Abstract
Background
The present study explored and compared the immediate responses in markers of inflammation and angiogenesis in maintenance heart transplant (HTx) recipients before, during and after sessions of high-intensity interval training (HIT) versus moderate-intensity continuous training (MICT). The study aimed to explain some of the trigger mechanisms behind HIT in HTx recipients.
Methods
This cross-over study included 14 HTx patients (mean±SD age: 53±13 years; time since HTx, 3±2 years). All participants underwent baseline blood samples and a cardiopulmonary exercise test during their first visit. The next two visits included one HIT session and one MICT session, in randomised order. Blood samples were taken during and after each exercise session. Myokines and inflammatory markers related to vascular inflammation, blood-platelet activation and modulation of angiogenesis were analysed.
Results
The main findings in this study were (1) exercise, regardless of intensity, induced a significant immediate response in several vascular, angiogenetic and in particular platelet-derived inflammatory mediators in HTx recipients. (2) HIT showed trends to induce an increased response in von Willebrand factor, vascular endothelial growth factor-1 and angiopoetin-2, and a decreased response in growth differentiation factor-15, compared with MICT.
Conclusions
This pattern and in particular the trend towards an increased angiogenetic mediator response could contribute to the beneficial effects of HIT in HTx recipients.
Trial registration number
Keywords: heart transplant, inflammation, exercise therapy for cardiac transplant recipients, vascular endothelial growht factor, high intensity interval training[title]
Key Questions.
What is already known about this subject?
Heart transplant (HTx) recipients show a limited exercise capacity compared with the general population. Recent exercise intervention studies have shown that high-intensity interval training (HIT) efficiently increases both exercise capacity and muscular endurance in this patient group. Little is known on the mechanisms behind this ‘HIT effect’ and in particular about the immediate inflammatory effects of different exercise intensities in a HTx population.
What does this study add?
This study showed that HTx recipients had a similar response in blood-platelet activation both during exercise with moderate and high intensity. For markers of angiogenesis, the trend showed an increased response during HIT compared with exercise with moderate intensity.
How might this impact on clinical practice?
This pattern, and in particular the trend towards an increased angiogenetic mediator response, could contribute to explain the beneficial effects of HIT in HTx recipients.
Introduction
Heart transplantation (HTx) is an established treatment for patients with end-stage heart failure (HF), with severely impaired exercise capacity. HTx is shown to markedly improve prognosis with a 50% survival past 11 years.1 Although the failing pump has been replaced with a vital donor graft, the exercise capacity is still impaired with a peak oxygen uptake (Vo2peak) of 50%–70% of estimated age matched values.2 Assessing and improving the HTx recipients’ physical capacity is therefore essential; it has been shown to predict outcome3 and is also of importance for quality of life.4 5
Both central haemodynamic and peripheral physiological factors contribute to the limited exercise capacity in HTx recipients.6 The dominant central limitation seems to be the impaired chronotropic response, which is most pronounced the first 6 months, with normalisation in the majority of patients thereafter.7 As for the peripheral limitations, skeletal muscles have a great impact on the effect of exercise trough capillary density, mitochondrial enzyme levels and muscle diffusion capacity.6 8
Regular exercise improves physical capacity, and the exercise intensity is found to be crucial for efficiency.9 10 These results are found in both patients with HF and patients with cardiovascular disease explained dominantly by an improvement in cardiac function.10 11 Exercise in HTx patients also shows superior effects of high-intensity interval training (HIT),12–14 but with increased peripheral factors as the dominant effect12 15 including improvement in endothelial function.14 However, the underlying triggers behind the positive effect of HIT in HTx recipients are poorly understood. Studies comparing inflammatory biomarkers before and after exercise interventions in HTx recipients are mostly neutral,14 16 17 but so far, these studies have focused on long-term steady-state levels of inflammatory markers rather than immediate responses during a HIT session.
Dall et al showed that HIT was superior to increase exercise capacity compared with moderate-intensity continuous training (MICT).13 Herein we examined if this beneficial effect was associated with an altered inflammatory response to exercise training. We explored the different immediate inflammatory responses in HTx patients before, during and after a HIT and a MICT session. Our research questions were (1) whether exercise in general triggers an early release of selected vascular, angiogenetic and platelet-derived inflammatory mediators, and (2) whether the response is different between HIT and MICT sessions performed by HTx patients. As a reference, we also compared the HTx inflammatory biomarkers at baseline to a group of healthy controls.
Methods
Patients and settings
All HTx recipients with acceptable travel distances to Oslo University Hospital received written information about the study (n=26), during spring 2015. Other inclusion criteria were age above 18, 1 to 10 years since HTx, stable medical condition and no recent changes in the immunosuppressive treatment. HTx recipients who met the inclusion criteria and were willing to participate were included (n=15). In addition, five participants with no history of heart disease were enrolled as a reference group. One HTx patient got injured after inclusion and was prevented from further participation, resulting in 14 HTx patients and five controls completing the study.
The study was designed as an experimental cross-over study to evaluate the immediate responses in inflammatory mediators during a HIT session compared with a MICT session, carried out in our test laboratory at the Department of Cardiology. The study was approved by the South-East Regional Committee for Medical and Health Research Ethics in Norway (approval no. 2015/97) and by the Department of Privacy and Data Security at Oslo University Hospital.
