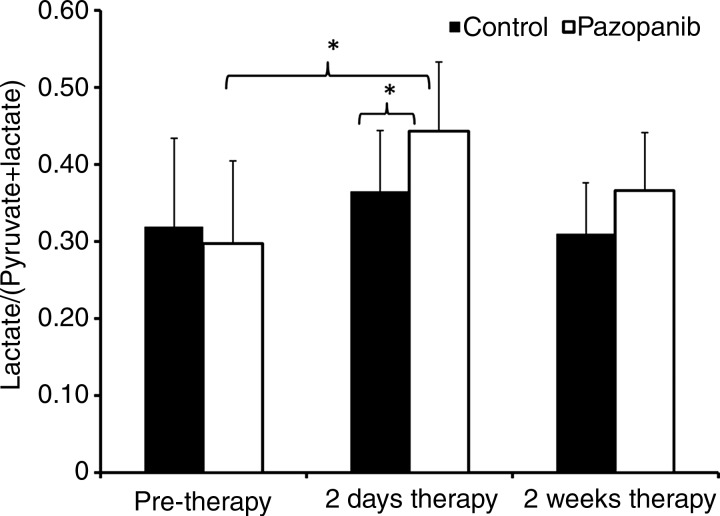
Intravenous administration of hyperpolarized 13C-pyruvate (200-μL injection, 80 mmol/L hyperpolarized 13C-pyruvate) resulted in readily detectable signal. As expected before therapy, no significant difference in 13C-pyruvate–to–13C-lactate conversion, observed by the normalized hyperpolarized lactate value, was observed in tumors between the prepazopanib therapy group and the control group (Figs 2, 3; Table E3 [online]). The flux of 13C-label from hyperpolarized 13C-pyruvate at 171 parts per million to 13C-lactate at 183 parts per million was increased in tumors 2 days after pazopanib therapy as suggested by the larger normalized area under the 13C-lactate curve (Fig 2, D). Two days after initiation of therapy, tumors from mice treated with pazopanib (n = 6) significantly increased conversion of 13C-pyruvate to 13C-lactate compared with before therapy (n = 7; 0.46 ± 0.07 vs 0.31 ± 0.14, respectively; P < .05). This was not observed in the control mice (Figs 2, 3). Complementing these findings, conversion of 13C-pyruvate to 13C-lactate was significantly increased 2 days after initiation of pazopanib therapy (n = 6) compared with control mice (n = 7; 0.46 ± 0.07 vs 0.35 ± 0.07, respectively; P < .05). No statistically significant difference was observed at 2 weeks.
Figure 2:
Graphs of dynamic MR imaging curves of tumor lactate and pyruvate peak intensities. Dynamic signal curves of MR spectroscopic imaging of hyperpolarized 13C-pyruvate and its metabolite 13C-lactate before (pre-therapy; A, B) or 2 days (C, D) and 2 weeks (E, F ) after control (A, C, E) or pazopanib (B, D, E) treatment. The mean signal for lactate and pyruvate was normalized to peak signal for each injection (inj ). Total hyperpolarized 13C signal was estimated by summing signal from hyperpolarized 13C-lactate and hyperpolarized 13C-pyruvate. s = seconds.
Figure 3:
Box plot shows ratio of lactate-to–pyruvate-and-lactate signal intensity. 13C-pyruvate–to–13C-lactate conversion before or 2 days and 2 weeks after control or pazopanib treatment. * P < .05.
