Abstract
Purpose
A Quality-adjusted Time WIthout Symptoms of disease or Toxicity (QTWiST) analysis was carried out to assess quality-adjusted survival time in the RECOURSE trial of trifluridine/tipiracil versus placebo in pretreated metastatic colorectal cancer (mCRC).
Methods
Duration of overall survival in the RECOURSE trial (n=798 patients) was partitioned into three discrete health states: toxicity (TOX), time without symptoms or toxicity (TWIST) and relapse (REL). TOX was defined as time spent with grade 3 or 4 treatment-related adverse events (AEs) after randomisation and before progression or censoring. AEs were limited to those related to trifluridine/tipiracil and known to affect quality of life (QoL) (ie, nausea, vomiting, diarrhoea, fatigue/asthaenia, anorexia and febrile neutropaenia). The estimated mean duration of each state, weighted by a utility coefficient representing QoL, was combined into a global QTWiST score.
Results
In the RECOURSE trial, overall survival was 7.1 months with trifluridine/tipiracil versus 5.3 months with placebo. Patients receiving trifluridine/tipiracil spent longer in each health state than placebo recipients. Using assumed utility coefficients of 1 for TWIST and 0.5 for TOX and REL, the QTWiST was 5.48 months for the trifluridine/tipiracil group and 3.98 months for the placebo group, a difference of 1.5 (95% CI 1.49 to 1.52) months in favour of trifluridine/tipiracil. A sensitivity analysis using large variations in utility coefficients for TOX and REL produced a range of only approximately 0.5 months from minimum to maximum QTWiST.
Conclusions
Quality-adjusted survival, as measured by QTWiST, shows clinically meaningful improvements in patients treated with trifluridine/tipiracil versus placebo in pretreated mCRC.
Keywords: metastatic colorectal cancer, trifluridine/tipiracil, QTWIST, quality of life
Key questions.
What is already known about this subject?
The impact of trifluridine/tipiracil on patients with pretreated metastatic colorectal cancer (mCRC) was assessed in the phase III, randomised, double-blind, placebo-controlled RECOURSE trial. Though the trial reported positive results in terms of survival and safety, it did not include direct measurement of quality of life (QoL) through standard questionnaires.
Quality-adjusted Time WIthout Symptoms of disease or Toxicity (QTWiST) is a method for incorporating QoL into survival estimates by establishing a trade-off between time spent with treatment-related adverse events and improvement in progression-free survival.
What does this study add?
Patients receiving trifluridine/tipiracil spent longer in all three QTWiST health states (toxicity, time without symptoms or toxicity and relapse) than placebo recipients.
Using assumed utility coefficients of 1 for time without symptoms or toxicity, 0.5 for toxicity and 0.5 for relapse, QTWiST was 5.48 months for trifluridine/tipiracil patients and 3.98 months for placebo patients; that is, a difference of 1.5 months in favour of trifluridine/tipiracil.
Analysis by QTWiST indicates clinically meaningful improvements in quality-adjusted survival in patients with mCRC treated with trifluridine/tipiracil versus placebo.
How might this impact on clinical practice?
QoL is an important consideration in the management of patients in the later lines of treatment of mCRC.
Further research is necessary, with more formal measurement of QoL in clinical trials and observational studies of trifluridine/tipiracil, to confirm our findings.
