Abstract
Background
Enterovirus D68 (EV-D68) caused a widespread outbreak of respiratory illness in the United States in 2014, predominantly affecting children. We describe EV-D68 rates, spectrum of illness, and risk factors from prospective, population-based acute respiratory illness (ARI) surveillance at a large US pediatric hospital.
Methods
Children <13 years of age with ARI and residence in Hamilton County, Ohio were enrolled from the inpatient and emergency department (ED) settings at a children’s hospital in Cincinnati, Ohio, from 1 July to 31 October 2014. For each participant, we interviewed parents, reviewed medical records, and tested nasal and throat swabs for EV-D68 using real-time reverse-transcription polymerase chain reaction assay.
Results
EV-D68 infection was detected in 51 of 207 (25%) inpatients and 58 of 505 (11%) ED patients. Rates of EV-D68 hospitalization and ED visit were 1.3 (95% confidence interval [CI], 1.0–1.6) and 8.4 per 1000 children <13 years of age, respectively. Preexisting asthma was associated with EV-D68 infection (adjusted odds ratio, 3.2; 95% CI, 2.0–5.1). Compared with other ARI, children with EV-D68 were more likely to be admitted from the ED (P ≤ .001), receive supplemental oxygen (P = .001), and require intensive care unit admission (P = .04); however, mechanical ventilation was uncommon (2/51 inpatients; P = .64), and no deaths occurred.
Conclusions
During the 2014 EV-D68 epidemic, high rates of pediatric hospitalizations and ED visits were observed. Children with asthma were at increased risk for medically attended EV-D68 illness. Preparedness planning for a high-activity EV-D68 season in the United States should take into account increased healthcare utilization, particularly among children with asthma, during the late summer and early fall.
Keywords: enterovirus D68, acute respiratory illness, respiratory virus
Enterovirus D68 (EV-D68), a member of the Picornaviridae family, was first described in 1962 in the United States among children with severe lower respiratory tract infection [1]. Subsequent reports of EV-D68 had been infrequent and primarily limited to small clusters until 2014, when the largest documented nationwide epidemic of EV-D68 respiratory disease in the United States occurred [2–4]. From mid-August 2014 through 15 January 2015, a total of 1153 EV-D68 cases were confirmed in 49 US states and the District of Columbia [5]. Children were predominantly affected, and the epidemic resulted in a substantial resource burden at certain hospitals and emergency departments [6, 7].
EV-D68 infection primarily manifests as a respiratory illness, including upper and lower respiratory tract disease and asthma exacerbation. During the 2014 outbreak, a history of asthma or reactive airway disease was common among children with medically attended EV-D68 infection [8] and in some studies was associated with more severe illness [4, 9].
Coincident with the timing of the US EV-D68 epidemic, Cincinnati Children’s Hospital Medical Center (CCHMC) conducted prospective, population-based surveillance for acute respiratory illness (ARI) among children <13 years of age in the emergency department (ED) and inpatient hospital settings. We describe rates, clinical features, and severity of EV-D68 infection and evaluate the association between EV-D68 and asthma.
METHODS
Study Population and Design
Children <13 years of age with ARI and residence in Hamilton County, Ohio, which includes Cincinnati, were prospectively enrolled from the hospital inpatient and ED settings at CCHMC, an academic, tertiary care pediatric hospital with >600 inpatient beds and 35 pediatric intensive care unit (ICU) beds. Inpatient admissions and ED visits average >33 000 and >102 000, respectively, annually. Hospitalized children were eligible if they had been admitted within the previous 48 hours with ARI, defined by admission diagnosis of acute respiratory illness, apnea, asthma exacerbation, bronchiolitis, croup, cystic fibrosis exacerbation, febrile neonate, febrile seizure, fever without localizing signs, hypothermia, influenza, otitis media, other respiratory infection, paroxysmal cough, pharyngitis, pneumonia, respiratory distress, respiratory syncytial virus (RSV), rule-out sepsis, sinusitis, strep throat, tonsillitis, upper respiratory illness, or wheezing. Children presenting to the ED were eligible if they had a chief complaint of fever or respiratory symptoms (cough, earache, nasal congestion or runny nose, shortness of breath or rapid or shallow breathing, sore throat, vomiting after cough, or wheezing). Hospitalized children were excluded if they had a known nonrespiratory cause for admission, had been previously hospitalized during the past 4 days, had remained hospitalized since birth, or had febrile neutropenia related to cancer treatment. Children presenting to the ED were excluded if they had been previously enrolled in the ED within the past 6 days.
Surveillance was conducted in the hospital Monday through Friday, excluding public holidays, and in the ED, 4–6 days per week in shifts of 6–8 hours per day. CCHMC accounts for >95% of hospitalizations and ED visits for children from Hamilton County.
Study Period
Children were enrolled from 1 July–31 October 2014. The study period was selected to capture EV-D68 circulation in the hospital catchment area during the 2014 US EV-D68 outbreak [4]. Specimens collected in June and November 2014 from hospitalized patients enrolled in ARI surveillance at CCHMC all tested negative for EV-D68.
Data Collection and Definitions
Study personnel conducted parent or guardian interviews and medical record reviews using standardized study forms. The child’s sex, race, birth history, and history of current illness (duration, symptoms) were obtained by parental report. Medical history, hospital course, admission diagnosis, and insurance information were obtained by chart review.
For each participant preliminarily classified as having asthma or reactive airway disease on the basis of parental report or chart review, the CCHMC electronic medical record was reviewed by a pediatrician (M. A. S.) to confirm the classification. We defined asthma as a documented diagnosis in the medical record by a qualified healthcare provider prior to the child’s current illness. When available, data on asthma severity (intermittent or persistent) were also collected. Premature birth was defined as parental report of birth >1 month early. Chest radiographic findings were categorized as normal or abnormal based on the radiologist’s impression in the report.