18F-FDG PET/CT Imaging
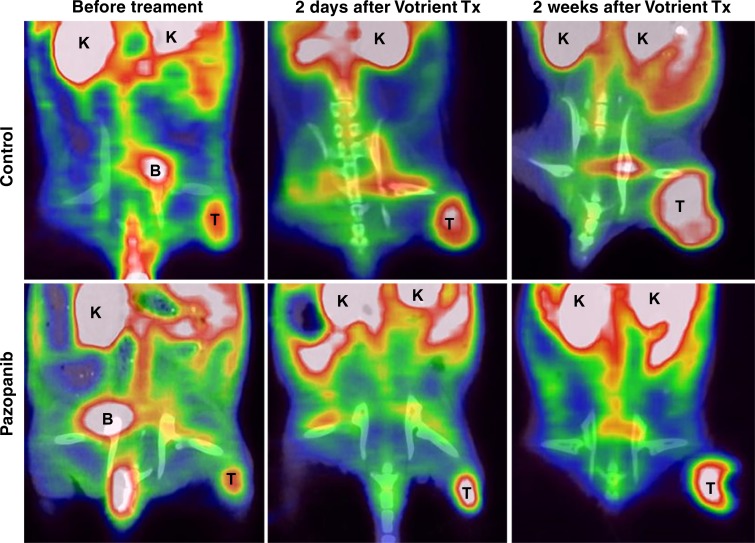
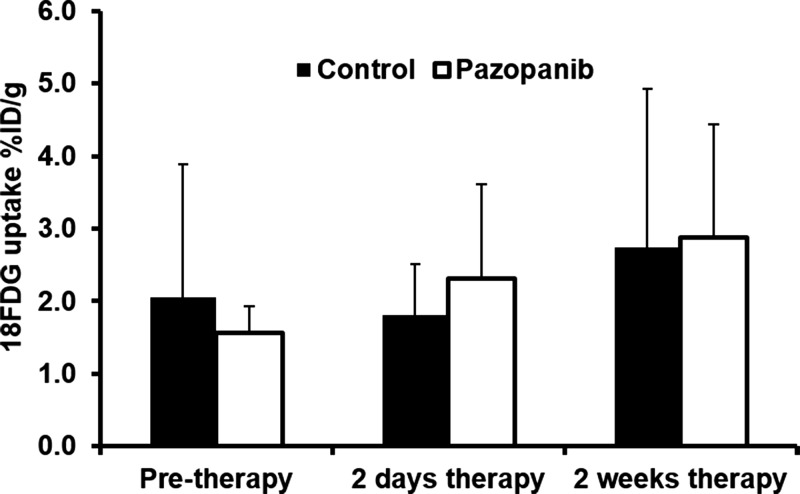
No significant difference was observed regarding 18F-FDG uptake for the pazopanib-treated mice compared with control mice before therapy (n = 7; Fig 4, Table E4 [online]). There was not a significant difference after therapy between pazopanib-treated animals (n = 6) and control animals (n = 7) after 2 days or after 2 weeks.
Figure 4a:
18F-FDG PET/CT imaging did not indicate response to pazopanib treatment. (a) Representative coronal 18F-FDG PET/CT images of mice before or 2 days and 2 weeks after pazopanib or control treatment. B = bladder; K = kidney; T = tumor. (b) Box plot shows 18F-FDG uptake in percent injected dose per gram (%ID/g) before or 2 days and 2 weeks after pazopanib treatment or for control mice.
Figure 4b:
18F-FDG PET/CT imaging did not indicate response to pazopanib treatment. (a) Representative coronal 18F-FDG PET/CT images of mice before or 2 days and 2 weeks after pazopanib or control treatment. B = bladder; K = kidney; T = tumor. (b) Box plot shows 18F-FDG uptake in percent injected dose per gram (%ID/g) before or 2 days and 2 weeks after pazopanib treatment or for control mice.
Discussion
We found significant increase in hyperpolarized 13C-lactate signal relative to the 13C-pyruvate substrate signal in vivo in a SKOV3 human ovarian tumor model 2 days after starting therapy with pazopanib. This corresponded to tumor growth inhibition, which suggested that in the ovarian cancer model, hyperpolarized pyruvate imaging can serve as an early response indicator to pazopanib therapy. Data suggested greater enzymatic activity and/or amount of lactate dehydrogenase drives the process.
Interestingly, at 2 weeks, increased pyruvate-to-lactate conversion was not observed in pazopanib-treated tumors corresponding with regrowth of pazopanib tumors starting at day 10. Thus, both hyperpolarized pyruvate MR imaging and tumor growth data suggested decreased pazopanib efficacy at these later times.