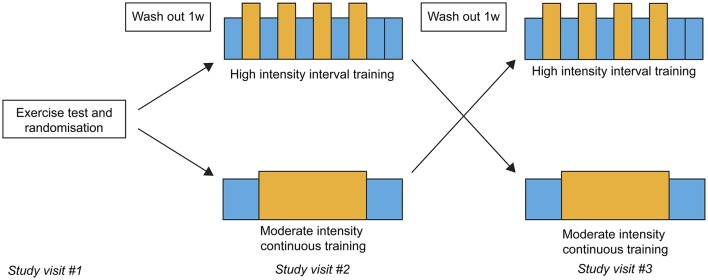
Each study participant had three study visits with 1-week wash-out between each visit (figure 1). The first study visit consisted of baseline blood samples and a maximal cardiopulmonary exercise test (CPET). The next two visits consisted of one HIT session and one MICT session. The order of the exercise sessions was organised using simple randomisation and the patients picked one of the two similar envelopes at their first visit. The CPET results were used to define each patient’s intensity zones: the moderate-intensity and high-intensity zone was defined as their actual heart rate (HR) at 60%–70% and 85%–95% of their peak Vo2, respectively.
Figure 1.
Illustration of the cross-over design of the study.
Cardiopulmonary exercise testing
The participants were tested on a treadmill (Woodway, Waukesha, Wisconsin, USA), monitored with continuous ECG, and blood pressure was measured at regular intervals (Tango-plus; SunTech Medical, Morrisville, North Carolina, USA). ‘Breath by breath’ gas exchange was examined with Jaeger systems (Jaeger-BD, CareFusion, Hoechberg, Germany). We used an individual stepwise protocol, with a 10 min warm-up, based on a modified test protocol from the Working Group on Cardiac Rehabilitation and Exercise Physiology and Working Group on Heart Failure of the European Society of Cardiology.18 The participants reported their rate of perceived exhaustion (RPE) on the Borg scale,6–20 and the speed was increased until the patients reported RPE 11–12 towards the end of the warm-up. To push the participants to peak exercise, the incline or the speed was increased every second minute until exhaustion, defined as RPE 18–19 and respiratory exchange rate (RER) >1.10. The aim of test duration was 8–12 min. The predicted Vo2peak values were based on the American College of Sports Medicine 2014 guidelines.19
Exercise sessions and blood sample management
The blood samples at baseline were drawn at the same time as the initial CPET, 1 week ahead of the first exercise session.
The exercise sessions were monitored with HR sensors (RCX3F; Polar Electro Oy, Kempele, Finland) to ensure that each patient exercised in the correct training zones. Each patient performed both exercise sessions with a 1-week wash-out period between the two sessions. Before exercise, the participant got an intravenous line in their forearm. The patients had four blood samples taken during each session (figure 2A,B): midway through the exercise session, at the end of exercise, and after 1 and 2 hours of recovery. Each sample started with one collection tube that got destroyed, and then two EDTA glasses (chilled on ice) and one collection tube with serum. The two first blood samples were taken while the patients were standing on the treadmill, with a maximum of 1 min interruption of the session.
Figure 2.
A, Illustration of the moderate-intensity continuous exercise session with timing of the blood samples. B, Illustration of the high-intensity interval exercise session with timing of the blood samples.
Blood was centrifuged immediately (plasma) or allowed to clot (serum) for 1 to 2 hours before centrifugation. Plasma was centrifuged at 2100×g for 20 min to obtained platelet-poor plasma and serum at 1900×g for 15 min after clotting, both at 4 ˚C. Plasma and serum were frozen in aliquots at −80 ˚C until analysis and thawed less than three times.
The MICT session (figure 2A) consisted of a 30 min exercise at 60%–70% of peak effort. The first and second blood samples were drawn midway through the session and at the end of the session.
The HIT session (figure 2B) consisted of four blocks of 4 min intervals at 85%–95% of peak effort, intermittent by a 3 min active pause between the intervals. The first blood sample was drawn immediately after the second interval (midway through the session), and the second blood sample was drawn immediately after the last interval. For both sessions, the third and fourth blood samples were drawn after 1 and 2 hours of recovery.
To investigate whether the exercise sessions were completed as per protocol, the main investigator (MY) analysed each patient’s HR graph using the Polar software available online (www.polarpersonaltrainer.com; Polar Electro Oy) and the results were compared with the defined intensity zones for each patient and each healthy control.
