Abstract
Background
Previous surveillance methods to monitor the prognoses of patients with bronchiectasis are too complex for use in daily practice. The 6-minute walk test (6MWT) is a simple exercise test to predict the prognosis of chronic obstructive airway disease and numerous chronic lung diseases, including idiopathic pulmonary fibrosis. No studies have investigated exercise-induced oxygen desaturation (EID) and distance-saturation product (DSP) of 6MWT to predict the prognoses of patients with bronchiectasis.
Methods
This was a prospective study to identify correlations between variables of 6MWT and mortality in patients with bronchiectasis over a 6-year period. The study cohort included 69 patients with stable non-cystic fibrosis (non-CF) bronchiectasis who were regularly evaluated for functional status via 6-minute walk distance (6MWD), spirometry, BODE index, EID, and DSP.
Results
Of the 69 patients, 9 (13%) died and 60 (87%) survived during the 6-year follow-up period. The percentage of EID was higher [7 of 9 patients (78%) vs. 22 of 60 patients (27%), P=0.003] in the non-survivors group. The 6MWD (467.9±77.1 vs. 363.7±126.7 m, P=0.001) was higher in the survivors group. DSP was significantly lower in the non-survivors group (411.0±78.4 vs. 283.9±90.0 m%, P<0.001). Multivariate analysis showed that DSP (OR =0.983; 95% CI: 0.974–0.993, P=0.001) was the best parameter of 6MWT to predict mortality. Patients with a lower DSP of <280 m% were at a 66.5-fold greater risk (OR =66.5; 95% CI: 9.4–469.2) of 6-year mortality compared with those with DSP >280 m% (P<0.001).
Conclusions
DSP is a simple parameter to predict 6-year mortality in patients with non-CF bronchiectasis.
Keywords: Non-cystic fibrosis (non-CF) bronchiectasis, 6-minute walk test (6MWT), distance-saturation product (DSP), mortality
Introduction
Non-cystic fibrosis (non-CF) bronchiectasis is a chronic progressive pulmonary disease characterized by airway inflammation and infection (1). Pulmonary function and high-resolution computed tomography (HRCT) are used to evaluate the severity of non-CF bronchiectasis (2-4). However, pulmonary spirometry and radiological interpretation are insufficient to capture the complexity of this disease (5) as patients with similar prognoses may have variable pulmonary spirometry and radiological findings. At present, there is no simple parameter to accurately evaluate the prognosis of non-CF bronchiectasis.
The identification of risk factors for disease can help clinicians to identify high-risk patients and design treatment plans. Pulmonary function, symptom scores, frequency of exacerbation, and number of hospital admissions are presently used to predict the outcomes of patients with chronic obstructive pulmonary disease (COPD). Two similar severity scoring systems were applied for non-CF bronchiectasis (6,7). Chalmers et al. derived and validated the bronchiectasis severity index (BSI) to predict mortality in patients with bronchiectasis. BSI includes age, predicted forced expiratory volume for 1 s (FEV1%, percentage of actual FEV1/predicted FEV1), body mass index (BMI), prior hospitalization, prior exacerbation, Medical Research Council dyspnea score, colonization of Pseudomonas aeruginosa or other pathogenic organisms, and involvement of three or more lobes on HRCT (6). Another index, the FACED score, includes predicted FEV1%, age, and chronic colonization by Pseudomonas aeruginosa, radiological extension, and Medical Research Council scale (7). The above two severity scoring systems are reportedly useful, but too difficult and complex for use in daily practice of pulmonary physicians. Therefore, a simple and accurate parameter is needed to identify patients at a high risk of mortality.
The progression of clinical disease in patients with non-CF bronchiectasis is a complex issue in some patients and is characterized by decreased lung function, chronic colonization of microorganisms, and hypoxemia or respiratory failure. Activity-related dyspnea and lower exercise tolerance occur in response to exercise-induced oxygen desaturation (EID) and often develop before resting hypoxemia at the end stage of disease. Activity-related dyspnea and EID are characteristic of chronic pulmonary diseases, such as bronchiectasis and COPD (8). Oxygen saturation during exercise has been shown to decrease with increased distance walked in patients with pulmonary hypertension and interstitial lung disease (9,10). These clinical presentations are the same as for patients with non-CF bronchiectasis.