In comparison, no difference was observed in tumors at 18F-FDG PET imaging between pazopanib-treated versus control-treated animals at either time. 18F-FDG PET imaging helps to show early events in glycolysis, whereas pyruvate-to-lactate conversion is a late event. Like glucose, 18F-FDG is brought into cells via glucose receptors and phosphorylated by hexokinase (primarily type 2), the first enzyme in glycolysis; in cancer, glucose transporters are upregulated and are often a more important factor than increased hexokinase activity (13) for greater 18F-FDG uptake. Phosphorylated 18F-FDG is not a good substrate for the next enzyme phosphoglucose isomerase; therefore, 18F-FDG imaging only represents early events of glycolysis. The altered metabolic phenotype of cancer, called the Warburg effect (14), includes increased activation of the glycolytic pathway even with aerobic conditions, including downstream increased lactate dehydrogenase activity and lactate production (15).
Because hyperpolarized MR imaging enables study of 13C-pyruvate and its metabolite 13C-lactate, lactate dehydrogenase activity can be assessed. Our data suggested that even when early glycolysis imaging is not informative, late events may still be informative regarding a response to a particular therapy. However, we do realize that as they are developed, more sensitive tests of early glycolytic events may show better correspondence with late events, including in our tumor model. Our data suggest that treatment with pazopanib preferentially affected late events in glycolysis measured by hyperpolarized pyruvate spectroscopy.
Regulation of metabolic activity is complex and can include altered cellular signaling and altered oxygen and nutrient supply. Pazopanib is a multityrosine kinase inhibitor that affects receptors found in tumor cells and cells involved in angiogenesis. Angiogenesis brings oxygen and nutrients to tumor cells and therefore regulates hypoxia and glycolysis. In gastrointestinal stromal tumors, the tyrosine kinase Kit drives growth and early glycolysis; therefore, these tumors have increased 18F-FDG uptake. Within 48 hours or 1 week of therapy with imatinib, which blocks Kit phosphorylation and turns off downstream signaling, 18F-FDG uptake was decreased in patients (16) and there was a corresponding decrease in expression of glucose transporters (17). However, gastrointestinal stromal tumors represent a special case where Kit activity is the primary tumor driver (18). Ovarian cancer is multifactorial and, as with most cancers, multiple pathways and receptors are involved. Pazopanib also inhibits angiogenesis.
Decreased pyruvate-to-lactate conversion has been suggested to be a marker of response in other models, for example colon cancer after radiation therapy (19), glioblastoma 7 days after mechanistic target of rapamycin inhibitor everolimus (20) or 1–2 days after alkylating agent temozolomide (21), and lymphoma 1 day after etoposide therapy (22). Decreased pyruvate-to-lactate conversion has also been noted at direct inhibition of glycolysis in p53-deficient lymphoma (23). These treatments directly affected tumor cells and resulted in decreased lactate production. However, we found increased 13C-pyruvate–to–13C-lactate conversion in our model. Tumor cell receptors affected by pazopanib, such as cKit (24) cFms (25), and fibroblast growth factor receptor (26,27) are not noted to be essential drivers of ovarian cancer cells. Platelet-derived growth factor receptor–α inhibition does not inhibit SKOV3 cell proliferation (28).
Pazopanib can affect vascular endothelial growth factor receptors on ovarian tumor cells and inhibit SKOV3 cell proliferation (29) but the effects in tumors are primarily paracrine and effect angiogenesis (30). Pazopanib inhibits ovarian tumor growth through its antiangiogenic activity and this may have led to an altered hypoxic state. This suggests that the antiangiogenic effect is the more likely cause of the increased lactate production in response to pazopanib.
Findings are supported by Fack et al (31) who injected 13C6-glucose into mice with glioblastoma and performed metabolic flux analysis and immunohistochemistry in excised tissues; they found that the angiogenesis inhibitor bevacizumab (an antibody that binds vascular endothelial growth factor) increased glycolysis, increased lactate dehydrogenase activity, and lowered levels of Krebs cycle metabolites.
Our findings and those previously reported lead us to propose a model of differential responses observed by hyperpolarized 13C-pyruvate MR imaging depending on the mechanism of therapeutic response. In the proposed model, agents that effect tumor cell growth and metabolism signaling or result in DNA damage directly to tumor cells tend to decrease pyruvate-to-lactate conversion, whereas antiangiogenic agents affect the tumor microenvironment and alter hypoxia with resulting early increase in such conversion. This increase or decrease in pyruvate-to-lactate conversion may be evaluated in vivo by hyperpolarized 13C-pyruvate MR imaging.