Markers of inflammation and angiogenesis
To explore the inflammatory and angiogenetic response to exercise, selected mediators were analysed in duplicate by enzyme immunoassays from R&D Systems (Stillwater, Minnesota, USA) except von Willebrand factor (vWF) (Dako Cytomation, Glostrup, Denmark). For general inflammation, C reactive protein (CRP) and soluble tumour necrosis factor receptor (sTNFr) 1 were measured. For vascular inflammation, we analysed vascular cell adhesion molecule (VCAM)-1 and vWF. To explore platelet-derived inflammation, we analysed platelet-derived growth factor (PDGF), soluble CD40 ligand (sCD40L) and the Dickkopf-related protein 1 (DKK-1). To explore angiogenetic mediators, we analysed vascular endothelial growth factor (VEGF)-1, endostatin, angiopoietin-2 (Ang-2) and its receptor (tyrosine kinase with immunoglobulin-like and EGF-like domains 2 (Tie-2)). The levels of growth differentiation factor (GDF)-15, interleukin 1 receptor-like 1 (ST2), and secreted protein acidic and rich in cysteine (SPARC) were measured as selected cardiokines and myokines. The levels of VCAM-1, CRP, GDF-15 and vWF were measured in plasma, and all remaining biomarkers were analysed in serum. Intra-assay coefficient of variation was <10%.
Statistical analyses
All statistical analyses were performed using SPSS V.22 (IBM, Armonk, New York, USA). Descriptive statistics for continuous variables were presented in mean±SD or median with first and third quartiles (Q1, Q3). Categorical variables were presented in percentages. To compare baseline demographic and inflammatory mediators between HTx and controls, t-test or Mann-Whitney U test was used as appropriate. Categorical variables were compared with Pearson’s χ2 or Fisher’s exact test. The low number of healthy controls limited the possibility to analyse and compare the response to exercise in this group. To evaluate the effect of exercise and the potential differences between HIT and MICT in the HTx population, a two-way repeated-measures analysis of variance (ANOVA) was performed. To evaluate normal distribution, Shapiro-Wilk normality test was used, and variables with skew distribution were log transformed prior to ANOVA. For the evaluation of the time and exercise factor, P values <0.05 were considered significant. For the evaluation of the interaction effect, <0.1 was used as level of significance. Missing data (0.02%) were replaced by the mean value from the other observations and included in the analysis.
Results
Descriptive statistics of the HTx patients and healthy controls are listed in table 1.
Table 1.
Descriptive statistics; heart transplant recipients and healthy controls
| Variables* | HTx (n=14) | Healthy controls (n=5) | P value |
| Sex (% men) | 86 | 80 | 1.000 |
| Age (years) | 53 (13) | 52 (7) | 0.764 |
| In work (% yes) | 71 | 100 | 0.530 |
| Time after HTx (years) | 3 (2) | – | |
| Smoking (% never smoked) | 79 | 100 | 0.530 |
| Exercise variables | |||
| Vo2peak (mL/kg/min) | 31.0 (6.8) | 40.2 (2.7) | 0.001 |
| % of estimated Vo2 | 85 (17) | 111 (15) | 0.014 |
| Peak heart rate | 153 (17) | 182 (14) | 0.004 |
| RER | 1.16 (0.10, 1.22) | 1.23 (1.20, 1.24) | 0.087 |
| VE/VCo2slope | 24 (12) | 25 (3) | 0.791 |
| Peak respiration rate | 46 (9) | 49 (8) | 0.567 |
| O2pulse | 15.7 (3.1) | 17.9 (2.3) | 0.131 |
| Peak systolic BP | 211 (25) | 215 (20) | 0.744 |
| Peak diastolic BP | 92 (30) | 90 (9) | 0.824 |
| Self-reported physical activity | |||
| Exercise frequency (%) ≤once weekly/2–3 weekly/approximately daily |
7/57/36 | 20/80/0 | 0.263 |
| Exercise duration (%) <30 min/>30 min |
14/86 | 25/75 | 1.000 |
| Exercise intensity (%) Low intensity/moderate intensity/maximum intensity |
21/72/7 | 0/75/25 | 0.416 |
| Meeting the recommendations for daily activity† (% yes) | 86 | 60 | 0.272 |
| Inactivity daily (hours) | 6 (3) | 8 (5) | 0.331 |
*Variables are presented as mean (SD) or median (1st quartile, 3rd quartile).
†≥30 min.
BP, blood pressure; HTx, heart transplant recipients; RER, respiratory equivalent rate; VE, ventilation.
The HTx patients were in average 53±13 years, 86% were men and time since HTx was 3±2 years. The healthy controls were in average 52±7 years, and 80% were men. Both completed a CPET, with RER values above 1.10 and a respiratory rate above 40. The HTx recipients had a significantly lower maximal HR and Vo2peak than the healthy controls, with an average Vo2peak level of 31.0±6.8 mL/kg/min representing 85% of the gender-matched and age-matched values. The healthy controls had a Vo2peak level of 40.2±2.7 mL/kg/min, corresponding to 111% of expected values.
Exercise sessions
MICT session
Nine of the total 19 participants were randomised to start with the MICT session. All participants exercised in the correct intensity zone; 60%–70% of their peak capacity. The HTx patients exercised in average at 65%±4% of their peak capacity and the healthy controls at 61%±1% of their peak capacity. During the MICT sessions, we succeeded in drawing all the planned blood samples from all patients.
HIT session
Ten of the total 19 participants were randomised to start with the HIT session. All participants exercised in the correct intensity zone, 85%–95% of their peak capacity. The HTx patients exercised in average at 89%±3% of their peak capacity and the healthy controls at 90%±2% of their peak capacity during the last 2 min of each interval. We missed two of the planned blood samples from one of the HTx recipients due to no flow in the intravenous line after exercise and because other potential veins already were punctured.