The 6-minute walk test (6MWT) is a simple exercise test to evaluate the general functional responses of the pulmonary, cardiovascular, and muscular systems to evaluate daily physical activities and quality of life of patients with non-CF bronchiectasis (11,12). However, few studies have investigated the prognostic value of variables derived from 6MWT for patients with a wide range of severity of non-CF bronchiectasis. Also, previous reports failed to identify a simple parameter from 6MWT to accurately predict the prognoses of patients with non-CF bronchiectasis.
The distance-saturation product (DSP, m%), which included surveillance of both the walking distance and lowest oxygen saturation of patients during 6MWT, may be a predictive factor of worsening condition and survival of patients with idiopathic pulmonary fibrosis (13).
The aim of this study was to determine whether DSP (m%) of 6MWT could provide a simple measurement to predict the outcome of patients with non-CF bronchiectasis.
Methods
Patients
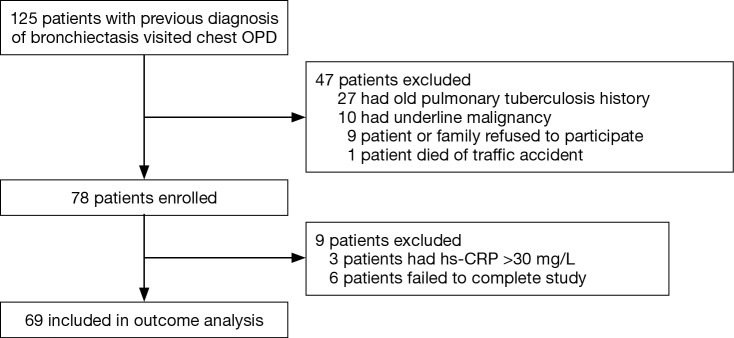
Patients with non-CF bronchiectasis were enrolled from our outpatient clinic in 2006 to investigate the correlation between clinical outcome and clinical surveillances, including several spirometry biomarkers, HRCT, and 6MWT. Initially, 125 patients with bronchiectasis were recruited from the Thoracic Outpatient Clinic of Chang Gung Memorial Hospital in Taiwan from January 2006 to December 2007. Our inclusion criteria were as follows: bronchiectasis documented on chest HRCT, idiopathic etiology of bronchiectasis (none of the patients with background suggests cystic fibrosis such as chronic dysfunction of the pancreas or liver or intestine, or an electrolyte imbalance, disease onset before adolescence and family history), chronic sputum production (daily sputum ≥10 mL), absence of other major pulmonary diagnoses, and a steady state defined by the absence of changes in symptoms noted by the patient over the past 3 weeks. The exclusion criteria were as follows: bronchiectasis with defined etiology (i.e., primary ciliary dyskinesia, allergic bronchopulmonary aspergillosis), common variable immunodeficiency, and use of antibiotics within the last three weeks. Patients with hepatic failure, malignancy, or pregnancy were also excluded (Figure 1). The Linko Chang Gung Memorial Hospital is a tertiary care medical center in north Taiwan. The following-up visit interval is 3 or 6 months at our out-patient clinic. Six years after recruitment, these patients were reassessed and factors identified in the original assessments were reanalyzed to determine the usefulness of these parameters to predict survival. There is no single method to predict the clinical outcomes of patients with non-CF bronchiectasis. The aim of the present study was to investigate whether 6MWT-derived variables predicted outcomes, with a particular focus on DSP during 6MWT.
Figure 1.
Flow chart of patient enrollment.
All patients submitted written informed consent and the study protocol was approved by the Institutional Review Board of Chang Gung Medical Foundation (approval no. 97-1105A3).
6MWT
All patients underwent 6MWT under supervision of well-trained technicians at our pulmonary rehabilitation center. 6MWT was performed in accordance with the standard protocol of the American Thoracic Society (11). Variables from 6MWT, including pulmonary spirometry, BODE (body-mass index, airflow obstruction, dyspnea, and exercise) index, EID, and DSP, were recorded.
