Abstract
Background
In the pivotal phase III, randomised, double-blind, placebo-controlled RECOURSE study, treatment with trifluridine/tipiracil was well tolerated and associated with prolonged progression-free and overall survival in patients with metastatic colorectal cancer (mCRC). There was no formal analysis of quality of life (QoL) in RECOURSE. The aim of the present analysis was to assess proxies of QoL during the RECOURSE treatment period, in terms of adverse events (AEs) likely to affect QoL and Eastern Cooperative Oncology Group performance status (ECOG PS).
Patients and methods
Enrolled patients had documented, previously treated (≥2 prior chemotherapy lines) mCRC and an ECOG PS of 0 or 1. Patients received best supportive care plus trifluridine/tipiracil 35 mg/m2 twice daily (n=534) or placebo (n=266) in a 28-day cycle. AEs analysed included nausea, vomiting, diarrhoea, dysgeusia and fatigue/asthenia. ECOG PS was determined at baseline, on day 1 of each treatment cycle, at treatment end and 30 days post-treatment discontinuation.
Results
AEs that affect QoL were more frequent in patients treated with trifluridine/tipiracil than placebo. Median treatment duration for patients experiencing at least one of these AEs was longer than that observed for the overall RECOURSE population (trifluridine/tipiracil: 12 vs 7 weeks; placebo: 10 vs 6 weeks). Versus placebo, the duration of most AEs was longer in trifluridine/tipiracil recipients; however, all AEs except nausea and vomiting occupied a lower proportion of the total treatment period. Of the patients who had their PS recorded at discontinuation, PS was maintained in 67% and 63% of trifluridine/tipiracil and placebo recipients, and 84% and 81% of the trifluridine/tipiracil and placebo patients remained at a PS of 0 or 1 at discontinuation.
Conclusions
Analysis of ECOG PS and AEs thought to affect QoL in the RECOURSE patient population suggests that trifluridine/tipiracil treatment does not result in a deterioration of patient QoL versus placebo.
Keywords: advanced colorectal cancer, trifluridine/tipiracil, quality of life, performance status
Key questions.
What is already known about this subject?
Quality of life (QoL) is an important issue for patients with cancer, since it is impacted by both the disease and therapies.
The pivotal RECOURSE trial investigated the efficacy and safety of trifluridine/tipiracil in refractory metastatic colorectal cancer, but did not include QoL questionnaires.
What does this study add?
In patients receiving trifluridine/tipiracil in RECOURSE, the occurrence of non-haematological, symptomatic adverse events did not result in premature treatment discontinuation.
Evaluation of performance status suggests that trifluridine/tipiracil preserves QoL versus placebo while prolonging overall survival.
Overall, patients treated with trifluridine/tipiracil in RECOURSE had preserved performance status in 84% of cases.
In patients with metastatic colorectal cancer receiving trifluridine/tipiracil or placebo in RECOURSE, occurrence of adverse events known to affect QoL did not result in premature treatment discontinuation, and ~70% of patients had unchanged performance status, suggesting that trifluridine/tipiracil preserves QoL versus placebo while prolonging overall survival.
How might this impact on clinical practice?
Our results may raise awareness of the importance of taking QoL into consideration in the management of patients with colorectal cancer, ultimately improving the experience of patients.
Formal assessment of QoL for trifluridine/tipiracil is currently underway in observational trials and should confirm these results.