Laboratory Testing
Midturbinate nasal and posterior pharyngeal swabs were collected from each participant and combined for testing. Respiratory virus testing using a multiplex polymerase chain reaction (PCR) assay (xTAG Respiratory Viral Panel, Luminex Molecular Diagnostics, Austin, Texas) was performed at CCHMC. All specimens were subsequently tested at the US Centers for Disease Control and Prevention (CDC) using a sensitive, CDC-developed EV-D68 real-time reverse-transcription PCR assay [10]. Children were classified as having EV-D68 infection if the nasal/throat specimen tested positive using the CDC assay with a cycle threshold (Ct) value ≤43; Ct values >43 were classified as equivocal, and specimens with no Ct were classified as negative. In the case of a specimen with a negative RNaseP assay result (RNA extraction control), the test result was considered inconclusive.
Statistical Analysis
Data from the study forms were entered into Redcap [11] at CCHMC and analyzed using Stata software version 12 (StataCorp, College Station, Texas). Characteristics of children with EV-D68 infection were compared to those of children with EV-D68–negative ARI. Patients enrolled from the ED and inpatients were analyzed separately, except when combined for multivariate analysis of demographic and clinical factors associated with medically attended EV-D68 infection. Pearson χ2, Fisher exact test, or Wilcoxon rank-sum test were used as appropriate to compare categorical or continuous variables. All P values were 2-sided and evaluated for statistical significance at P < .05.
Rate Calculations
The number of EV-D68 hospitalizations and ED visits per 1000 children was calculated using weighted case numbers divided by the 2014 age-specific Hamilton County population according to the US Census Bureau [12]. For inpatients, individual records were inflated to account for the proportion of eligible children enrolled and the number of inpatient surveillance days out of all possible days during the study period [13]. The 95% confidence intervals (CIs) were calculated by using the 2.5th and 97.5th percentile values for EV-D68 positive hospitalizations resulting from 1000 bootstrap samples [14]. ED visit rates were determined by using the number of ARI-related ED visits among study-eligible children for the 24-hour surveillance periods, inflating for days when surveillance was not conducted. The monthly totals of ARI-related ED visits were multiplied by the monthly proportion of study participants testing positive for EV-D68. Because of differences in the weighting methodology for the ED setting, 95% CIs were not calculated. Children testing positive for EV-D68 and admitted from the ED on an inpatient surveillance day were included in rate estimates for both ED visits and hospitalizations.
Ethics
The study was approved by the CCHMC institutional review board. Informed consent was obtained from each child’s parent or guardian before enrollment, and assent was obtained from children 7 years of age and older.
RESULTS
Study Population and EV-D68 Detection
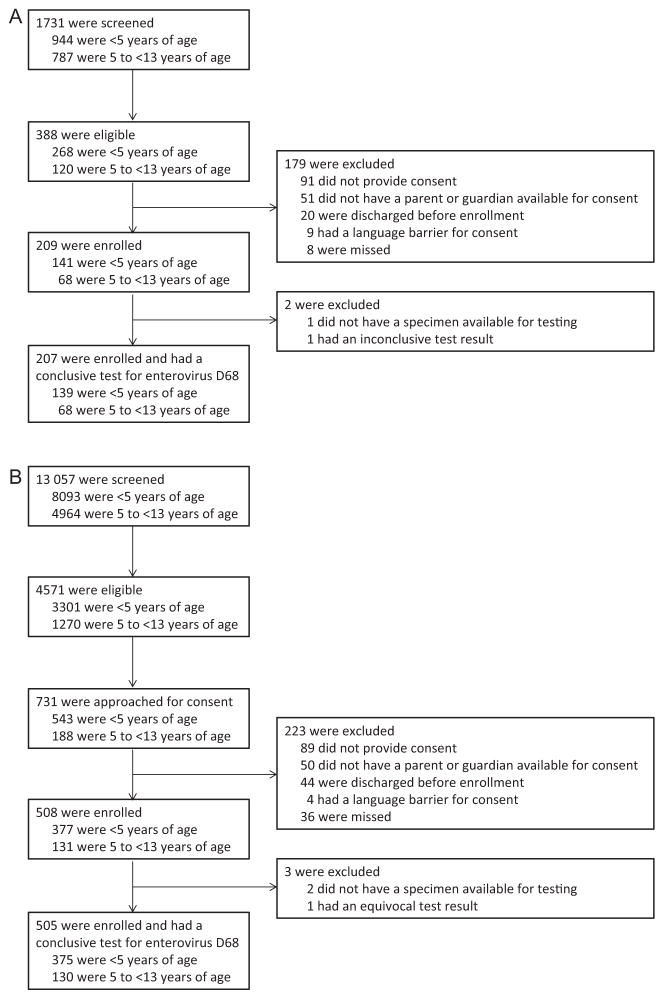
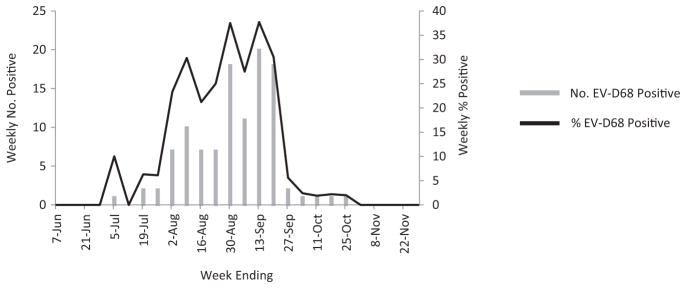
From 1 July through 31 October 2014, 505 ED patients and 207 hospitalized patients were enrolled and had a conclusive test for EV-D68 (Figure 1). EV-D68 infection was detected in 58 of 505 (11%) ED patients and 51 of 207 (25%) hospitalized patients. Most EV-D68 infections occurred during August and September (Figure 2).