DSP was defined as the product of the final 6-minute walk distance (6MWD) in meters and the lowest oxygen saturation in room air during 6MWT (EID) (13). For example, a patient walking a total of 300 m with a lowest oxygen saturation of 88% during 6MWT would have a DSP of 264 m% (e.g., 300×0.88).
Statistical analysis
Data are presented as means ± SD. All statistical analyses were performed using SPSS version 14.0 software (IBM-SPSS Inc., Chicago, IL, USA). The following variables were recorded: age (years), BMI (kg/m2), forced vital capacity (FVC in L), FEV1 (L), FEV1/FVC (%), immunoglobulin E (IgE, KU/L), eosinophilic cationic protein (ECP, µg/L), O2 saturation (%) during test, presence of bacterial colonization, 6MWD (m), lowest O2 saturation (%) during 6MWT, EID (O2 saturation <88%), and DSP (m%) for univariate analysis. Independent Student t-tests or chi-square tests were performed to compare the clinical parameters. A P value of <0.05 was considered statistically significant. Using Cox proportional hazards model, multivariate analysis of factors with significance in univariate analysis was performed using a stepwise ascending method. Receiver operating characteristic (ROC) curves were used to evaluate ability of the predictive power of the various factors to predict 6-year mortality. The ROC curves were evaluated via comparisons of the area under the curve (AUC). After identifying DSP value that generated the highest AUC, actuarial survival curves were then constructed for comparisons with DSPs above this threshold to those falling below this breakpoint. Survival analysis was performed using the Kaplan-Meier method. In further analyses, the participants were stratified into two groups according to the best AUC measurement using a DSP cut-off point of 280 m%.
Results
Patient Characteristics
The patients were followed-up from 2006 to June 2013. During the 6-year follow-up period (66.8±18.0 months), 9 (13.0%) of 69 patients died. The cause of death in the majority of patients (8, 88.9%) was respiratory infection or respiratory failure. One male patient with a history of chronic hepatitis B viral infection died of hepatic cellular carcinoma. This 35-year-old male of the non-survival group was the only one who died of non-respiratory factors. He died suddenly due to massive hematemesis.
Table 1 shows the baseline demographic characteristics of the study participants and 6MWT-derived variables. There was no significant difference in age or sex distribution between the groups of survivors and non-survivors. There were also no significant differences in serum IgE levels, ECP, and the presence of bacterial colonization between the two groups. The survivor group had higher FVC (2.1±0.7 vs. 1.3±0.5 L, P=0.003), FEV1 (1.6±0.7 vs. 0.8±0.4 L, P=0.002), and FEV1/FVC (72.1%±11.4% vs. 63.1%±11.6%, P=0.030). Oxygen saturation during exercise was lower in the non-survivors than the survivors (79.7%±9.6% vs. 87.9%±6.5%, P=0.001), and there was also a significant difference in EID (O2 <88%) between the two groups [seven of nine patients (78%) vs. 22 of 60 patients (27%), P=0.003]. 6MWD was significantly longer in the survivors (466.1±78.0 vs. 359.4±113.6 m, P=0.001). DSP was significantly correlated with outcomes, reflecting the cumulative contribution of component parts (Table 1). The mean DSP was only 283.9±90.0 m% among the non-survivors compared to 410.5±78.9 m% among the survivors (P<0.000). The distribution of DSP among the cohort is shown in Figure 2.
Table 1. Univariate survival analysis of patients with non-CF bronchiectasis.