Introduction
Globally, colorectal cancer (CRC) is the third most commonly diagnosed cancer (~10% of new cases) and is responsible for 8% of cancer-related deaths.1 It is now well known that quality of life (QoL) is an important issue for individuals with cancer, with QoL affected by both the disease and therapies used to treat the disease.2–4 In advanced CRC, the main aims of treatment are to prolong overall survival (OS) and maintain QoL.5
Analysis of QoL assessment in trials of advanced CRC has shown that formal assessment of QoL occurred in only 27% of phase III trials conducted between 2007 and 2014.2 Despite this lack of formal analysis, investigation of patient symptoms and treatment-related side effects remains important in the study of cancer treatments and as part of high-quality, patient-centred care. Primarily, physician-rated, functional performance status (PS) and patient-reported adverse events (AEs) are used to assess these treatment effects. Data suggest a significant correlation between PS and patient-reported AEs, both in cancer in general and in advanced CRC specifically,6 7 including both measures deteriorating concurrently in patients with advanced CRC.8
Trifluridine/tipiracil is a combination of the thymidine-based nucleoside analogue trifluridine and the thymidine phosphorylase inhibitor tipiracil.9 Trifluridine/tipiracil inhibits thymidylate synthase, disrupting DNA synthesis, but its predominant antitumour activity results from the incorporation of trifluridine into DNA, while tipiracil improves trifluridine bioavailability. Trifluridine/tipiracil is approved in the USA and Europe for the treatment of patients with metastatic CRC (mCRC) who have been previously treated with currently available therapies.10 11 In Japan, it is indicated for the treatment of unresectable advanced or recurrent CRC.12 13
The efficacy and safety of trifluridine/tipiracil in patients with refractory mCRC have been investigated in the large, international RECOURSE (Randomized, Double-Blind, Phase 3 Study of TAS-102 plus Best Supportive Care (BSC) versus Placebo plus BSC in Patients with Metastatic Colorectal Cancer Refractory to Standard Chemotherapies) study.14 Median OS in RECOURSE was significantly longer in patients treated with trifluridine/tipiracil plus BSC versus placebo plus BSC (7.1 vs 5.3 months; HR: 0.68; 95% CI 0.58 to 0.81; p<0.001). Median progression-free survival (PFS) was 2.0 vs 1.7 months (HR: 0.48; 95% CI 0.41 to 0.57; p<0.001). Common AEs in the RECOURSE population included dysgeusia, gastrointestinal AEs (nausea and vomiting, diarrhoea) and fatigue/asthenia. These AEs are considered likely to affect QoL.15–18
No formal assessment of QoL was undertaken in the RECOURSE study. Therefore, the aim of the present analysis was to assess AEs likely to affect QoL during the course of the trial and Eastern Cooperative Oncology Group (ECOG) PS.
Methods
RECOURSE study design, patient population and treatment regimen
Detailed methods of the RECOURSE study have been previously published.14 Briefly, RECOURSE was a phase III, randomised, double-blind, placebo-controlled trial conducted in Japan, Europe, the USA and Australia. Patients enrolled were those with documented mCRC treated with ≥2 prior lines of standard chemotherapy (including anti-epidermal growth factor receptor antibody if KRAS wild-type) and an ECOG PS of 0 or 1. All patients received BSC and either trifluridine/tipiracil or placebo on days 1–5 and 8–12 of each 28-day cycle. Trifluridine/tipiracil was administered at a dose of 35 mg/m2 twice daily, and three dose reductions (in 5 mg/m2 steps) were allowed.
Patients continued study treatment until disease progression, death, unacceptable toxicity or patient refusal of treatment. Patients were evaluated every 2 weeks during the treatment period and every 8 weeks from treatment cessation until the trial data collection cut-off date or death.
Collection of AE and PS data
AEs were classified and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, V.4.03.19 PS was evaluated using the ECOG score20 at baseline, on day 1 of each treatment cycle, at the end of treatment and 30 days after treatment discontinuation. Patients who died (PS=5) before treatment discontinuation were not included in the analysis of PS at discontinuation.
Statistical analysis
AEs for all patients who received at least one dose of study drug were summarised using descriptive statistics. The AEs included in this analysis were those of high prevalence and which were likely to affect QoL: nausea and vomiting, diarrhoea, dysgeusia and fatigue/asthenia. ECOG PS at treatment discontinuation (ECOG scores of 0–4) was compared with PS at baseline in the trifluridine/tipiracil and placebo groups using descriptive statistics. Time to worsening of ECOG PS was analysed in the intent-to-treat population using a two-sided, stratified log-rank test, and basing the HR and two-sided 95% CIs on a stratified Cox model and associated Kaplan-Meier survival estimates.14 SAS V.9.1 was used for statistical analyses.