Although the normalized lactate ratio is commonly thought to reflect lactate dehydrogenase activity, other factors may play a role, including perfusion (32), intracellular-reducing potential, and activity and number of monocarboxylate transporter 1, which is involved in bringing exogenous pyruvate into the cell (33). Hypoxia influences monocarboxylate transporter 1 (33) activity and lactate dehydrogenase activity (34). These are akin to glucose transporters and hexokinase at 18F-FDG imaging.
Differential uptake of 18F-FDG and 13C-pyruvate has been noted in a dog model, where the former was observed as uptake in muscle and liposarcoma, and lactate production observed preferentially in tumor (35). Note that 18F-FDG uptake is imaged commonly 30 to 90 minutes after injection, whereas 13C-pyruvate is imaged over approximately 2 minutes; there is a temporal disparity that can confound interpretation of differences between the two imaging techniques in addition to mechanistic differences of assessing early versus late metabolic events. However, increased glucose uptake may also be because of increased aerobic respiration, such as in muscle, brain, and sometimes inflammation. Clinically in ovarian cancer patients, Avril et al (36) noted that decreased uptake of 18F-FDG can suggest response to carboplatin-based therapy after one cycle in terms of progression-free but not overall survival. In a small phase I–II study, 18F-FDG PET was not found to be useful for detecting response in patients who received the angiogenesis inhibitor aflibercept (a decoy receptor that binds vascular endothelial growth factor) with docetaxel (37). Current findings suggest the measurement of 13C-pyruvate conversion to lactate may be more useful for evaluation of response to angiogenesis inhibitors, particularly pazopanib, in the setting of ovarian cancer. 13C-pyruvate MR imaging has been performed in clinical trials, which suggests potential for translation (38).
Limitations of our study include variability of measurements within tumors and between animals, which is common in animal models. The surface coil was centered on the tumor, but some normal tissue may have been included in the measurements. Also, cognates of clinical machines were used. Translation to humans may introduce technical problems, but a completed first-in-man study (38) provides some confidence.
In summary, our findings suggest that hyperpolarized 13C-pyruvate–to-lactate conversion can serve as an early indicator of response to multityrosine kinase inhibitor pazopanib in an ovarian cancer model and that evaluation of late events in glycolysis, such as by hyperpolarized 13C-pyruvate MR, may be predictive of response even when the imaging of early events, such as by 18F-FDG PET, is not predictive.
Advance in Knowledge
■ Findings suggest that hyperpolarized carbon 13 (13C)-pyruvate MR spectroscopy may serve as an early indicator of response to tyrosine kinase (ie, angiogenesis) inhibitors such as pazopanib in an ovarian cancer model (13C-pyruvate–to-lactate conversion, 0.46 ± 0.07 vs 0.035 ± 0.07; six mice with pazopanib, seven control mice; P < .05) even when fluorine 18 (18F) fluorodeoxyglucose (FDG) PET/CT imaging does not indicate a response.
Implications for Patient Care
■ Hyperpolarized 13C-pyruvate MR spectroscopy has the potential to serve as an indicator of tumor response even when 18F-FDG PET/CT does not indicate a response.
SUPPLEMENTAL TABLES
Acknowledgments
Acknowledgments
We acknowledge Nan Li, PhD, and Wei Wei, MS, from the Department of Biostatistics (University of Texas M.D. Anderson Cancer Center) for their significant contribution in performing the statistical analysis in this manuscript.
Received August 11, 2016; revision requested October 18; revision received February 21, 2017; accepted March 20; final version accepted May 4.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the study design, the data collection and analysis, the decision to publish, or the preparation of the manuscript.
Study supported by National Institutes of Health (CA016672), National Cancer Institute (1R01CA159042), Cancer Prevention Institute of Texas (RP101243-P5, RP140021-P5), and the Bernard W. Biedenharn Chair in Cancer Research at U.T. MD Anderson Cancer Center.