Introduction
Metastatic colorectal cancer (mCRC) is typically associated with a high symptom burden and poor quality of life (QoL). Patients may suffer CRC symptoms, such as constipation, diarrhoea, abdominal pain, anorexia and fatigue, as well as additional symptoms related to the location of metastases,1 such as nausea, malaise and jaundice caused by liver metastases.2 The side effects of treatment also frequently contribute to symptom burden. During chemotherapy, adverse events (AEs), such as nausea, vomiting, diarrhoea, dysgeusia and fatigue or asthaenia can severely affect patients’ QoL.3–6 In the setting of mCRC in patients who have progressed after more than one previous line of therapy, survival gains with further treatment are generally modest.7 There is increasing interest in determining the effects of later-stage treatments on QoL as well as survival, so that patients and physicians can make informed decisions about the trade-off between extending survival and the likely quality of the extra life gained.7 8
Trifluridine/tipiracil is an orally administered combination of the antineoplastic, thymidine-based nucleoside analogue trifluridine and the thymidine phosphorylase inhibitor tipiracil, in a molar ratio of 1:0.5.9 10 It has been approved in Europe, the USA and Japan for the treatment of patients who had been previously treated with, or are not considered candidates for, available therapies, including fluoropyrimidine-based, oxaliplatin-based and irinotecan-based chemotherapies, anti-VEGF agents and (if RAS wild type) anti-epidermal growth factor receptor (EGFR) agents.11 The efficacy and safety of trifluridine/tipiracil was assessed in the RECOURSE trial, a phase III, randomised, double-blind, placebo-controlled multicentre trial in patients with pretreated mCRC.12 Median overall survival (OS) was 7.1 months, compared with 5.3 months with placebo (hazard ratio (HR) for death 0.68 (95% CI 0.58 to 0.81); P<0.0001). However, the study end points in the RECOURSE trial did not include a measurement of QoL. To explore OS in the RECOURSE trial adjusted for QoL, we carried out a post hoc analysis using the Quality-adjusted Time WIthout Symptoms of disease or Toxicity (QTWiST) method.13 QTWiST is an established method for incorporating a measure of health-related QoL into survival estimates and is frequently used to evaluate outcomes in oncology trials.14
Methods
Study population and treatments
The data source for this analysis was the RECOURSE trial.12 Patients enrolled in the RECOURSE trial had documented mCRC and had received prior treatment with ≥2 lines of chemotherapy, including an anti-EGFR antibody in those who had wild-type KRAS tumours. Patients were randomised in a 2:1 ratio to receive trifluridine/tipiracil or placebo in addition to best supportive care and received treatment on days 1–5 and 8–12 of each 28-day cycle. Patients randomised to trifluridine/tipiracil received a dose of 35 mg/m2 twice daily. If dose reduction was required, this occurred in 5 mg/m2 steps no more than three times over the treatment period. Patients continued in the study until they experienced unacceptable toxicity or disease progression, or until they decided to stop treatment. The primary end point was OS. Patients treated with trifluridine/tipiracil experienced an average 1.8-month improvement in survival versus placebo (median OS was 7.1 months and 5.3 months, respectively).
QTWiST analysis
QTWiST is, in principle, a quality-adjusted life-year metric.15 The duration of OS is partitioned into three discrete health states: toxicity (TOX), time without symptoms or toxicity (TWIST) and relapse (REL). An estimate of the mean duration of each health state, weighted by a utility coefficient representing QoL, is combined into a global QTWiST score. Thus, the measure incorporates a trade-off between time spent with treatment-related AEs and improvement in progression-free survival (PFS).
Definition of health states
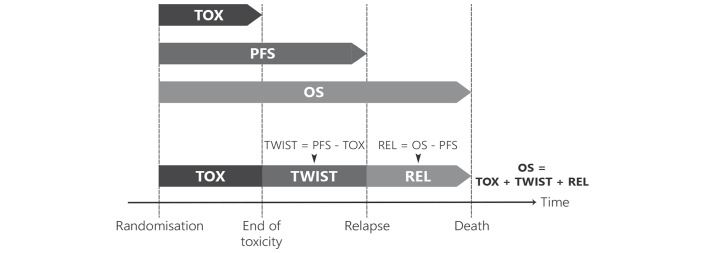
The duration of the three health states was derived as described below; a schematic is shown in figure 1. TOX was defined as time spent with grade 3 or 4 treatment-related AEs after randomisation and before disease progression or censoring for progression. AEs used in the definition were limited to those known to be related to trifluridine/tipiracil and to have an impact on QoL: these were nausea, vomiting, diarrhoea, fatigue/asthaenia, anorexia and febrile neutropaenia. For the TOX state, the number of days with grade 3/4 qualifying AEs before disease progression or censoring date was summed for each patient, based on the AE onset and end dates reported in the trial database. TOX counted calendar time: if a patient experienced several qualifying AEs on the same day then the day was counted once. For patients with censored progression, the duration of TOX was also censored, as the total duration of TOX was unknown.
Figure 1.
Definition of health states. OS, overall survival; PFS, progression-free survival; REL, relapse state (from progression until death); TOX, toxicity related to grade 3/4 adverse nausea, vomiting, diarrhea, fatigue/asthaenia, anorexia, or febrile neutropaenia before progression; TWIST, time without symptoms or toxicity.
TWIST was defined as the time without symptoms or toxicity before disease progression (symptoms are defined as clinical or radiological progression). REL was defined as the time between disease progression and either death or censoring.