Results
Patient population
RECOURSE enrolled 800 patients, with 534 and 266 randomised to trifluridine/tipiracil and placebo, respectively.14 The characteristics of the population have been previously described14; the main characteristics were as follows: median age 63 years, ~61% male and predominantly white (~57%) or Asian (~34%). ECOG PS was 0 or 1 in ~55% and ~45% of patients, respectively. The colon was the primary site of disease in the majority (~62% of each group), and most patients (79%) were 18 months or more from a diagnosis of first metastases. The mean (±SD) duration patients received the study drug was 12.7±12.0 weeks in the trifluridine/tipiracil group and 6.8±6.1 weeks in the placebo group.
AEs affecting QoL
Trifluridine/tipiracil patients were more likely to experience AEs that affect QoL than placebo patients (table 1). The overall incidence in the RECOURSE population as well as incidence at the highest grade are given for each AE likely to affect QoL.
Table 1.
Frequency of adverse events experienced during the RECOURSE trial that are likely to affect patient quality of life
| Adverse event | Trifluridine/tipiracil (n=533) | Placebo (n=265) | ||||
| Overall incidence, % | Highest grade | Incidence at highest grade, n (%) | Overall incidence, % | Highest grade | Incidence at highest grade, n (%) | |
| Nausea | 48.4 | 3 | 10 (1.9) | 23.8 | 3 | 3 (1.1) |
| Vomiting | 27.8 | 3 | 11 (2.1) | 14.3 | 3 | 1 (0.4) |
| Diarrhoea | 31.9 | 4 | 16 (3.0)* | 12.5 | 3 | 1 (0.4) |
| Dysgeusia | 6.8 | 2 | 3 (0.6) | 2.3 | 2 | 1 (0.4) |
| Fatigue | 35.3 | 3 | 21 (3.9) | 23.4 | 3 | 15 (5.7) |
| Asthenia | 18.2 | 3 | 18 (3.4) | 11.3 | 3 | 8 (3.0) |
*One incidence of grade 4 diarrhoea was observed in trifluridine/tipiracil patients. This number indicates the total number of grade 3 and 4 diarrhoea events.
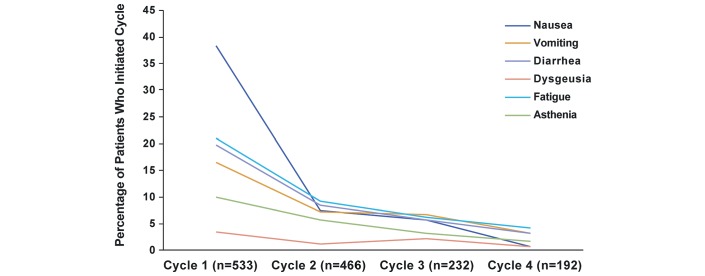
The frequency of AEs was higher during cycle 1 than in subsequent cycles for patients treated with trifluridine/tipiracil (figure 1).
Figure 1.
Incidence of adverse events by trifluridine/tipiracil treatment cycle.
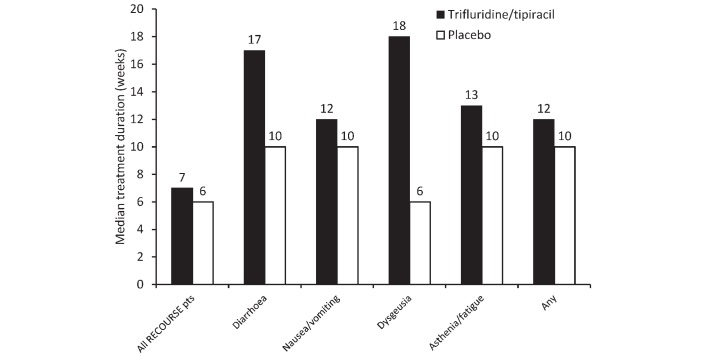
The median treatment duration for all patients in the RECOURSE study was 6.7 weeks for the trifluridine/tipiracil patients and 5.7 weeks for the placebo patients (figure 2).14 Notably, the median treatment duration for patients experiencing at least one AE likely to affect QoL was longer than that for the whole RECOURSE population regardless of treatment allocation (figure 2). Patients in the trifluridine/tipiracil group had longer median treatment durations than patients in the placebo group.