Figure 1.
A, Inpatient enrollment flow diagram. B, Emergency department enrollment flow diagram.
Figure 2.
Enterovirus D68 (EV-D68)–positive tests among hospitalized and emergency department patients <13 years of age with acute respiratory illness, weekly number and percentage positive, Cincinnati, Ohio, 1 June–30 November 2014. Test results for June and November represent hospitalized children only.
Characteristics of Children With EV-D68 Infection
The median age of all patients (ED and hospitalized) with EV-D68 infection was 2.9 (range, 0.2–12.9) years and 62 of 109 (57%) were male. Sociodemographic characteristics of children with EV-D68 infection were similar to those of children with ARI from other causes, with the exception of race/ethnic group among ED patients (Table 1).
Table 1.
Characteristics of Hospitalized and Emergency Department Patients <13 Years of Age With Enterovirus D68 (EV-D68)–Positive and EV-D68–Negative Acute Respiratory Illness, Cincinnati, Ohio, 1 July–31 October 2014
| Characteristic | Emergency Department | Hospitalized | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| EV-D68 Positive (n = 58) | EV-D68 Negative (n = 447) | P Value | EV-D68 Positive (n = 51) | EV-D68 Negative (n = 156) | P Value | |
| Age, y | ||||||
| Median | 2.2 | 2.1 | .79 | 3.7 | 2.7 | .05 |
| Range | 0.4–11.1 | 0.1–12.3 | 0.2–12.9 | 0.04–12.6 | ||
|
| ||||||
| Age group, No. (%) | ||||||
| 0 to <6 mo | 2 (3) | 30 (7) | .91 | 1 (2) | 29 (19) | .06 |
| 6 to <12 mo | 10 (17) | 75 (17) | 5 (10) | 12 (8) | ||
| 12 to <24 mo | 14 (24) | 112 (25) | 11 (22) | 24 (15) | ||
| 24 to <60 mo | 16 (28) | 116 (26) | 15 (29) | 42 (27) | ||
| 60 mo to <13 y | 16 (28) | 114 (26) | 19 (37) | 49 (31) | ||
|
| ||||||
| Sex, No. (%) | ||||||
| Female | 26 (45) | 204 (46) | .91) | 21 (41 | 57 (37) | .55 |
| Male | 32 (55) | 243 (54) | 30 (59) | 99 (63) | ||
|
| ||||||
| Race or ethnic group, No. (%)a | ||||||
| White, non-Hispanic | 5 (9) | 92 (21) | .006 | 14 (27) | 54 (34) | .08 |
| Black, non-Hispanic | 46 (79) | 313 (70) | 33 (65) | 71 (46) | ||
| Hispanic | 5 (9) | 10 (2) | 2 (4) | 11 (7) | ||
| Other | 2 (3) | 32 (7) | 2 (4) | 20 (13) | ||
|
| ||||||
| Insurance type, No. (%) | ||||||
| Public | 54 (93) | 384 (86) | .21 | 39 (76) | 103 (66) | .28 |
| Private | 3 (5) | 39 (9) | 9 (18) | 39 (25) | ||
| Both | 0 | 16 (4) | 2 (4) | 13 (8) | ||
| None | 1 (2) | 8 (2) | 1 (2) | 1 (1) | ||
|
| ||||||
| Maternal education | ||||||
| Less than high school | 13 (23) | 91 (21) | .29 | 10 (20) | 19 (12) | .20 |
| Completion of high school | 37 (65) | 259 (58) | 25 (50) | 71 (46) | ||
| More than high school | 7 (12) | 94 (21) | 15 (30) | 66 (42) | ||
|
| ||||||
| Lives with smoker, No. (%) | 19 (33) | 146 (33) | .99 | 17 (33) | 55 (35) | .80 |
|
| ||||||
| Attends day care or preschool, No. (%) | 32 (56) | 211 (47) | .31 | 13 (25) | 48 (31) | .37 |
|
| ||||||
| Attends elementary school, No. (%)b | 14 (24) | 97 (22) | .62 | 17 (33) | 47 (30) | .67 |
Abbreviation: EV-D68, enterovirus D68.
Other includes Asian, American Indian/Alaska Native, Native Hawaiian/Other Pacific Islander, and ≥2 races.
Excludes home school.
Compared with other causes of ARI, cough, difficulty breathing, and wheezing were significantly more common among ED and hospitalized patients with EV-D68 infection; coryza or rhinorrhea was more common among children with EV-D68 infection in the ED only (Table 2). Wheezing was significantly associated with EV-D68 infection even among children without preexisting asthma. Children with EV-D68 infection presenting to the ED were less likely to have fever or sore throat compared to children with other causes of ARI (P = .02 and P = .007, respectively).
Table 2.