QTWiST calculation
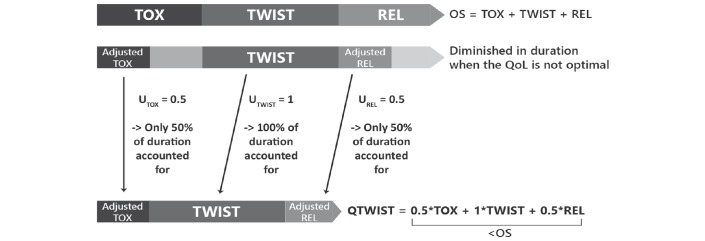
An overview of the QTWiST method is shown in figure 2. The product-limit method was used to estimate the mean duration of TOX, PFS and OS. Kaplan-Meier survival curves corresponding to each survival outcome were plotted on a single graph for each treatment group, with areas between curves representing the restricted mean durations of the health states. Estimates were restricted to the median follow-up time of the RECOURSE trial. The analysis was carried out using the safety-analysis population from the RECOURSE trial, which consisted of all randomised patients who received at least one dose of study treatment.12
Figure 2.
Overview of the QTWiST method. OS, overall survival; PFS, progression-free survival; QoL, quality of life; QWiST, Quality-adjusted Time WIthout Symptoms of disease or Toxicity; REL, relapse state (from progression until death); TOX, toxicity related to grade 3/4 adverse nausea, vomiting, diarrhea, fatigue/asthaenia, anorexia, or febrile neutropaenia before progression; TWIST, time without symptoms or toxicity.
For each treatment arm, QTWiST was calculated as follows: QTWiST = =(uTOXxTOX) + (uTWISTxTWIST) + (uRELxREL).
uTOX, uTWIST and uREL are utility coefficients and represent the QoL associated with each health state. They can vary between 0 and 1, with 0 being death and 1 being the best QoL experienced during the study period. Since QoL is assumed to be best during the TWIST period and diminished during the TOX and REL health states, uTWIST is generally set as 1 for the purpose of QTWiST analyses. We assigned a utility coefficient of 0.5 to TOX and REL (figure 2).
To test the null hypothesis of no difference in QTWiST between treatment groups, a 95% CI and two-sided P values were calculated based on normal approximations, with standard errors calculated using the bootstrap method. To test the sensitivity of the results to the utility coefficient assumptions, a threshold analysis was performed in which the utility coefficients for TOX and REL were varied between 0 and 1.
Results
The RECOURSE safety population consisted of 798 patients in total: 533 treated with trifluridine/tipiracil and 265 with placebo.12 Median follow-up for the purposes of this analysis was 11.8 months.
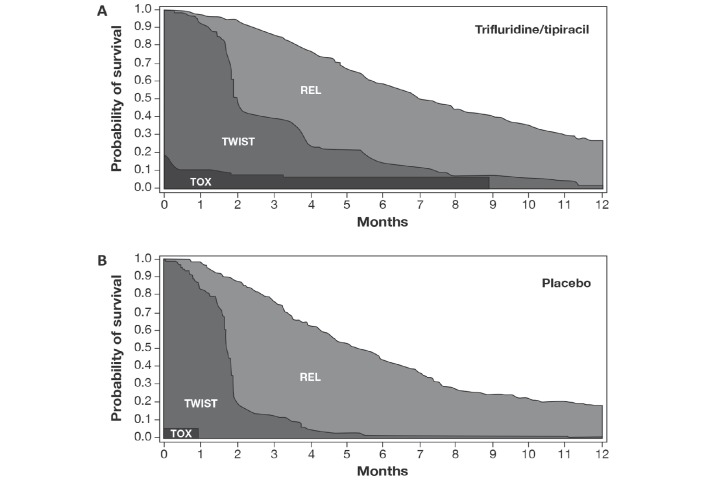
Partitioned survival plots for each treatment group are presented in figure 3 and the mean duration of each health state is shown in table 1. Patients receiving trifluridine/tipiracil spent longer in each health state than placebo recipients. Using the health state durations in table 1 with the assumed utility coefficients of 1 for uTWIST and 0.5 for uTOX and uREL, the QTWiST was 5.48 months for the trifluridine/tipiracil group and 3.98 months for the placebo group.
Figure 3.
Partitioned survival plots for the (A) trifluridine/tipiracil group and (B) placebo group in RECOURSE. REL, relapse state (from progression until death); TOX, toxicity related to grade 3/4 adverse nausea, vomiting, diarrhea, fatigue/asthaenia, anorexia, or febrile neutropaenia before progression; TWIST, time without symptoms or toxicity.
Table 1.