Figure 2.
Median duration of treatment in patients in the RECOURSE trial, stratified by adverse event and in the whole population. Pts, patients.
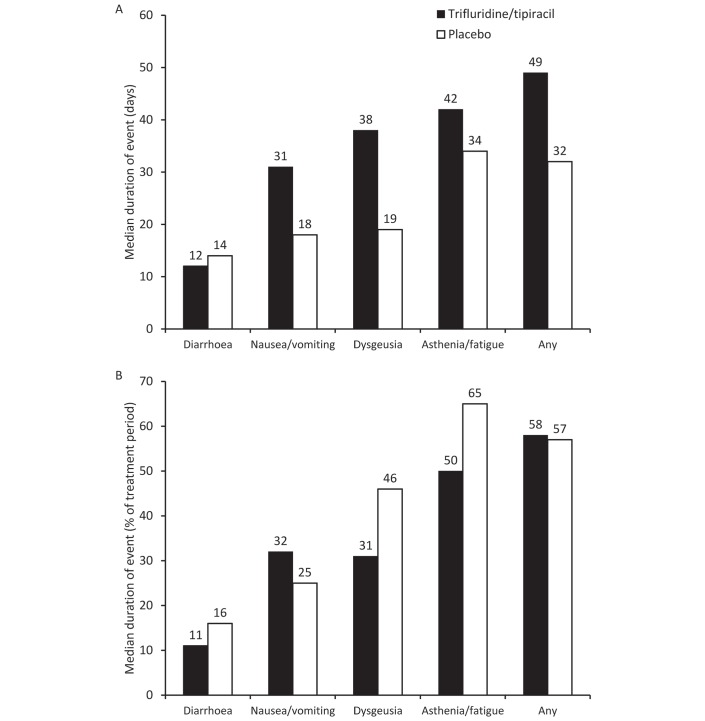
While most of the AEs experienced by trifluridine/tipiracil recipients were of longer duration than those experienced by placebo recipients (figure 3A), the AEs occupied a lower proportion of the total treatment period (with the exception of nausea/vomiting) (figure 3B).
Figure 3.
Median duration of adverse events experienced in the RECOURSE trial presented in (A) days and (B) proportion of the treatment period.
PS at discontinuation
A total of 759 patients had their PS recorded at discontinuation (table 2). Two hundred and seventy-eight and 146 patients receiving trifluridine/tipiracil and placebo, respectively, had a PS of 0 at baseline; 180 (65%) and 86 (60%) of these patients maintained a PS of 0 at discontinuation. Of the patients receiving trifluridine/tipiracil or placebo with a PS of 1 at baseline (n=218 and n=117), 152 (70%) and 80 (68%) had a PS of 1 at discontinuation; overall, 67% and 63% of trifluridine/tipiracil and placebo recipients maintained their PS throughout the study. The percentage of patients with a PS of 1 at baseline who improved to PS 0 at discontinuation was 4% in the trifluridine/tipiracil group and 3% in the placebo group. Overall, the percentage of patients who were at PS 0 or 1 at treatment discontinuation was 84% and 81% in the trifluridine/tipiracil and placebo groups, respectively. In addition, trifluridine/tipiracil significantly delayed the median time to an ECOG PS of ≥2 versus placebo (5.7 vs 4.0 months; HR: 0.66; 95% CI 0.56 to 0.78; p<0.001).14
Table 2.