Disclosures of Conflicts of Interest: M.K.R. disclosed no relevant relationships. S.P.S. disclosed no relevant relationships. J.L. disclosed no relevant relationships. J.A.B. disclosed no relevant relationships. V.K. disclosed no relevant relationships.
Abbreviation:
- FDG
- fluorodeoxyglucose
References
- 1.American Cancer Society . Cancer facts & figures 2015. Atlanta, Ga: American Cancer Society, 2015. [Google Scholar]
- 2.Munk Jensen M, Erichsen KD, Björkling F, et al. Imaging of treatment response to the combination of carboplatin and paclitaxel in human ovarian cancer xenograft tumors in mice using FDG and FLT PET. PLoS One 2013;8(12):e85126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim TJ, Ravoori M, Landen CN, et al. Antitumor and antivascular effects of AVE8062 in ovarian carcinoma. Cancer Res 2007;67(19):9337–9345. [DOI] [PubMed] [Google Scholar]
- 4.Berg JM, Tymoczko JL, Stryer L. Biochemistry. 7th ed. New York, NY: Freeman, 2012. [Google Scholar]
- 5.Ardenkjaer-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A 2003;100(18):10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Geel RM, Beijnen JH, Schellens JH. Concise drug review: pazopanib and axitinib. Oncologist 2012;17(8):1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris PA, Boloor A, Cheung M, et al. Discovery of 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methyl-benzenesulfonamide (Pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J Med Chem 2008;51(15):4632–4640. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Knick VB, Rudolph SK, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther 2007;6(7):2012–2021. [DOI] [PubMed] [Google Scholar]
- 9.Friedlander M, Hancock KC, Rischin D, et al. A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol Oncol 2010;119(1):32–37. [DOI] [PubMed] [Google Scholar]
- 10.Sandulache VC, Skinner HD, Wang Y, et al. Glycolytic inhibition alters anaplastic thyroid carcinoma tumor metabolism and improves response to conventional chemotherapy and radiation. Mol Cancer Ther 2012;11(6):1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu D, Wolf JK, Scanlon M, Price JE, Hung MC. Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res 1993;53(4):891–898. [PubMed] [Google Scholar]
- 12.Yang D, Han L, Kundra V. Exogenous gene expression in tumors: noninvasive quantification with functional and anatomic imaging in a mouse model. Radiology 2005;235(3):950–958. [DOI] [PubMed] [Google Scholar]
- 13.Jadvar H, Alavi A, Gambhir SS. 18F-FDG uptake in lung, breast, and colon cancers: molecular biology correlates and disease characterization. J Nucl Med 2009;50(11):1820–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol 1927;8(6):519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004;4(11):891–899. [DOI] [PubMed] [Google Scholar]
- 16.Stroobants S, Goeminne J, Seegers M, et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur J Cancer 2003;39(14):2012–2020. [DOI] [PubMed] [Google Scholar]
- 17.Van den Abbeele AD, Gatsonis C, de Vries DJ, et al. ACRIN 6665/RTOG 0132 phase II trial of neoadjuvant imatinib mesylate for operable malignant gastrointestinal stromal tumor: monitoring with 18F-FDG PET and correlation with genotype and GLUT4 expression. J Nucl Med 2012;53(4):567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cullinane C, Dorow DS, Kansara M, et al. An in vivo tumor model exploiting metabolic response as a biomarker for targeted drug development. Cancer Res 2005;65(21):9633–9636. [DOI] [PubMed] [Google Scholar]
- 19.Saito K, Matsumoto S, Takakusagi Y, et al. 13C-MR spectroscopic imaging with hyperpolarized [1-13C]pyruvate detects early response to radiotherapy in SCC tumors and HT-29 tumors. Clin Cancer Res 2015;21(22):5073–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaumeil MM, Ozawa T, Park I, et al. Hyperpolarized 13C MR spectroscopic imaging can be used to monitor Everolimus treatment in vivo in an orthotopic rodent model of glioblastoma. Neuroimage 2012;59(1):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park I, Bok R, Ozawa T, et al. Detection of early response to temozolomide treatment in brain tumors using hyperpolarized 13C MR metabolic imaging. J Magn Reson Imaging 2011;33(6):1284–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher FA, Kettunen MI, Day SE, et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 2008;453(7197):940–943. [DOI] [PubMed] [Google Scholar]