| Characteristics | Survivors (n=60) | Non-survivors (n=9) | P |
|---|---|---|---|
| Age (years), mean ± SD | 56.8±13.7 | 60.8±15.1 | 0.424 |
| Sex (M/F) | 32/28 | 7/2 | 0.281 |
| BMI, mean ± SD | 22.3±3.4 | 20.2±2.5 | 0.081 |
| Smoking | |||
| Never | 50 | 5 | 0.075 |
| Ex/current | 10 | 4 | – |
| PFT | |||
| FVC (L), median (IQR) | 2.0 (1.5–2.5) | 1.4 (0.7–1.8) | 0.004 |
| FEV1 (L), median (IQR) | 1.4 (1.1–1.9) | 0.8 (0.4–1.2) | 0.001 |
| FEV1/FVC (%), median (IQR) | 75.9 (63.9–81.3) | 65.3 (55.2–72.8) | 0.029 |
| Total IgE, median (IQR) | 42.1 (16.2–91.6) | 71.2 (20.2–100.7) | 0.537 |
| ECP (serum), median (IQR) | 12.1 (6.3–26.4) | 15.4 (8.7–28.3) | 0.656 |
| Bacterial colony | |||
| Pseudomonas aeruginosa | 13 | 3 | 0.671 |
| Others | 12 | 1 | – |
| Normal flora/no growth | 35 | 5 | – |
| 6MWT | |||
| Rest O2 sat (%), median (IQR) | 96.0 (95.0–97.0) | 94.0 (89.0–96.5) | 0.055 |
| Lowest O2 sat (%), median (IQR) | 89.5 (83.3–92.0) | 80.0 (74.0–88.0) | 0.011 |
| Walk distances (m), mean ± SD | 466.1±78.0 | 359.4±113.6 | 0.001 |
| DSP (m%), median (IQR) | 433.0 (340.0–482.0) | 249.0 (230.0–315.0) | 0.001 |
| EID | |||
| <88% | 22 | 7 | 0.030 |
| ≥88% | 38 | 2 | – |
A Shapiro-Wilk’s test (P>0.05) and a visual inspection of their histogram, normal Q-Q plots and box plots showed that the age, BMI, and walking distance were approximately normal distributed. These three parameters were expressed as mean and SD, with using independent Student t-test for comparison between study groups. 6MWT, 6-minute walk test; BMI, body mass index; CF, cystic fibrosis; DSP, distance-saturation product; ECP, eosinophilic cationic protein; EID, exercise-induced oxygen desaturation; FEV1, volume that has been exhaled at the end of the first second of forced expiration; FVC, forced vital capacity; IgE, immunoglobulin E; PFT, pulmonary function test.
Figure 2.
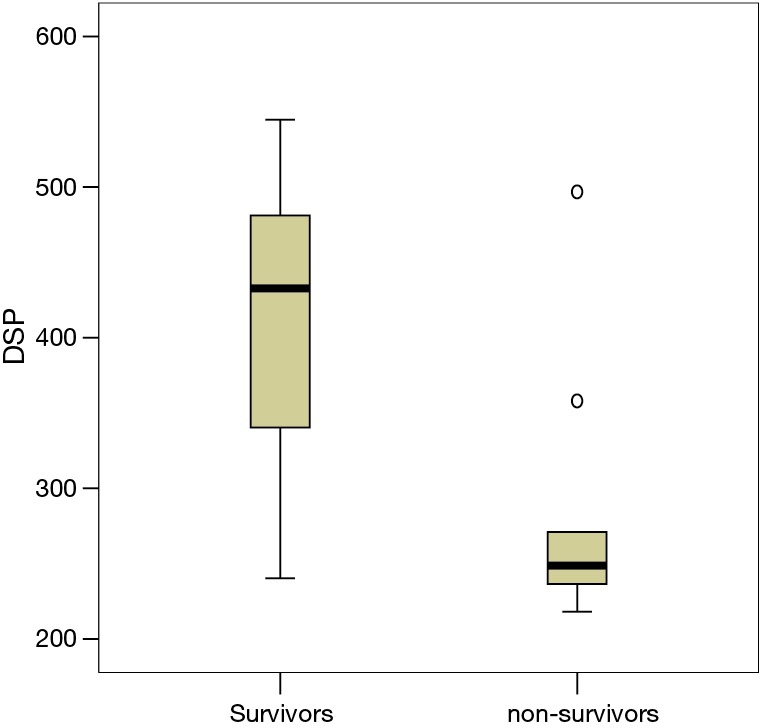
DSP in survivors and non-survivors. DSP was significantly higher in survivors (P<0.001) than that in the non-survivors. DSP, distance-saturation product.
Correlations between 6MWT-derived variables and 6-year survival
As shown by the univariate survival analysis results presented in Table 1, FVC, FEV1, FEV1/FVC, EID (saturation O2% <88%), lowest oxygen saturation during 6MWT, 6MWD, and DSP were significant prognostic factors for survival (with associated P<0.05).