Changes in Eastern Cooperative Oncology Group performance status from baseline to treatment discontinuation in the RECOURSE trial
| Baseline performance status | ||||
| Trifluridine/tipiracil | Placebo | |||
| 0 (n=278) | 1 (n=218) | 0 (n=146) | 1 (n=117) | |
| Performance status at discontinuation, n (%) | ||||
| 0 | 180 (65) | 8 (4) | 86 (60) | 4 (3) |
| 1 | 78 (28) | 152 (70) | 43 (30) | 80 (68) |
| 2 | 13 (5) | 40 (18) | 13 (9) | 22 (19) |
| 3 | 6 (2) | 17 (8) | 4 (3) | 8 (7) |
| 4 | 1 (0.4) | 1 (0.5) | 0 | 3 (3) |
Discussion
No formal assessment of QoL was performed during the RECOURSE study. The present analysis aimed to assess the type, timing and duration of AEs known to affect QoL and changes in physician-rated functional PS, and use these endpoints as surrogates for QoL in the RECOURSE population. Evidence suggests that chemotherapy-induced nausea and vomiting, diarrhoea, dysgeusia and fatigue/asthenia can have a significant negative impact on patients’ QoL.15–18 These AEs were reported more frequently in the trifluridine/tipiracil group than in the placebo group in RECOURSE. The majority of these AEs were mild to moderate in severity, and did not affect treatment continuity in a substantial way, being managed by dose reductions and/or treatment delays rather than hospitalisation.14 21 These AEs are expected in patients treated with a thymidine nucleotide analogue,21 are predictable and reversible, and were managed appropriately during RECOURSE.
Patients experiencing AEs that affect their QoL might be expected to discontinue therapy at a higher rate than those who were not; however, patients in RECOURSE who experienced these AEs had a longer treatment duration than the overall population, and the duration of these AEs was generally a lower proportion of the total treatment duration in patients receiving trifluridine/tipiracil than in those receiving placebo. These data indicate that patients treated with trifluridine/tipiracil remained in the study longer than those treated with placebo despite a higher likelihood of experiencing more frequent AEs of a longer duration that may have affected their QoL.
The original RECOURSE analysis showed that worsening of baseline PS from 0 to 1 to ≥2 was delayed in patients receiving trifluridine/tipiracil versus placebo.14 PS was unchanged in 67% of patients treated with trifluridine/tipiracil, which was a similar proportion to that seen in the placebo group (63%), suggesting that PS was maintained in patients treated with trifluridine/tipiracil despite a higher level of AEs versus placebo.
Assessment of QoL in clinical trials of cancer drugs is difficult, being hampered by patient and physician subjectivity, complexity and patient adherence.22 Over the past 20 years, formal assessment of QoL in advanced CRC has decreased such that during 1998–2006, 46% of trials formally assessed QoL, while during 2007–2014 this decreased to 27% of studies (p=0.04).2 Use of surrogate endpoints to assess efficacy in cancer studies can be a risk, with evidence suggesting that approvals based on endpoints such as tumour shrinkage or PFS can result in agents used for treatment that have no benefits on either OS and/or QoL.23 In contrast, several studies indicate that PS is related to QoL. In a study of patients with pancreatic cancer, those with a worse PS self-reported a worse QoL,24 while another analysis of patients with metastatic non-small cell lung cancer found that higher QoL was significantly associated with good PS, and patients who demonstrated a decline in QoL also had a decline in PS (p=0.0001 for both).25 A systematic review found good correlation between PS and patient-reported AEs (including QoL), and suggested that assessment of PS and patient-reported AEs may lead to more effective patient care.6 Deteriorating PS has been associated with a worsening of QoL-related symptoms such as fatigue, nausea and diarrhoea.7
The limitations of the current analysis include the subjective nature of QoL analysis, and the fact that using PS at discontinuation as a surrogate for QoL has not been validated and is not formally recognised. The strength of the analysis is its consistency. The lack of PS deterioration at discontinuation, plus the longer treatment duration in patients experiencing AEs thought to affect QoL, suggests that, in the RECOURSE patients, treatment with trifluridine/tipiracil did not affect their QoL to the extent where they wished to leave the study. In addition, a separate analysis using the Quality-adjusted Time WIthout Symptoms of disease or Toxicity (QTWIST) method26 has shown an improvement in quality-adjusted survival in patients treated with trifluridine/tipiracil versus placebo.27
In conclusion, analysis of PS and AEs thought to affect QoL in the RECOURSE patient population suggests that trifluridine/tipiracil treatment does not result in a deterioration of patient QoL versus placebo while prolonging OS. Formal assessment of QoL for trifluridine/tipiracil is currently underway in an observational, early-access programme.28
Acknowledgments
We would like to thank Sheridan Henness, PhD, of Springer Healthcare Communications, who drafted the outline and subsequent drafts of this manuscript, under the direction of the authors.