Mean duration of health states by treatment group (months)
| Duration of health state | Trifluridine /tipiracil (n=533) |
Placebo (n=265) |
Between-group difference |
| TOX | 0.92 | 0.70 | 0.22 |
| TWIST | 2.56 | 1.28 | 1.29 |
| REL | 4.92 | 4.70 | 0.22 |
REL, relapse state (from progression until death); TOX, toxicity related to grade 3/4 adverse nausea, vomiting, diarrhoea, fatigue/asthaenia, anorexia or febrile neutropaenia before progression; TWIST, time without symptoms or toxicity.
The difference in QTWiST between the two groups was 1.5 (95% CI 1.49 to 1.52) months in favour of trifluridine/tipiracil. This difference is statistically significant, as indicated by the fact that the lower bound of the 95% CI exceeds 1. Varying the TOX and REL utility coefficients produced a difference in QTWiST between groups ranging from 1.28 months to 1.73 months, always in favour of trifluridine/tipiracil (figure 4).
Figure 4.
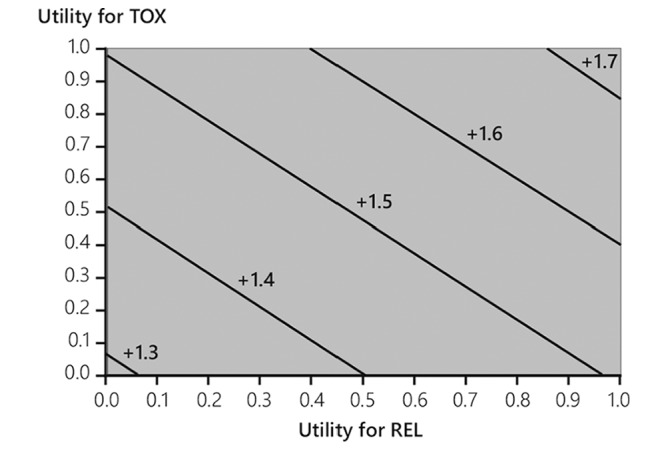
Threshold utility plot: Quality-adjusted Time WIthout Symptoms of disease or Toxicity difference (trifluridine/tipiracil vs placebo) after a median follow-up of 11.8 months. REL, relapse state (from progression until death); TOX, toxicity related to grade 3/4 adverse nausea, vomiting, diarrhea, fatigue/asthaenia, anorexia, or febrile neutropaenia before progression.
Discussion
Our analysis showed a statistically significant improvement of 1.5 months (95% CI 1.49 to 1.52) in quality-adjusted OS, as measured by QTWiST, for patients receiving trifluridine/tipiracil for refractory mCRC compared with placebo. The OS difference before quality adjustment was 1.8 months. The QTWiST analysis shows that most of this survival gain was spent in a health state in which patients were not experiencing major toxicities as measured by reported grade 3 or 4 AEs.
It has been suggested that the minimum clinically important difference in QTWiST values is 10% of the OS and that values of 15% are clearly clinically important.14 The QTWiST improvement with trifluridine/tipiracil in the RECOURSE trial was 1.5 months out of an OS difference of 1.8 months. Thus, by these criteria, trifluridine/tipiracil clearly confers a clinically meaningful improvement in quality survival time compared with placebo in this setting, which involved treatment of heavily pretreated metastatic patients with a poor prognosis. The sensitivity analysis examining the impact of variations in the utility coefficients assigned to the REL and TOX states implies that the results are robust to such variations.