Clinical Presentation and Medical History of Hospitalized and Emergency Department Patients <13 Years of Age With Enterovirus D68 (EV-D68)–Positive and EV-D68–Negative Acute Respiratory Illness, Cincinnati, Ohio, 1 July–31 October 2014
| Characteristic | Emergency Department
|
Hospitalized
|
||||
|---|---|---|---|---|---|---|
| EV-D68 Positive (n = 58) | EV-D68 Negative (n = 447) | P Value | EV-D68 Positive (n = 51) | EV-D68 Negative (n = 156) | P Value | |
| Duration of symptoms before enrollment, d | ||||||
| Median | 2 | 3 | .38 | 3 | 3 | .33 |
| Range | 1–10 | 1–60 | … | 1–11 | 1–21 | … |
|
| ||||||
| Symptoms, No. (%) | ||||||
| Fever | 26 (45) | 280 (63) | .02 | 30 (59) | 96 (62) | .69 |
| Myalgiaa | 3/16 (19) | 22/114 (19) | .92 | 4/19 (21) | 18/48 (38) | .20 |
| Poor appetite | 25 (43) | 190 (43) | .96 | 37 (73) | 90 (58) | .05 |
| Headachea | 6/16 (38) | 39/114 (34) | .85 | 8/19 (42) | 23/48 (48) | .67 |
| Coryza or rhinorrhea | 48 (83) | 260 (58) | <.001 | 46 (90) | 125 (80) | .10 |
| Earache | 8 (14) | 117 (26) | .07 | 11 (22) | 19 (13) | .12 |
| Sore throat | 5 (9) | 112 (25) | .007 | 18 (35) | 44 (29) | .39 |
| Cough | 52 (90) | 280 (63) | <.001 | 50 (98) | 130 (83) | .007 |
| Vomiting after cough | 15 (26) | 70 (16) | .05 | 24 (47) | 56 (36) | .16 |
| Shortness of breath or rapid or shallow breathing | 34 (59) | 95 (21) | <.001 | 51 (100) | 123 (79) | <.001 |
| Wheezing | 26 (45) | 74 (17) | <.001 | 51 (100) | 108 (69) | <.001 |
| Wheezing with preexisting asthma | 14/16 (88) | 23/64 (36) | <.001 | 33/33 (100) | 65/66 (98) | .48 |
| Wheezing with no asthma history | 12/42 (29) | 51/382 (13) | .008 | 18/18 (100) | 43/90 (48) | <.001 |
|
| ||||||
| Medical history, No. (%)b | ||||||
| Genetic/metabolic disorder | 0 | 1 (<1) | 1.00 | 2 (4) | 2 (1) | .26 |
| Heart disease | 0 | 1 (<1) | 1.00 | 1 (2) | 5 (3) | 1.00 |
| Cerebral palsy | 0 | 1 (<1) | 1.00 | 0 | 5 (3) | .34 |
| Seizure disorder | 0 | 4 (1) | 1.00 | 1 (2) | 7 (4) | .68 |
| Mental retardation or developmental delay | 0 | 5 (1) | 1.00 | 1 (2) | 10 (6) | .30 |
| Asthma | 16 (28) | 64 (14) | .009 | 33 (65) | 66 (42) | .005 |
| Intermittent asthmac | … | … | … | 16 (31) | 17 (11) | … |
| Persistent asthmac | … | … | … | 16 (31) | 43 (28) | … |
| Not documented | … | … | … | 1 (2) | 6 (4) | … |
| Bronchopulmonary dysplasia or chronic lung disease | 0 | 1 (<1) | 1.00 | 3 (6) | 7 (4) | .69 |
| Other chronic condition | 14 (3) | 3 (5) | .50 | 3 (6) | 12 (8) | 1.00 |
|
| ||||||
| Birth history, No. (%)d | ||||||
| Premature birth (born >1 mo early) | 4/42 (10) | 17/332 (5) | .24 | 10/33 (30) | 13/110 (12) | .01 |
| Needed supplemental oxygen after birth | 6/42 (14) | 27/332 (8) | .20 | 10/33 (30) | 17/110 (16) | .07 |
| Ever breastfed | 20/42 (48) | 197/332 (59) | .15 | 14/33 (42) | 65/110 (60) | .08 |
Abbreviation: EV-D68, enterovirus D68.
Asked for children ≥5 years of age.
On the basis of medical chart review; conditions affecting <5 participants not listed (blood disorder, cancer, diabetes mellitus, immunodeficiency, kidney disease, liver disease, sickle cell disease, cystic fibrosis).
Collected when available; not available for most emergency department patients.
Parental report, children <5 years of age. Premature birth was defined as parental report of birth >1 month early.
Preexisting asthma was associated with EV-D68 infection among ED and hospitalized patients (combined odds ratio [OR], 3.0; 95% CI, 1.9–4.5). This association remained significant after adjusting for age, sex, and race in multivariate analysis (adjusted OR, 3.2; 95% CI, 2.0–5.1).
Hospitalization and ED Visit Rates
The rates of EV-D68 hospitalization were 1.3 (95% CI, 1.0–1.6) per 1000 children <13 years of age, 1.9 (95% CI, 1.4–2.6) per 1000 children <5 years of age, and 0.9 (95% CI, .6–1.2) per 1000 children 5 to <13 years of age. Rates of hospitalization were highest among children 6 months to <5 years of age (Table 3). The rates of EV-D68–associated ED visit were 8.4 per 1000 children <13 years of age, 15.3 per 1000 children <5 years of age, and 4.0 per 1000 children 5 to <13 years of age.
Table 3.
Rates of Hospitalization and Emergency Department Visit for Enterovirus D68 Infection Among Children Residing in Hamilton County, Ohio, 1 July–31 October 2014, by Age
| Age | Emergency Department | Hospitalized |
|---|---|---|
| No. of EV-D68 Infections per 1000 Children (95% CI) | ||
| 0 to <6 mo | …a | 0.6 (.0–2.2) |
| 6 to <12 mo | …a | 2.8 (.6–5.6) |
| 12 to <24 mo | …a | 2.9 (1.3–4.6) |
| 24 to <60 mo | …a | 1.7 (1.0–2.4) |
| 0 to <60 mo | 15.3 | 1.9 (1.4–2.6) |
| 60 mo to <13 y | 4.0 | 0.9 (.6–1.2) |
| 0 to <13 y | 8.4 | 1.3 (1.0–1.6) |
Abbreviations: CI, confidence interval; EV-D68, enterovirus D68.