Footnotes
Contributors: All authors contributed to the collection of the data, interpretation of the results, the preparation of the manuscript and approved the decision to submit.
Funding: This work was supported by the Institut de Recherches Internationales Servier, Suresnes, France.
Competing interests: None declared.
Patient consent: Detail has been removed from this case description/these case descriptions to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
Ethics approval: The review board at each participating institute approved the study (Mayer et al, NEJM, 2015).
Provenance and peer review: Not commissioned; internally peer reviewed.
Data sharing statement: There are no unpublished data available for this post-hoc study.
References
- 1.Ferlay J, Shin HR, Bray F, et al. . Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 2.Adamowicz K, Saad ED, Jassem J. Health-related quality of life assessment in contemporary phase III trials in advanced colorectal cancer. Cancer Treat Rev 2016;50:194–9. 10.1016/j.ctrv.2016.09.015 [DOI] [PubMed] [Google Scholar]
- 3.Bottomley A. The cancer patient and quality of life. Oncologist 2002;7:120–5. 10.1634/theoncologist.7-2-120 [DOI] [PubMed] [Google Scholar]
- 4.Bottomley A, Aaronson NK. International perspective on health-related quality-of-life research in cancer clinical trials: the European Organisation for Research and Treatment of Cancer experience. J Clin Oncol 2007;25:5082–6. 10.1200/JCO.2007.11.3183 [DOI] [PubMed] [Google Scholar]
- 5.Funaioli C, Longobardi C, Martoni AA. The impact of chemotherapy on overall survival and quality of life of patients with metastatic colorectal cancer: a review of phase III trials. J Chemother 2008;20:14–27. 10.1179/joc.2008.20.1.14 [DOI] [PubMed] [Google Scholar]
- 6.Atkinson TM, Andreotti CF, Roberts KE, et al. . The level of association between functional performance status measures and patient-reported outcomes in cancer patients: a systematic review. Support Care Cancer 2015;23:3645–52. 10.1007/s00520-015-2923-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laird BJ, Fallon M, Hjermstad MJ, et al. . Quality of life in patients with advanced cancer: differential association with performance status and systemic inflammatory response. J Clin Oncol 2016;34:2769–75. 10.1200/JCO.2015.65.7742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward P, Hecht JR, Wang HJ, et al. . Physical function and quality of life in frail and/or elderly patients with metastatic colorectal cancer treated with capecitabine and bevacizumab: an exploratory analysis. J Geriatr Oncol 2014;5:368–75. 10.1016/j.jgo.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 9.Burness CB, Duggan ST. Trifluridine/tipiracil: a review in metastatic colorectal cancer. Drugs 2016;76:1393–402. 10.1007/s40265-016-0633-9 [DOI] [PubMed] [Google Scholar]
- 10.European Medicines Agency. Lonsurf (trifluridine/tipiracil) film-coated tablets. Summary of product characteristics. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003897/WC500206246.pdf (accessed 23 Nov 2016).