These results are in line with other analyses of QoL-related end points from the RECOURSE trial. Van Cutsem et al 16 carried out a post hoc descriptive analysis of changes in Eastern Cooperative Oncology Group (ECOG) performance status (PS) in the RECOURSE trial patients between baseline and treatment discontinuation. The ECOG PS was 0 at baseline in 56% of patients in the trifluridine/tipiracil group and 55% of patients in the placebo group. The remainder had an ECOG PS of 1 at baseline. Of the 496 patients in the trifluridine/tipiracil group who discontinued treatment during the study, 69% maintained their baseline PS, suggesting that treatment did not have a negative impact on PS. The proportion of placebo recipients who maintained their baseline PS at discontinuation was similar (65%). When patients with PS 0 and 1 at baseline were combined, 84% and 81% of the trifluridine/tipiracil and placebo groups, respectively, remained at a PS of 0–1 at discontinuation. Seventy-two per cent of patients in the trifluridine/tipiracil group and 81% in the placebo group had a worsening of their PS to 2 or higher during the study. However, this was significantly delayed in the trifluridine/tipiracil group: median time to PS 2 or higher was 5.7 months, compared with 4.0 months in the placebo group (HR: 0.66; 95% CI 0.56 to 0.78; p<0.001).16 Although PS scores are not always strongly correlated with patient-reported health-related QoL scores,17 Laird et al 18 found that a lower PS was associated with lower EORTC QLQ-C30 scores in a sample of 2520 patients with advanced cancer, of whom 22% had gastrointestinal cancers.18 Similarly, a study in 45 elderly patients with mCRC found that those with ECOG PS of 2 had significantly more functional limitations and lower QoL (as measured by FACT-C composite score and the visual-analogue scale component of the EQ-5D instrument) than those with PS 1.19
The impact of AEs on QoL and duration of treatment in the RECOURSE trial was also analysed.16 Patients in the trifluridine/tipiracil group were more likely to experience grade 3/4 AEs that affect QoL (nausea, vomiting, diarrhoea, fatigue, asthaenia and dysgeusia) than patients in the placebo group. However, onset of these events did not decrease treatment exposure. Patients in the trifluridine/tipiracil group had longer durations for most of these events than those in the placebo group but the durations occupied a lower proportion of the total treatment period (with the exception of nausea/vomiting). Notably, only 4% of patients in the trifluridine/tipiracil group discontinued the study due to AEs (vs 2% in the placebo group).12
Our analysis has some limitations. It is not a direct assessment of patients’ QoL in the RECOURSE trial. However, in the absence of a direct assessment, it adds useful information to the trial findings. One limitation is that hypothetical thresholds were defined for utility coefficients; coefficients were not directly elicited from patients. However, the analysis was not highly sensitive to the coefficient used, in that large variations in the coefficients assigned to the TOX and REL states produce a range of only approximately 0.5 months from minimum to maximum quality-adjusted survival (ie, from 1.28 to 1.73 months). Another limitation is that the analysis did not include all grade 3/4 AEs from the RECOURSE trial but was restricted to those that could be expected to have an impact on QoL. Other AEs were assumed not to affect QoL. On the other hand, this did not result in a large number of AEs being excluded. The only non-laboratory grade 3/4 AEs listed in the primary study publication that were not included were abdominal pain (reported in 2% of treated and 4% of placebo patients) and stomatitis (reported in <1% of treated patients).12 However, the analysis does not take into account the impact of grade 1 and 2 AEs on QoL. Grade 1 and 2 AEs were reported at markedly higher frequencies than grade 3/4 AEs in both study groups, and occurred at higher frequencies in the treated group than the placebo group. Although lower-grade AEs are classed as less severe, they may have an impact on patients’ QoL, particularly if experienced over a long duration.
The decision on whether to undertake further chemotherapy following progression after more than one previous line of treatment for mCRC is a complex one for both patients and physicians, and involves weighing potential benefits against potential risks. Potential risks of harm to QoL have been suggested from continuing chemotherapy as patients approach the end of life, even in those with good PS.20 Furthermore, gains in OS in the setting of salvage therapy for pretreated mCRC are generally small. However, Price noted that the clinical relevance of relatively small gains in survival might be clearer if they are combined with true gains in symptom control.7 Taken together, the QTWiST analysis and the additional findings on PS provide reassurance about the favourable benefit/risk profile of trifluridine/tipiracil in patients with mCRC who progressed on previous therapies.
Conclusion
Quality-adjusted survival showed clinically meaningful improvement in patients treated with trifluridine/tipiracil compared with placebo in pretreated mCRC.
Acknowledgments
Assistance with drafting of the manuscript was provided by Scinopsis (Frejus, France), funded by Servier.
Footnotes
Contributors: All authors contributed to the collection of the data, interpretation of the results, the preparation of the manuscript and approved the decision to submit.
Funding: This study was funded by Taiho and Institute de Recherches Internationales Servier.
Competing interests: JT reports consulting/advisory fees from Amgen, Boehringer Ingelheim, Celgene, Chugai, Imclone Systems Inc., Eli Lilly and Company, Merck & Co., Merck Serono, Millennium, Novartis, F. Hoffmann-La Roche Ltd., Sanofi, Symphogen and Taiho Oncology Inc. EVC has received research funding from Amgen, Bayer, Boehringer, Celgene, Ipsen, Lilly, Merck, Merck KgA, Novartis, Roche, Sanofi and Servier. RJM has performed a consulting/advisory role for Taiho. AO has an immediate family member who is an employee of Celgene. NA, SC, RF and BH are employees of Servier.