Markers of inflammation and angiogenesis
Baseline
At baseline, HTx patients had higher levels of most inflammatory mediators compared with the healthy controls, although only significant for GDF-15 and sTNFr1 potentially due to the low number of included participants (table 2).
Table 2.
Baseline values of inflammatory markers; heart transplant recipients and healthy controls
| Inflammatory biomarkers† | HTx (n=14) |
Healthy controls (n=5) |
P value |
| General inflammation | |||
| C reactive protein‡ | 4.0 (0.5, 6.9) |
1.7 (0.7, 4.7) |
0.687 |
| sTNFr1 | 3.07 (2.68, 3.31) |
2.18 (1.77, 2.62) |
0.026* |
| Vascular inflammation | |||
| von Willebrand factor§ | 187 (92, 288) |
111 (35, 146) |
0.156 |
| VCAM | 489 (402, 616) |
408 (390, 430) |
0.115 |
| Blood platelets | |||
| Platelet-derived growth factor | 1.97 (1.40, 2.57) |
2.87 (1.27, 4.16) |
0.559 |
| sCD40L | 0.98 (0.66, 1.49) |
1.59 (1.17, 1.88) |
0.070 |
| DKK-1 | 2.08 (1.03, 4.28) |
1.28 (0.58, 3.71) |
0.440 |
| Angiogenesis | |||
| Vascular endothelial growth factor-1 | 0.19 (0.14, 0.28) |
0.15 (0.14, 0.20) |
0.298 |
| Angiopoietin-2 | 1.30 (1.08, 2.11) |
1.47 (1.11, 1.98) |
0.823 |
| Tie-2 | 15.9 (13.0, 17.7) |
15.8 (13.4, 15.9) |
0.817 |
| Endostatin | 162 (135, 200) |
138 (114, 145) |
0.165 |
| Cardiokine/myokine | |||
| GDF-15 | 1.14 (0.61, 1.41) |
0.36 (0.32, 0.83) |
0.019* |
| ST2 | 17.5 (15.2, 26.9) |
22.4 (17.0, 33.1) |
0.247 |
| SPARC | 306 (127, 482) |
212 (98, 909) |
0.823 |
*P value ≤0.05 between groups.
†All variables are presented as median with (1st quartile, 3rd quartile) and measured in ng/mL serum if not otherwise stated. ‡Measured as μg/mL.
§Measured as per cent of pool.
DKK-1, Dickkopf-related protein 1; GDF-15, growth differentiation factor-15; HTx, heart transplant recipients; sCD40L, soluble CD40 ligand; SPARC, secreted protein acidic and rich in cysteine; ST2, interleukin 1 receptor-like 1; sTNFr1, soluble tumour necrosis factor receptor 1; Tie-2, tyrosine kinase with immunoglobulin-like and EGF-like domains 2; VCAM, vascular cell adhesion molecule.
Exercise
As shown in table 3, exercise, regardless of intensity, caused a significant response in systemic inflammation as assessed by sTNFr1, platelet-derived inflammation as assessed by PDGF, DKK-1 and sCD40L, angiogenetic activation as assessed by VEGF-1 and Ang-2, and release of myokines as assessed by SPARC.
Table 3.
Analysis of variance (ANOVA) results for all inflammatory markers in the heart transplant recipients
| Variables† n=14 HTx | Baseline‡ | 15 min | 30 min | 1 hour | 2 hours | Time | Group | Time×group | |
| General inflammation | |||||||||
| CRP§ | MICT | 3.40±1.10 | 2.85±0.80 | 2.74±0.78 | 2.47±0.61 | 2.93±0.69 | 0.601 | 0.362 | 0.422 |
| HIT | 4.10±0.96 | 3.9±0.94 | 3.58±0.88 | 3.46±0.83 | |||||
| sTNFr1¶ | MICT | 3.08±0.25 | 3.17±0.16 | 3.39±0.20 | 3.09±0.21 | 2.91±0.17 | <0.001** | 0.616 | 0.112 |
| HIT | 3.38±0.24 | 3.56±0.20 | 2.76±0.16 | 2.74±0.18 | |||||
| Vascular inflammation | |||||||||
| vWF†† | MICT | 191±31 | 117±17 | 131±20 | 138±23 | 173±28 | 0.175 | 0.044 | 0.075* |
| HIT | 179±29 | 239±48 | 151±26 | 151±23 | |||||
| VCAM | MICT | 511±36 | 527±36 | 486±29 | 458±29 | 468±26 | 0.069 | 0.672 | 0.422 |
| HIT | 495±23 | 507±32 | 446±20 | 460±25 | |||||
| Blood platelets | |||||||||