Not calculated.
Clinical Course of ED Patients
Of children enrolled in the ED, 29 of 505 (6%) required hospital admission; EV-D68 infection accounted for 13 of the 29 (45%) ARI admissions. Children with EV-D68 infection were more likely to be admitted (13/58 [22%]) than children with other causes of ARI (16/446 [3.6%]) (P ≤ .001). Most children admitted with EV-D68 infection had asthma (10/13 [77%]). Admission was more common among children with asthma who had EV-D68 infection compared to children with asthma and non-EV-D68 ARI (10/16 [63%] vs 6/64 [9%]; P < .001).
Clinical Course of Inpatients
Among hospitalized children, those with EV-D68 infection more often required ICU admission, received oxygen, and had a chest radiograph performed compared to children with other causes of ARI (Table 4). EV-D68 accounted for 33 of 99 (33%) admissions among children with asthma during the study period, and 29 of 62 (47%) during the months with highest EV-D68 incidence (August and September). Among children with EV-D68, no significant differences in hospital course were observed between children with and without preexisting asthma (Table 5). All children hospitalized with ARI survived.
Table 4.
Hospital Course and Outcomes for Children <13 Years of Age With Enterovirus D68 (EV-D68)–Positive and EV-D68–Negative Acute Respiratory Illness, Cincinnati, Ohio, 1 July–31 October 2014
| Characteristic | EV-D68 Positive (n = 51) | EV-D68 Negative (n = 156) | P Value |
|---|---|---|---|
| Inpatient length of stay, d | |||
| Median | 2 | 2 | .88 |
| Range | 1–6 | 0–11 | |
|
| |||
| Admission diagnosis, No. (%) | |||
| LRTIa | 51 (100) | 106 (68) | <.001 |
| Asthma exacerbation or RAD | 39 (76) | 68 (44) | <.001 |
| Bronchiolitis | 5 (10) | 15 (10) | … |
| Pneumonia or pneumonitis | 5 (10) | 14 (9) | … |
| Wheezing | 2 (4) | 9 (6) | … |
|
| |||
| Admitted to ICU, No. (%) | 15 (29) | 25 (16) | .04 |
| ICU length of stay, d | |||
| Median | 2 | 2 | .41 |
| Range | 1–8 | 1–7 | |
|
| |||
| Oxygen received, No. (%) | 38 (75) | 73 (47) | .001 |
|
| |||
| Intubated, No. (%) | 2 (4) | 4 (3) | .64 |
|
| |||
| CXR done, No. (%) | 35 (69) | 77 (49) | .02 |
| Abnormal CXR, No. (%)b | 27/35 (77) | 55/77 (71) | .53 |
|
| |||
| Outcome, No. (%) | |||
| Survived | 51 (100) | 156 (100) | … |
Abbreviations: CXR, chest radiograph; EV-D68, enterovirus D68; ICU, intensive care unit; LRTI, lower respiratory tract infection; RAD, reactive airway disease.
LRTI defined as asthma exacerbation or RAD, bronchiolitis, cystic fibrosis exacerbation, pneumonia or pneumonitis, or wheezing.
Chest radiograph findings were categorized as normal or abnormal based on the radiologist’s impression in the report.
Table 5.
Hospital Course and Outcomes for Children <13 Years of Age With Enterovirus D68 Infection With and Without Asthma, Cincinnati, Ohio, 1 July–31 October 2014
| Characteristic | Asthma (n = 33) | No Asthma (n = 18) | P Value |
|---|---|---|---|
| Length of stay, d | |||
| Median | 1 | 2 | .13 |
| Range | 1–6 | 1–6 | |
|
| |||
| Admission diagnosis, No. (%) | |||
| LRTIa | 33 (100) | 18 (100) | … |
| Asthma exacerbation or RAD | 31 (94) | 8 (44) | … |
| Bronchiolitis | 0 | 5 (28) | … |
| Pneumonia or pneumonitis | 0 | 5 (28) | … |
| Wheezing | 2 (6) | 0 | … |
|
| |||
| Admitted to ICU, No. (%) | 9 (27) | 6 (33) | .65 |
| ICU length of stay, d | |||
| Median | 2 | 2 | .39 |
| Range | 1–8 | 1–4 | |
|
| |||
| Oxygen received, No. (%) | 23 (70) | 15 (83) | .34 |
|
| |||
| Intubated, No. (%) | 1 (3) | 1 (6) | 1.00 |
|
| |||
| CXR done, No. (%) | 20 (61) | 15 (83) | .12 |
| Abnormal CXR, No. (%) | 16/20 (80) | 11/15 (73) | .70 |
Abbreviations: CXR, chest radiograph; ICU, intensive care unit; LRTI, lower respiratory tract infection; RAD, reactive airway disease.
LRTI defined as asthma exacerbation or RAD, bronchiolitis, cystic fibrosis exacerbation, pneumonia or pneumonitis, or wheezing.