Ethics approval: The review board at each participating study approved the study.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1. WebMD Colorectal cancer, metastatic or recurrent—symptoms. http://www.webmd.com/colorectal-cancer/tc/colorectal-cancer-metastatic-or-recurrent-symptoms (accessed 10 Mar 2017).
- 2. Cancer research UK About secondary liver cancer. 2017. http://www.cancerresearchuk.org/about-cancer/secondary-cancer/secondary-liver-cancer/about10
- 3. Hilarius DL, Kloeg PH, van der Wall E, et al. . Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer 2012;20:107–17. 10.1007/s00520-010-1073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maroun JA, Anthony LB, Blais N, et al. . Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the Canadian working group on chemotherapy-induced diarrhea. Curr Oncol 2007;14:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hofman M, Ryan JL, Figueroa-Moseley CD, et al. . Cancer-related fatigue: the scale of the problem. Oncologist 2007;12(Suppl 1):4–10. 10.1634/theoncologist.12-S1-4 [DOI] [PubMed] [Google Scholar]
- 6. Bernhardson BM, Tishelman C, Rutqvist LE. Taste and smell changes in patients receiving cancer chemotherapy: distress, impact on daily life, and self-care strategies. Cancer Nurs 2009;32:45–54. 10.1097/01.NCC.0000343368.06247.74 [DOI] [PubMed] [Google Scholar]
- 7. Price TJ. Advanced colorectal cancer treatment options beyond standard systemic therapy. Lancet Oncol 2017;18:157–9. 10.1016/S1470-2045(17)30002-5 [DOI] [PubMed] [Google Scholar]
- 8. Hickish T, Andre T, Wyrwicz L, et al. . MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2017;18:192–201. 10.1016/S1470-2045(17)30006-2 [DOI] [PubMed] [Google Scholar]
- 9. Emura T, Suzuki N, Fujioka A, et al. . Potentiation of the antitumor activity of alpha, alpha, alpha-trifluorothymidine by the co-administration of an inhibitor of thymidine phosphorylase at a suitable molar ratio in vivo. Int J Oncol 2005;27:449–55. [PubMed] [Google Scholar]
- 10. Temmink OH, Emura T, de Bruin M, et al. . Therapeutic potential of the dual-targeted TAS-102 formulation in the treatment of gastrointestinal malignancies. Cancer Sci 2007;98:779–89. 10.1111/j.1349-7006.2007.00477.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lonsurf summary of product characteristics (2016). https://www.medicines.org.uk/emc/medicine/32207 (accessed 13 Apr 2017).
- 12. Mayer RJ, Van Cutsem E, Falcone A, et al. . Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19. 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 13. Glasziou PP, Simes RJ, Gelber RD. Quality adjusted survival analysis. Stat Med 1990;9:1259–76. 10.1002/sim.4780091106 [DOI] [PubMed] [Google Scholar]
- 14. Revicki DA, Feeny D, Hunt TL, et al. . Analyzing oncology clinical trial data using the Q-TWiST method: clinical importance and sources for health state preference data. Qual Life Res 2006;15:411–23. 10.1007/s11136-005-1579-7 [DOI] [PubMed] [Google Scholar]
- 15. PROTECT Health indices: Q-TWiST (Quality-adjusted Time Without Symptoms and Toxicity). 2017. http://protectbenefitrisk.eu/Qtwist.html12.
- 16. Van Cutsem E, Falcone A, Garcia-Carbonero R, et al. . Proxies of quality of life in metastatic colorectal cancer: analyses in the RECOURSE trial. ESMO Open. In Press 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atkinson TM, Andreotti CF, Roberts KE, et al. . The level of association between functional performance status measures and patient-reported outcomes in cancer patients: a systematic review. Support Care Cancer 2015;23:3645–52. 10.1007/s00520-015-2923-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laird BJ, Fallon M, Hjermstad MJ, et al. . Quality of life in patients with advanced cancer: differential association with performance status and systemic inflammatory response. J Clin Oncol 2016;34:2769–75. 10.1200/JCO.2015.65.7742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ward P, Hecht JR, Wang HJ, et al. . Physical function and quality of life in frail and/or elderly patients with metastatic colorectal cancer treated with capecitabine and bevacizumab: an exploratory analysis. J Geriatr Oncol 2014;5:368–75. 10.1016/j.jgo.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 20. Prigerson HG, Bao Y, Shah MA, et al. . Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol 2015;1:778–84. 10.1001/jamaoncol.2015.2378 [DOI] [PMC free article] [PubMed] [Google Scholar]