| PDGF | MICT | 2.08±0.22 | 2.48±0.33 | 2.72±0.33 | 2.22±0.28 | 2.17±0.21 | 0.002** | 0.162 | 0.690 |
| HIT | 2.78±0.40 | 3.00±0.43 | 2.33±0.24 | 2.33±0.30 | |||||
| sCD40L | MICT | 1.07±0.15 | 1.29±0.18 | 1.26±0.18 | 1.04±0.15 | 1.06±0.17 | <0.001** | 0.047 | 0.237 |
| HIT | 1.41±0.20 | 1.46±0.21 | 1.20±0.18 | 1.14±0.16 | |||||
| DKK-1 | MICT | 2.85±0.62 | 3.15±0.84 | 3.49±0.87 | 3.00±0.69 | 2.80±0.71 | 0.023** | 0.995 | 0.130 |
| HIT | 3.42±0.86 | 4.13±0.83 | 2.15±0.49 | 2.74±0.44 | |||||
| Angiogenesis | |||||||||
| VEGF-1¶ | MICT | 0.24±0.03 | 0.26±0.04 | 0.26±0.04 | 0.24±0.03 | 0.26±0.03 | 0.020** | 0.275 | 0.077* |
| HIT | 0.29±0.04 | 0.29±0.04 | 0.24±0.03 | 0.24±0.03 | |||||
| Ang-2¶ | MICT | 1.60±0.20 | 1.97±0.26 | 2.06±0.22 | 1.74±0.20 | 1.75±0.21 | <0.001** | 0.415 | 0.095* |
| HIT | 1.97±0.26 | 2.06±0.22 | 1.75±0.20 | 1.64±0.21 | |||||
| Tie-2 | MICT | 15.8±1.3 | 15.2±1.7 | 15.2±1.4 | 16.5±1.5 | 15.9±1.2 | 0.632 | 0.362 | 0.250 |
| HIT | 16.2±1.6 | 16.3±1.6 | 16.3±1.5 | 15.4±1.7 | |||||
| Endostatin | MICT | 165±11 | 166±12 | 169±12 | 163±9 | 159±11 | 0.206 | 0.167 | 0.594 |
| HIT | 184±14 | 183±13 | 170±13 | 162±12 | |||||
| Cardiokine/myokine | |||||||||
| GDF-15 | MICT | 1.03±0.11 | 1.20±0.19 | 1.19±0.19 | 1.12±0.19 | 1.10±0.19 | 0.206 | 0.041 | 0.067* |
| HIT | 1.10±0.11 | 1.15±0.11 | 0.96±0.11 | 0.93±0.08 | |||||
| ST2 | MICT | 20.3±2.0 | 20.0±1.9 | 20.5±2.1 | 20.1±1.8 | 20.7±2.2 | 0.425 | 0.112 | 0.228 |
| HIT | 22.4±2.2 | 22.7±2.2 | 21.2±2.0 | 21.3±2.4 | |||||
| SPARC | MICT | 319±57 | 486±60 | 433±95 | 297±64 | 294±63 | 0.020** | 0.122 | 0.745 |
| HIT | 391±79 | 491±84 | 336±60 | 328±56 | |||||
*P value <0.10.
**Significant P values <0.05.
†All variables are measured in ng/mL serum if not otherwise stated.
‡Results from 1 week ahead of first exercise session.
§Measured as μg/mL.
¶Analysis of variance was performed with log-transformed variables.
††Measured as per cent of pool.
Ang-2, angiopoietin-2; CRP, C reactive protein; DKK-1, Dickkopf-related protein 1; GDF-15, growth differentiation factor-15; HIT, high-intensity interval training; HTx, heart transplant recipients; MICT, moderate-intensity continuous training; PDGF, platelet-derived growth factor; sCD40L, soluble CD40 ligand; SPARC, secreted protein acidic and rich in cysteine; ST2, interleukin 1 receptor-like 1; sTNF1, soluble tumour necrosis factor receptor 1; Tie-2, tyrosine kinase with immunoglobulin-like and EGF-like domains 2; VCAM, vascular cell adhesion molecule; VEGF-1, vascular endothelial growth factor-1; vWF, von Willebrand factor.
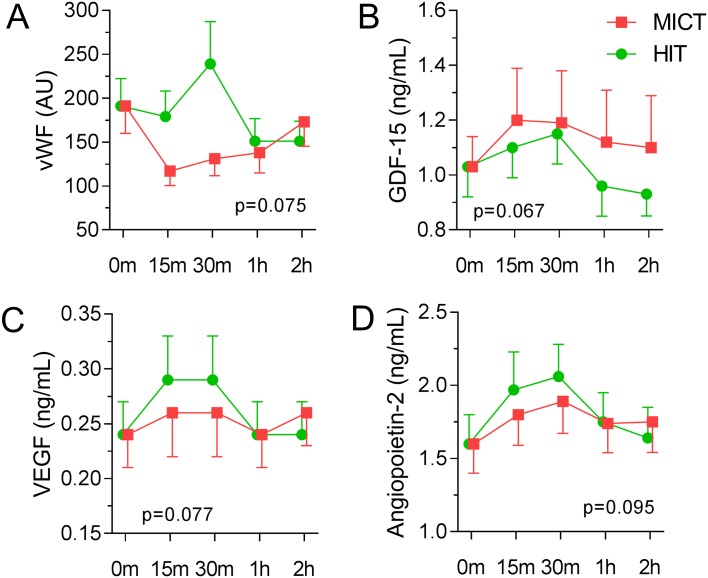
As shown in figure 3, we found trend towards significant differences when the temporal course of the marker was evaluated in relation to exercise intensity (evaluated as P values <0.1). First, vWF increased during HIT while a decrease was observed during MICT, resulting in a marked difference between the two exercise modalities. In contrast, GDF-15 showed a decrease during the recovery period of the HIT session with a significant interaction effect compared with the MICT session. Of the angiogenetic biomarkers, VEGF-1 and Ang-2 had a higher activation during the HIT session compared with the MICT session. The remaining biomarkers also showed a numerically higher response during HIT compared with MICT, although these were non-significant (table 3).