Other Respiratory Virus Detections
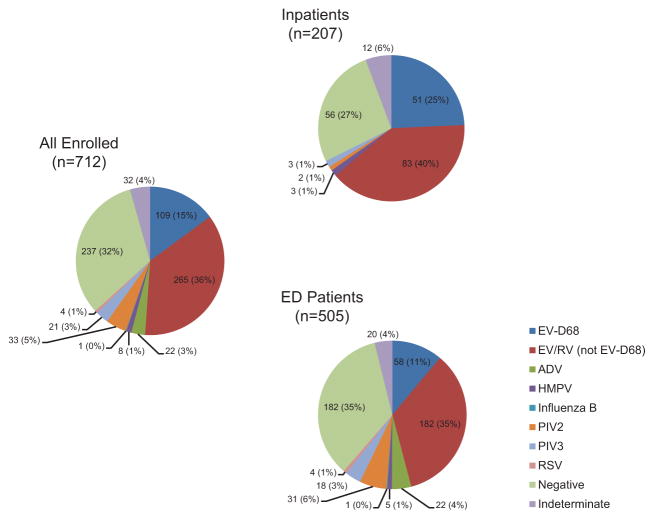
Respiratory viruses other than EV-D68 were detected in 245 of 505 (49%) children in the ED and 88 of 207 (43%) hospitalized children (Figure 3). Co-detection of other respiratory viruses was rare among children with EV-D68 infection, with 1 of 109 (<1%) also testing positive for human metapneumovirus (hMPV). The multiplex assay detected enterovirus/rhinovirus in 106 of the 109 (97%) EV-D68 infections detected by the EV-D68-specific assay.
Figure 3.
Respiratory viruses detected among hospitalized and emergency department (ED) patients <13 years of age with acute respiratory illness using a commercial multiplex assay, 1 July–31 October 2014. Labels display No. (%). Among hospitalized patients, co-detection of >1 respiratory virus occurred in 3 of 207 (1%) children. Among ED patients, co-detection of >1 respiratory virus occurred in 17 of 505 (3%) children. Abbreviations: ADV, adenovirus; EV-D68, enterovirus D68; EV/RV, enterovirus/rhinovirus (excluding EV-D68 in this figure); HMPV, human metapneumovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus.
DISCUSSION
During a season of high circulation, EV-D68 was detected in 25% of ARI hospitalizations and 11% of ARI ED visits among children <13 years of age at a large children’s hospital. We estimated the rate of hospitalization associated with EV-D68 infection among children <5 years of age in Hamilton County, Ohio, to be 1.9 per 1000 children; in studies using comparable methodology, this is higher than estimates for parainfluenza viruses (1.0–1.2 per 1000 children), hMPV (1 per 1000 children), and influenza virus during seasons in 2000–2011 (0.2–1.6 per 1000 children), which included the pandemic H1N1 season, but lower than for RSV (3–3.5 per 1000 children) [13, 15–22]. For children 5 to <13 years of age, published comparison data are limited; however, EV-D68 hospitalization rates were higher than those estimated for pneumonia attributed to influenza and other respiratory viruses among similar age groups during 2010–2012 [23]. The rate of ED visits for EV-D68 among children <5 years of age was similar to estimates for hMPV (6–17 per 1000 children) but lower than for RSV (21.5–28 per 1000 children) [17, 20, 24]; comparisons with influenza varied on the basis of season, but were lower than age-specific ED visit rates for the pandemic H1N1 season in one county with available estimates (19–29 per 1000 children) [15, 18, 25].
Preexisting asthma was significantly associated with medically attended EV-D68 infection, confirming observations from other studies [4, 9, 26, 27]. The prospective study design with systematic testing for EV-D68 is a strength of our study and reduced the potential for selection bias compared with case series or retrospective studies in which testing was performed on the basis of clinician discretion. During the peak of the outbreak, nearly half of ARI hospitalizations and a majority of ED admissions among children with asthma were associated with EV-D68 infection. Among children without a history of asthma, we observed significantly more wheezing in those with EV-D68 infection compared to other causes of ARI, a finding also previously reported from other studies and outbreak descriptions [27, 28].
The association between asthma exacerbation or acute wheezing and respiratory viruses, particularly rhinoviruses, is well documented [29–32]. Rhinovirus infection in patients with asthma has been associated with increased lower respiratory tract symptoms and clinical severity [33, 34]. Although rhinoviruses are most commonly implicated in acute wheezing hospitalizations, the relative contributions of rhinoviruses and respiratory enteroviruses might not be fully appreciated because of limitations of diagnostic tests. Commercial molecular assays do not differentiate between rhinoviruses and enteroviruses, and advances in molecular methods that allow more reliable differentiation are relatively recent. EV-D68 and rhinoviruses are both members of the picornavirus family (and Enterovirus genus) and are phenotypically similar [35], which could explain similarities in clinical presentation in children with asthma.
In our study, the hospital course and outcome among children admitted with EV-D68 infection did not differ on the basis of asthma status. Although children with asthma were more likely to have medically attended EV-D68 infection and were more often admitted from the ED, hospital outcomes were similar between children with and without preexisting asthma. Some reports have shown a more severe clinical course among hospitalized children with EV-D68 infection who have preexisting asthma [4, 9], whereas others have not observed this difference [8]. It is possible that differences in study design or power could explain differences in findings. With the exception of asthma, underlying medical conditions were uncommon among children in our study and no significant associations with EV-D68 infection were detected.
In both the ED and inpatient settings, children with EV-D68 infection had significantly more cough, difficulty breathing, and wheezing compared to children with other causes of ARI. Other reports have also documented these symptoms as predominant features of EV-D68 ARI [4, 26, 28, 36]. This triad of symptoms was reported nearly universally among inpatients. In the ED, cough was most prominent (90%), whereas wheezing and difficulty breathing were present in 45% and 59%, respectively. A majority of ED patients with EV-D68 illness also had nasal congestion or rhinorrhea, whereas fever and sore throat were less common compared with other causes of ARI. Our findings contribute to the limited available data on the spectrum of EV-D68 illness observed in the outpatient setting. Surveillance reports using influenza-like illness criteria (requiring objective fever) are likely to underestimate the number of EV-D68 cases and might not capture the full spectrum of respiratory illness seen in the outpatient setting given our finding that fever was reported in less than half of ED patients with EV-D68 illness.