Even including age and smoking as parameters in multivariate model, using forward analysis, only DSP (OR =0.983; 95% CI: 0.974–0.993; P=0.001) was an independent significant factor for survival. Next, ROCs were constructed for the three most powerful variables as a function of their ability to predict 6-year mortality (Figure 3). The study participants were stratified using a DSP cut-off value of 280 m% into two groups according to the best AUC value. As shown in Table 2, there were no statistically significant differences in sex distribution, BMI, FEV1/FVC in pulmonary function tests, or oxygen saturation at rest between the two groups. Bacteriology and regular treatment regimens (data not shown) were also similar between groups. Patient age was significantly lower in the higher DSP group compared with the lower DSP group. Some components of pulmonary function (FVC and FEV1) were higher in the higher DSP group. The outcomes were significantly different between these two groups (Figure 4).
Figure 3.
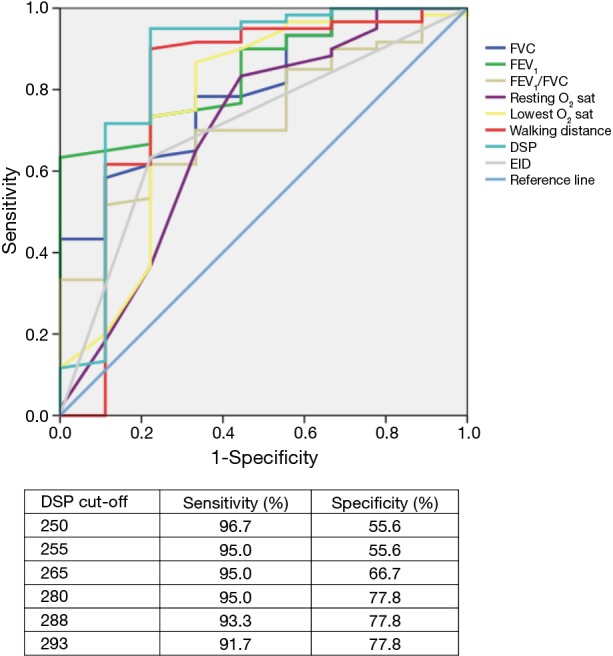
ROC curves of survival. ROC, receiver operating characteristic; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; O2 sat, oxygen saturation; DSP, distance saturation product; EID, exercise-induced oxygen desaturation.
Table 2. The relationship between DSP, characteristics, and outcomes of patients with non-CF bronchiectasis.
| Characteristics | DSP <280 (n=10) | DSP ≥280 (n=59) | P |
|---|---|---|---|
| Age (years) | 68.9±10.8 | 55.4±13.4 | 0.003 |
| Sex (M/F) | 7/3 | 32/27 | 0.281 |
| BMI | 20.9±4.5 | 22.2±3.2 | 0.286 |
| Smoking | |||
| Never | 5 | 50 | 0.024 |
| Ex/current | 5 | 9 | – |
| PFT | |||
| FVC (L) | 1.2±0.4 | 2.1±0.7 | 0.000 |
| FEV1 (L) | 0.8±0.3 | 1.6±0.7 | 0.001 |
| FEV1/FVC (%) | 64.6±12.4 | 72.0±11.4 | 0.063 |
| Total IgE | 56.9±65.2 | 70.2±72.7 | 0.607 |
| ECP (serum) | 17.8±11.1 | 21.4±29.9 | 0.708 |
| Bacterial colony | |||
| Pseudomonas aeruginosa | 3 | 13 | 0.255 |
| Others | 0 | 13 | – |
| Normal flora/no growth | 7 | 33 | – |
| 6MWT | |||
| Rest O2 sat (%) | 93.2±4.3 | 95.8±2.0 | 0.098 |
| Lowest O2 sat (%) | 81.7±10.0 | 87.7±6.6 | 0.017 |
| Walk distances (m) | 302.3±38.6 | 477.5±68.9 | 0.000 |
| Mortality | |||
| Survivors | 3 | 57 | 0.000 |
| Non-survivors | 7 | 2 | – |
6MWT, 6-minute walk test; BMI, body mass index; CF, cystic fibrosis; DSP, distance-saturation product; ECP, eosinophilic cationic protein; EID, exercise-induced oxygen desaturation; FEV1, volume that has been exhaled at the end of the first second of forced expiration; FVC, forced vital capacity; IgE, immunoglobulin E; PFT, pulmonary function test.