Figure 3.
Graphic presentation of growth differentiation factor-15 (GDF-15), von Willebrand factor (vWF), vascular endothelial growth factor (VEGF)-1 and angiopoietin-2 during exercise and recovery with high and moderate intensity in the heart transplant recipients, with inserted P value for the analysis of variance interaction effect, exercise session×time. Level of significance: P value <0.1. HIT, high-intensity interval training; MICT, moderate-intensity continuous training.
Discussion
The main findings in this study were that (1) exercise, regardless of intensity, induced a significant immediate response in several systemic, angiogenetic and in particular platelet-derived inflammatory mediators in HTx recipients. (2) HIT showed trends to induce an increased response in vWF, VEGF-1 and Ang-2, and a decreased response in GDF-15, compared with MICT. The trend towards the increased angiogenetic mediator response during HIT could contribute to the beneficial effects of this form of exercise in HTx recipients.
In our study, exercise training, irrespective of intensity, led to an increase in multiple systemic, angiogenetic and platelet-derived inflammatory mediators. These results are in line with published research showing a procoagulation state with blood-platelet activation potentially reflecting an increase in catecholamines20 and endothelial activation, promoting NO production related to the increased shear stress.21 These effects are shown to improve the endothelium-dependent vasodilatation and can regulate the growth and repair of blood vessels.22 When evaluating the angiogenesis in skeletal muscles, VEGF has been found to be the most central factor and upregulates with exercise.23 This study explored for the first time the immediate differences between HIT and MICT in HTx recipients. The finding of HIT causing a clear trend towards an increase in mediators of angiogenesis, including VEGF, provides new knowledge about the potential mechanisms behind the effect of HIT in HTx recipients.
The endothelial activation from exercise, measured as increased vWF, was only present during the HIT session in HTx recipients. Exercise in healthy individuals is shown to result in a rapid rise of circulating vWF,24 and the rise in vWF as a response to exercise has been suggested as a novel and reliable test of endothelial function.25 Our results may suggest that in HTx patients, who have a higher degree of endothelial dysfunction,26 higher exercise intensity is needed in order to successfully activate the endothelium. However, an increase in vWF during HIT could also reflect an inappropriate activation of these cells, and the impact of this finding is at present unclear. In contrast, HIT was associated with a decrease in GDF-15, potentially reflecting improved endothelial function and decreased vascular stiffness.27 However, the effect of HIT on endothelial function in HTx recipients is at present unclear, and this issue should be further clarified in larger studies.
Activation of the endothelium and thereby induction of capillary growth in skeletal muscle through proangiogenetic mechanisms may play an important role in the beneficial effects of HIT. Kilian et al demonstrated an increase in mRNA for VEGF in whole blood during HIT in healthy children.28 Herein, we have, to the best of our knowledge, for the first time observed trends towards a higher response in both Ang-2 and VEGF-1 during HIT in HTx recipients potentially promoting neovascularisation. VEGF is dominantly secreted by working skeletal muscles, an essential factor to increase capillary density, oxygen delivery and thereby exercise performance.29 30 Ang-2, in the presence of VEGF, facilitates sprouting of new vessels from existing vessels.31 In healthy individuals, increased capillary density is documented as a result of endurance training,32 and we can assume that this effect is present in HTx recipients as they achieve improved muscular exercise capacity after a HIT intervention.12 The trends towards increased angiogenetic response by Ang-2 and VEGF shown in the present study could potentially contribute to the higher Vo2peak levels after a HIT intervention. In relation to this, the exercise-induced response of VEGF is also shown to stimulate neurogenesis in cerebellar tissue33 and as a potential drug to decrease infarct size.34
Limitations
The study was designed as a cross-over study and the risk of a carry-over effect is possible. Based on our findings, we evaluate this risk as low due to the numerical fall in the inflammation markers to baseline levels already at 2 hours after exercise cessation and the 1-week wash-out time between the visits. The physical capacity measured after HTx was higher than documented in international publications and could possibly be a result of selection bias. On the contrary, we would expect a somewhat higher performance in our population due to worldwide differences.35 36 The number of included patients and in particular healthy controls were rather low and the number of mediators analysed was relatively high, and some of the findings (both negative and positive) could be by chance. Moreover, there are considerable overlap between the different ‘cytokine categories’ (eg, VEGF are derived from platelets with angiogenetic effects), but the division into these categories may still be meaningful from a functional point of view. Finally, the correlation between different responses does not necessarily imply a causal relationship, and more mechanistic studies are needed to substantiate if the beneficial effects of HIT could be mediated through angiogenetic factors in HTx recipients.