- 23.Venkatanarayan A, Raulji P, Norton W, et al. IAPP-driven metabolic reprogramming induces regression of p53-deficient tumours in vivo. Nature 2015;517(7536):626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson JW, Nucci MR, Brodsky J, Crum CP, Hirsch MS. Biomarker-assisted diagnosis of ovarian, cervical and pulmonary small cell carcinomas: the role of TTF-1, WT-1 and HPV analysis. Histopathology 2007;51(3):305–312. [DOI] [PubMed] [Google Scholar]
- 25.Kakiuchi-Kiyota S, Lappin PB, Heintz C, et al. Expression of proto-oncogene cFMS protein in lung, breast, and ovarian cancers. Appl Immunohistochem Mol Morphol 2014;22(3):188–199. [DOI] [PubMed] [Google Scholar]
- 26.Byron SA, Gartside MG, Wellens CL, et al. FGFR2 mutations are rare across histologic subtypes of ovarian cancer. Gynecol Oncol 2010;117(1):125–129. [DOI] [PubMed] [Google Scholar]
- 27.Fearon AE, Gould CR, Grose RP. FGFR signalling in women’s cancers. Int J Biochem Cell Biol 2013;45(12):2832–2842. [DOI] [PubMed] [Google Scholar]
- 28.Matsuo K, Nishimura M, Komurov K, et al. Platelet-derived growth factor receptor alpha (PDGFRα) targeting and relevant biomarkers in ovarian carcinoma. Gynecol Oncol 2014;132(1):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruz-Muñoz W, Di Desidero T, Man S, et al. Analysis of acquired resistance to metronomic oral topotecan chemotherapy plus pazopanib after prolonged preclinical potent responsiveness in advanced ovarian cancer. Angiogenesis 2014;17(3):661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merritt WM, Nick AM, Carroll AR, et al. Bridging the gap between cytotoxic and biologic therapy with metronomic topotecan and pazopanib in ovarian cancer. Mol Cancer Ther 2010;9(4):985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fack F, Espedal H, Keunen O, et al. Bevacizumab treatment induces metabolic adaptation toward anaerobic metabolism in glioblastomas. Acta Neuropathol (Berl) 2015;129(1):115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bankson JA, Walker CM, Ramirez MS, et al. Kinetic modeling and constrained reconstruction of hyperpolarized [1-13C]-pyruvate offers improved metabolic imaging of tumors. Cancer Res 2015;75(22):4708–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris T, Eliyahu G, Frydman L, Degani H. Kinetics of hyperpolarized 13C1-pyruvate transport and metabolism in living human breast cancer cells. Proc Natl Acad Sci U S A 2009;106(43):18131–18136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bluff JE, Reynolds S, Metcalf S, et al. Measurement of the acute metabolic response to hypoxia in rat tumours in vivo using magnetic resonance spectroscopy and hyperpolarised pyruvate. Radiother Oncol 2015;116(3):392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutte H, Hansen AE, Henriksen ST, et al. Simultaneous hyperpolarized (13)C-pyruvate MRI and (18)F-FDG-PET in cancer (hyperPET): feasibility of a new imaging concept using a clinical PET/MRI scanner. Am J Nucl Med Mol Imaging 2014;5(1):38–45. [PMC free article] [PubMed] [Google Scholar]
- 36.Avril N, Sassen S, Schmalfeldt B, et al. Prediction of response to neoadjuvant chemotherapy by sequential F-18-fluorodeoxyglucose positron emission tomography in patients with advanced-stage ovarian cancer. J Clin Oncol 2005;23(30):7445–7453. [DOI] [PubMed] [Google Scholar]
- 37.Coleman RL, Duska LR, Ramirez PT, et al. Phase 1-2 study of docetaxel plus aflibercept in patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer. Lancet Oncol 2011;12(12):1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson SJ, Kurhanewicz J, Vigneron DB, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci Transl Med 2013;5(198):198ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.