EV-D68 infection was associated with an increased likelihood of admission from the ED and, among hospitalized patients, increased ICU admission compared with other causes of ARI. Children with EV-D68 illness were more often treated with oxygen and were more likely to receive a chest radiograph; however, differences in some other measures of disease severity such as the need for mechanical ventilation or duration of ICU stay were not observed. Consistent with our findings, hypoxia or the need for supplemental oxygen has been associated with EV-D68 infection in numerous reports [27, 28, 37, 38]. In a recent retrospective study comparing children with EV-D68 and H1N1 influenza illness, those with EV-D68 were more likely to require admission to the ICU and to receive noninvasive positive pressure ventilation (bilevel positive airway pressure or continuous positive airway pressure) but less likely to require intubation or to have severe outcomes or complications [26]. Our study supports other published data, and suggests that an EV-D68 epidemic may result in considerable respiratory morbidity and utilization of hospital resources, including intensive care, particularly among children with asthma; however, severe complications, prolonged hospitalization, and death have not been common features of EV-D68 respiratory illness. These findings have implications for epidemic planning. In particular, planning for an increased patient census in pediatric wards and ICUs, and increased demand for asthma therapeutics and respiratory equipment, may be important [6, 26, 39].
Our study was subject to limitations. Surveillance was conducted at a single tertiary-level hospital, and rates of EV-D68 infection might not be generalizable to other geographic locations or to hospitals serving different populations. EV-D68 rates represent a single epidemic season, and might not be directly comparable to rates of other respiratory viruses estimated over several years of surveillance at multiple sites. To calculate population- based rates, we assumed that eligible children who were not enrolled were similar to those enrolled. Our age criterion for enrollment (<13 years old) excluded older children who could have had EV-D68 infection, and this is likely reflected in the lower median age for EV-D68 infection in our study compared with studies that included all ages [4]. We excluded children with febrile neutropenia, which may have limited our ability to evaluate the association between EV-D68 infection and immunosuppression. We included only symptoms typically associated with respiratory infections on our interview form; therefore, it is possible that we did not capture the full spectrum of symptoms associated with EV-D68 respiratory illness.
In conclusion, during the 2014 EV-D68 epidemic, population- based rates of hospitalization were generally higher than estimates for other respiratory viruses during their typical seasons, with the exception of RSV among children <5 years of age. These rates reflect a period of high EV-D68 circulation, which has not been observed in subsequent years (2015 or 2016) [5]. Children with preexisting asthma appear to be at increased risk for medically attended EV-D68 illness. Continued surveillance and vigilance among clinicians for EV-D68 infection, particularly during late summer and early autumn in the United States, is important. In the instance of a season with high EV-D68 activity, anticipating increased healthcare utilization and increased demand for asthma therapeutics and respiratory equipment may be prudent.
Acknowledgments
We thank Sahle Amsalu, Brittney Cassell, Jacqueline DellaTorre, Krista Doerflein, Vanessa Florian, Chelsea Rohlfs, Michelle Roth, Amy Singh, Joseph Sorter, Elizabeth Stahl, Kathryn Weiskircher, and Michael Whalen from CCHMC (Cincinnati Children’s Hospital Medical Center) for their assistance with patient enrollment, chart review, data management, and laboratory analysis.
Financial support. This work was supported by the CDC through a contract with Cincinnati Children’s Medical Center.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Potential conflicts of interest. M. S. O. and W. A. N. report a potential patent application on enterovirus D68 diagnostic assay. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol. 1967;85:297–310. doi: 10.1093/oxfordjournals.aje.a120693. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Clusters of acute respiratory illness associated with human enterovirus 68— Asia, Europe, and United States, 2008–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1301–4. [PubMed] [Google Scholar]
- 3.Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus D68—Missouri and Illinois, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:798–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Midgley CM, Watson JT, Nix WA, et al. Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA: a descriptive epidemiological investigation. Lancet Respir Med. 2015;3:879–87. doi: 10.1016/S2213-2600(15)00335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. [Accessed 1 August 2016];Enterovirus D68. Available at: http://www.cdc.gov/non-polio-enterovirus/about/EV-D68.html.
- 6.Messacar K, Hawkins SM, Baker J, et al. Resource burden during the 2014 enterovirus D68 respiratory disease outbreak at Children’s Hospital Colorado: an unexpected strain. JAMA Pediatr. 2016;170:294–7. doi: 10.1001/jamapediatrics.2015.3879. [DOI] [PubMed] [Google Scholar]
- 7.Conners GP, Doyle SJ, Fowler MA, Jr, Schroeder LL, Tryon TW. System stresses in 2 pediatric emergency departments and 2 pediatric urgent care centers during the 2014 enterovirus-D68 outbreak. Pediatr Emerg Care. 2016 doi: 10.1097/PEC.0000000000000856. [DOI] [PubMed] [Google Scholar]
- 8.Schuffenecker I, Mirand A, Josset L, et al. Epidemiological and clinical characteristics of patients infected with enterovirus D68, France, July to December 2014. Euro Surveill. 2016:21. doi: 10.2807/1560-7917.ES.2016.21.19.30226. [DOI] [PubMed] [Google Scholar]
- 9.Moyer K, Wang H, Salamon D, Leber A, Mejias A. Enterovirus D68 in hospitalized children: sequence variation, viral loads and clinical outcomes. PLoS One. 2016;11:e0167111. doi: 10.1371/journal.pone.0167111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. [Accessed 2 August 2016];Enterovirus D68 2014 real-time RT-PCR assay. Available at: http://www.fda.gov/downloads/MedicalDevices/Safety/EmergencySituations/UCM446784.pdf.