Conclusions
To the best of our knowledge, this study is the first that compare the immediate inflammatory responses of HIT and MICT sessions in HTx recipients. In HTx recipients, exercise, regardless of intensity, induces an immediate inflammatory response, especially through activation of blood platelets and modulation of angiogenesis and neovascularisation. The trends in our findings suggest that HIT can induce a rise in proangiogenetic mediators that are beneficial for new blood vessel formations. Future studies with larger sample sizes are necessary to verify our results and to evaluate if these immediate responses of inflammatory biomarkers may have an impact on long-term outcomes in this population.
Acknowledgments
We would like to express special thanks to the study participants who completed the study with enthusiasm.
Footnotes
Contributors: MY (corresponding author) was the main researcher of this cross-over study and contributed to the design of the study, inclusion of patients, data collection with the exercise sessions and physical tests, data analysis and drafting the article. TU evaluated the markers of inflammation and angiogenesis that were analysed, contributed with the statistics and the data interpretation, tables and figures, and critically revised the manuscript. AM contributed to the analysis of the inflammatory markers from all blood samples and critically revised the manuscript. PA contributed with the data interpretation and critically revised the manuscript. EB contributed with the coordination of the study participants and drawing blood samples at study visits, and critically revised the manuscript. LG contributed to the design of the study and data interpretation, and critically revised the manuscript. KN contributed to the design and the data interpretation, and drafted and critically revised the manuscript.
Funding: This work was funded by student research grants from the Norwegian Health Association and the South-East Regional Health Authority in Norway.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: South-East Regional Committee for Medical and Health Research Ethics in Norway (approval no. 2015/97).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Stehlik J, Edwards LB, Kucheryavaya AY, et al. . The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report—2012. J Heart Lung Transplant 2012;31:1052–64. 10.1016/j.healun.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 2. Hsieh PL, Wu YT, Chao WJ. Effects of exercise training in heart transplant recipients: a meta-analysis. Cardiology 2011;120:27–35. 10.1159/000332998 [DOI] [PubMed] [Google Scholar]
- 3. Yardley M, Havik OE, Grov I, et al. . Peak oxygen uptake and self-reported physical health are strong predictors of long-term survival after heart transplantation. Clin Transplant 2016;30:161–9. 10.1111/ctr.12672 [DOI] [PubMed] [Google Scholar]
- 4. Karapolat H, Eyigor S, Durmaz B, et al. . The relationship between depressive symptoms and anxiety and quality of life and functional capacity in heart transplant patients. Clin Res Cardiol 2007;96:593–9. 10.1007/s00392-007-0536-6 [DOI] [PubMed] [Google Scholar]
- 5. Buendía F, Almenar L, Martínez-Dolz L, et al. . Relationship between functional capacity and quality of life in heart transplant patients. Transplant Proc 2011;43:2251–2. 10.1016/j.transproceed.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 6. Marconi C, Marzorati M. Exercise after heart transplantation. Eur J Appl Physiol 2003;90:250–9. 10.1007/s00421-003-0952-x [DOI] [PubMed] [Google Scholar]
- 7. Nytrøen K, Myers J, Chan KN, et al. . Chronotropic responses to exercise in heart transplant recipients: 1-yr follow-up. Am J Phys Med Rehabil 2011;90:579–88. 10.1097/PHM.0b013e31821f711d [DOI] [PubMed] [Google Scholar]
- 8. Squires RW. Exercise therapy for cardiac transplant recipients. Prog Cardiovasc Dis 2011;53:429–36. 10.1016/j.pcad.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 9. Elliott AD, Rajopadhyaya K, Bentley DJ, et al. . Interval training versus continuous exercise in patients with coronary artery disease: a meta-analysis. Heart, Lung and Circulation 2015;24:149–57. 10.1016/j.hlc.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 10. Wisløff U, Støylen A, Loennechen JP, et al. . Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 2007;115:3086–94. 10.1161/CIRCULATIONAHA.106.675041 [DOI] [PubMed] [Google Scholar]
- 11. Kemi OJ, Wisloff U. High-intensity aerobic exercise training improves the heart in health and disease. J Cardiopulm Rehabil Prev 2010;30:2–11. 10.1097/HCR.0b013e3181c56b89 [DOI] [PubMed] [Google Scholar]
- 12. Nytrøen K, Rustad LA, Aukrust P, et al. . High-intensity interval training improves peak oxygen uptake and muscular exercise capacity in heart transplant recipients. Am J Transplant 2012;12:3134–42. 10.1111/j.1600-6143.2012.04221.x [DOI] [PubMed] [Google Scholar]
- 13. Dall CH, Snoer M, Christensen S, et al. . Effect of high-intensity training versus moderate training on peak oxygen uptake and chronotropic response in heart transplant recipients: a randomized crossover trial. Am J Transplant 2014;14:2391–9. 10.1111/ajt.12873 [DOI] [PubMed] [Google Scholar]