- 11.Taiho Oncology Inc. LONSURF (trifluridine and tipiracil) tablets for oral use: US prescribing infomation. 2015. https://www.taihooncology.com/us/prescribing-information.pdf (accessed 14 Dec 2016).
- 12.Yoshino T, Mizunuma N, Yamazaki K, et al. . TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 2012;13:993–1001. 10.1016/S1470-2045(12)70345-5 [DOI] [PubMed] [Google Scholar]
- 13.Pharmaceuticals and Medical Devices Agency. Lonsurf combination tablet: Japanese prescribing information [Japanese]. 2015. http://www.info.pmda.go.jp/downfiles/ph/PDF/400107_4299100F1026_1_03.pdf (accessed 20 Apr 2017).
- 14.Mayer RJ, Van Cutsem E, Falcone A, et al. . Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19. 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 15.Hilarius DL, Kloeg PH, van der Wall E, et al. . Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer 2012;20:107–17. 10.1007/s00520-010-1073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maroun JA, Anthony LB, Blais N, et al. . Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the Canadian Working Group on Chemotherapy-Induced Diarrhea. Curr Oncol 2007;14:13–20. 10.3747/co.v14i1.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofman M, Ryan JL, Figueroa-Moseley CD, et al. . Cancer-related fatigue: the scale of the problem. Oncologist 2007;12(Suppl 1):4–10. 10.1634/theoncologist.12-S1-4 [DOI] [PubMed] [Google Scholar]
- 18.Bernhardson BM, Tishelman C, Rutqvist LE. Taste and smell changes in patients receiving cancer chemotherapy: distress, impact on daily life, and self-care strategies. Cancer Nurs 2009;32:45–54. 10.1097/01.NCC.0000343368.06247.74 [DOI] [PubMed] [Google Scholar]
- 19.Department of Health and Human Services National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.03. 2010. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (accessed 14 Dec 2016).
- 20.ECOG-ACRIN Cancer Research Group. ECOG performance status. 2016. http://ecog-acrin.org/resources/ecog-performance-status (accessed 14 Dec 2016).
- 21.European Medicines Agency. Lonsurf: European public assessment report. 2016. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003897/human_med_001975.jsp&mid=WC0b01ac058001d124 (accessed 15 Dec 2016).
- 22.Tassinari D. Surrogate end points of quality of life assessment: have we really found what we are looking for? Health Qual Life Outcomes 2003;1:71 10.1186/1477-7525-1-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rupp T, Zuckerman D. Quality of life, overall survival, and costs of cancer drugs approved based on surrogate endpoints. JAMA Intern Med 2017;177:276–7. 10.1001/jamainternmed.2016.7761 [DOI] [PubMed] [Google Scholar]
- 24.Moningi S, Walker AJ, Hsu CC, et al. . Correlation of clinical stage and performance status with quality of life in patients seen in a pancreas multidisciplinary clinic. J Oncol Pract 2015;11:e216–e221. 10.1200/JOP.2014.000976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finkelstein DM, Cassileth BR, Bonomi PD, et al. . A pilot study of the Functional Living Index-Cancer (FLIC) Scale for the assessment of quality of life for metastatic lung cancer patients: an Eastern Cooperative Oncology Group study. Am J Clin Oncol 1988;11:630–3. 10.1097/00000421-198812000-00007 [DOI] [PubMed] [Google Scholar]
- 26.Glasziou PP, Simes RJ, Gelber RD. Quality adjusted survival analysis. Stat Med 1990;9:1259–76. 10.1002/sim.4780091106 [DOI] [PubMed] [Google Scholar]
- 27.Tabernero J, Van Cutsem E, Ohtsu A, et al. . QTWIST analysis of the RECOURSE trial of trifluridine/tipiracil in metastatic colorectal cancer. ESMO Open. In Press 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laboratorios Servier SL. An open-label early access phase IIIb study of trifluridine / tipiracil (S 95005/TAS-102) in patients with a pretreated metastatic colorectal cancer. 2016. Available at http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016-002311-18-ES (accessed 15 Dec 2016).