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics. Vintage 2014 postcensal estimates of the resident population of the United States (April 1, 2010, July 1, 2010–July 1, 2014), by year, county, single-year of age (0, 1, 2, .., 85 years and over), bridged race, Hispanic origin, and sex. [Accessed 2 September 2016]; Prepared under a collaborative arrangement with the US Census Bureau. Available at: http://www.cdc.gov/nchs/nvss/bridged_race.htm.
- 13.Iwane MK, Edwards KM, Szilagyi PG, et al. New Vaccine Surveillance Network. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113:1758–64. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 14.Rust KF, Rao JN. Variance estimation for complex surveys using replication techniques. Stat Methods Med Res. 1996;5:283–310. doi: 10.1177/096228029600500305. [DOI] [PubMed] [Google Scholar]
- 15.Jules A, Grijalva CG, Zhu Y, et al. Influenza-related hospitalization and ED visits in children less than 5 years: 2000–2011. Pediatrics. 2015;135:e66–74. doi: 10.1542/peds.2014-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinberg GA, Hall CB, Iwane MK, et al. New Vaccine Surveillance Network. Parainfluenza virus infection of young children: estimates of the population- based burden of hospitalization. J Pediatr. 2009;154:694–9. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 17.Edwards KM, Zhu Y, Griffin MR, et al. New Vaccine Surveillance Network. Burden of human metapneumovirus infection in young children. N Engl J Med. 2013;368:633–43. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poehling KA, Edwards KM, Weinberg GA, et al. New Vaccine Surveillance Network. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 19.Jules A, Grijalva CG, Zhu Y, et al. Estimating age-specific influenza-related hospitalization rates during the pandemic (H1N1) 2009 in Davidson Co, TN. Influenza Other Respir Viruses. 2012;6:e63–71. doi: 10.1111/j.1750-2659.2012.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poehling KA, Edwards KM, Griffin MR, et al. The burden of influenza in young children, 2004–2009. Pediatrics. 2013;131:207–16. doi: 10.1542/peds.2012-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jhung MA, Swerdlow D, Olsen SJ, et al. Epidemiology of 2009 pandemic influenza A (H1N1) in the United States. Clin Infect Dis. 2011;52(suppl 1):S13–26. doi: 10.1093/cid/ciq008. [DOI] [PubMed] [Google Scholar]
- 23.Jain S, Williams DJ, Arnold SR, et al. CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–45. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourgeois FT, Valim C, McAdam AJ, Mandl KD. Relative impact of influenza and respiratory syncytial virus in young children. Pediatrics. 2009;124:e1072–80. doi: 10.1542/peds.2008-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Self WH, Grijalva CG, Zhu Y, et al. Emergency department visits for influenza A(H1N1) pdm09, Davidson County, Tennessee, USA. Emerg Infect Dis. 2012;18:863–5. doi: 10.3201/eid1805.111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao S, Messacar K, Torok MR, et al. Enterovirus D68 in critically ill children: a comparison with pandemic H1N1 influenza. Pediatr Crit Care Med. 2016;17:1023–31. doi: 10.1097/PCC.0000000000000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasegawa S, Hirano R, Okamoto-Nakagawa R, Ichiyama T, Shirabe K. Enterovirus 68 infection in children with asthma attacks: virus-induced asthma in Japanese children. Allergy. 2011;66:1618–20. doi: 10.1111/j.1398-9995.2011.02725.x. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson LM, Redd JT, Schneider E, et al. Outbreak of lower respiratory tract illness associated with human enterovirus 68 among American Indian children. Pediatr Infect Dis J. 2012;31:309–12. doi: 10.1097/INF.0b013e3182443eaf. [DOI] [PubMed] [Google Scholar]
- 29.Khetsuriani N, Kazerouni NN, Erdman DD, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119:314–21. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jartti T, Lehtinen P, Vuorinen T, et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minor TE, Dick EC, DeMeo AN, Ouellette JJ, Cohen M, Reed CE. Viruses as precipitants of asthmatic attacks in children. JAMA. 1974;227:292–8. [PubMed] [Google Scholar]
- 32.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Message SD, Laza-Stanca V, Mallia P, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105:13562–7. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corne JM, Marshall C, Smith S, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–4. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 35.Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol. 2004;85(Pt 9):2577–84. doi: 10.1099/vir.0.79925-0. [DOI] [PubMed] [Google Scholar]
- 36.Meijer A, van der Sanden S, Snijders BE, et al. Emergence and epidemic occurrence of enterovirus 68 respiratory infections in the Netherlands in 2010. Virology. 2012;423:49–57. doi: 10.1016/j.virol.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 37.Vazquez-Perez JA, Ramirez-Gonzalez JE, Moreno-Valencia Y, et al. EV-D68 infection in children with asthma exacerbation and pneumonia in Mexico City during 2014 autumn. Influenza Other Respir Viruses. 2016;10:154–60. doi: 10.1111/irv.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calvo C, Cuevas MT, Pozo F, et al. Respiratory infections by enterovirus D68 in outpatients and inpatients Spanish children. Pediatr Infect Dis J. 2016;35:45–9. doi: 10.1097/INF.0000000000000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuster JE, Miller JO, Selvarangan R, et al. Severe enterovirus 68 respiratory illness in children requiring intensive care management. J Clin Virol. 2015;70:77–82. doi: 10.1016/j.jcv.2015.07.298. [DOI] [PubMed] [Google Scholar]