- 14. Hermann TS, Dall CH, Christensen SB, et al. . Effect of high intensity exercise on peak oxygen uptake and endothelial function in long-term heart transplant recipients. Am J Transplant 2011;11:536–41. 10.1111/j.1600-6143.2010.03403.x [DOI] [PubMed] [Google Scholar]
- 15. Rustad LA, Nytrøen K, Amundsen BH, et al. . One year of high-intensity interval training improves exercise capacity, but not left ventricular function in stable heart transplant recipients: a randomised controlled trial. Eur J Prev Cardiol 2014;21:181–91. 10.1177/2047487312469477 [DOI] [PubMed] [Google Scholar]
- 16. Nytrøen K, Rustad LA, Erikstad I, et al. . Effect of high-intensity interval training on progression of cardiac allograft vasculopathy. J Heart Lung Transplant 2013;32:1073–80. 10.1016/j.healun.2013.06.023 [DOI] [PubMed] [Google Scholar]
- 17. Dall CH, Gustafsson F, Christensen SB, et al. . Effect of moderate- versus high-intensity exercise on vascular function, biomarkers and quality of life in heart transplant recipients: a randomized, crossover trial. J Heart Lung Transplant 2015;34:1033–41. 10.1016/j.healun.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 18. Working Group on Cardiac Rehabilitation & Excercise Physiology and Working Group on Heart Failure of the European Society of Cardiology. Recommendations for exercise testing in chronic heart failure patients. Eur Heart J 2001;22:37–45. 10.1053/euhj.2000.2388 [DOI] [PubMed] [Google Scholar]
- 19. Pescatello L, Arena R, Riebe D, et al. . American College of Sports Medicine ACSM’s guidelines for exercise testing and prescription. 9th ed Baltimore: Wolters Kluwer, Lippincott, Williams, 2014. [DOI] [PubMed] [Google Scholar]
- 20. Wallén NH, Goodall AH, Li N, et al. . Activation of haemostasis by exercise, mental stress and adrenaline: effects on platelet sensitivity to thrombin and thrombin generation. Clin Sci 1999;97:27–35. 10.1042/cs0970027 [DOI] [PubMed] [Google Scholar]
- 21. Crimi E, Ignarro LJ, Cacciatore F, et al. . Mechanisms by which exercise training benefits patients with heart failure. Nat Rev Cardiol 2009;6:292–300. 10.1038/nrcardio.2009.8 [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Li M, Dong F, et al. . Physical exercise-induced protection on ischemic cardiovascular and cerebrovascular diseases. Int J Clin Exp Med 2015;8:19859–66. [PMC free article] [PubMed] [Google Scholar]
- 23. Olfert IM, Baum O, Hellsten Y, et al. . Advances and challenges in skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol 2016;310:H326–H336. 10.1152/ajpheart.00635.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wagner DD. Cell biology of von Willebrand factor. Annu Rev Cell Biol 1990;6:217–42. 10.1146/annurev.cb.06.110190.001245 [DOI] [PubMed] [Google Scholar]
- 25. Balen S, Ruzić A, Mirat J, et al. . Exercise induced von Willebrand Factor release—new model for routine endothelial testing. Med Hypotheses 2007;69:1320–2. 10.1016/j.mehy.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 26. Holm T, Aukrust P, Andreassen AK, et al. . Peripheral endothelial dysfunction in heart transplant recipients: possible role of proinflammatory cytokines. Clin Transplant 2000;14:218–25. 10.1034/j.1399-0012.2000.140307.x [DOI] [PubMed] [Google Scholar]
- 27. Andersson C, Enserro D, Sullivan L, et al. . Relations of circulating GDF-15, soluble ST2, and troponin-I concentrations with vascular function in the community: the Framingham heart study. Atherosclerosis 2016;248:245–51. 10.1016/j.atherosclerosis.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kilian Y, Wehmeier UF, Wahl P, et al. . Acute response of circulating vascular regulating microRNAs during and after high-intensity and high-volume cycling in children. Front Physiol 2016;7:92 10.3389/fphys.2016.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoier B, Hellsten Y. Exercise-induced capillary growth in human skeletal muscle and the dynamics of VEGF. Microcirculation 2014;21:301–14. 10.1111/micc.12117 [DOI] [PubMed] [Google Scholar]
- 30. Delavar H, Nogueira L, Wagner PD, et al. . Skeletal myofiber VEGF is essential for the exercise training response in adult mice. Am J Physiol Regul Integr Comp Physiol 2014;306:R586–95. 10.1152/ajpregu.00522.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arany Z, Foo SY, Ma Y, et al. . HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 2008;451:1008–12. 10.1038/nature06613 [DOI] [PubMed] [Google Scholar]
- 32. Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev 1992;72:369–417. [DOI] [PubMed] [Google Scholar]
- 33. Fabel K, Fabel K, Tam B, et al. . VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 2003;18:2803–12. 10.1111/j.1460-9568.2003.03041.x [DOI] [PubMed] [Google Scholar]
- 34. Sun Y, Jin K, Xie L, et al. . VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 2003;111:1843–51. 10.1172/JCI200317977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaminsky LA, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the Fitness Registry and the Importance of Exercise National Database. Mayo Clin Proc 2015;90:1515–23. 10.1016/j.mayocp.2015.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loe H, Rognmo Ø, Saltin B, et al. . Aerobic capacity reference data in 3816 healthy men and women 20–90 years. PLoS One 2013;8:e64319 10.1371/journal.pone.0064319 [DOI] [PMC free article] [PubMed] [Google Scholar]