Figure 4.
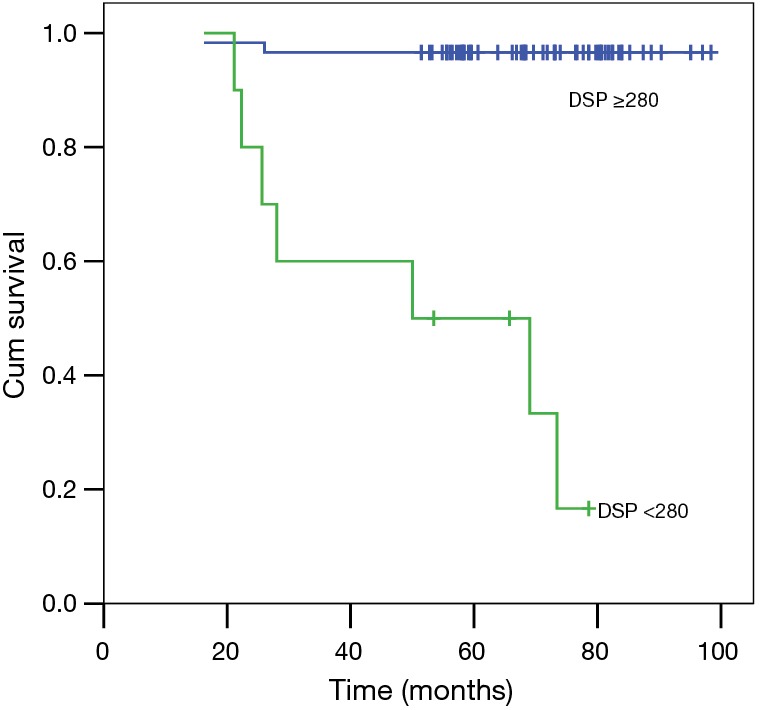
Kaplan-Meier survival curve for patients with non-CF bronchiectasis grouped by distance-saturation product (DSP, cut-off value: 280 m%) during the 6MWT (blue line: higher group; P<0.001). Non-CF, non-cystic fibrosis; 6MWT, 6-minute walk test.
Discussion
Few studies have reported the outcomes of adult non-CF bronchiectasis (6,14-16). Two severity scoring systems were recently reported as useful to predict mortality in patients with bronchiectasis, but are thought to be too complicated for use in daily clinical practice (6,7). Our study is unique in that it included patients with very well-defined bronchiectatic, all of whom had extensive disease at baseline, particularly indicated by 6MWT measurements. Furthermore, all patients were followed prospectively until death or the end of this 6-year study. To the best of our knowledge, this is the first study to identify the value of DSP to predict mortality of adult patients with non-CF bronchiectasis.
Non-CF bronchiectasis is a progressive disease characterized by chronic airway infection and inflammation (1,17). The results of this study showed a mortality rate of 13.0% over the 6-year study period. This finding is consistent with the results of a prospective analysis conducted by Loebinger et al., in which the 4-year mortality rate was 9% and the 8.8-year mortality rate was 16.5% (15). The primary causes of death among the patients included in the present study were respiratory infection and respiratory failure, suggesting that non-CF bronchiectasis was a main cause of death.
Univariate survival analysis showed that mortality was associated with the degree of restrictive and obstructive disease, which is consistent with 2009 report by Loebinger et al. (15). A recent study by Goeminne et al. reported that the mortality rate of patients with non-CF bronchiectasis and associated COPD was higher than those with non-CF bronchiectasis alone (16). Using the definition of pulmonary function in COPD as FEV1/FVC <70% among smokers, the trend in our cohort was similar, although it did not reach statistical significance (data not shown).
6MWT is a standardized and reproducible method to measure functional capacity in various diseases (10,18-20). However, the value of this test to predict prognoses of patients with non-CF bronchiectasis remains unknown. This is the first study to specifically evaluate 6MWD combined with simultaneously measured lowest oxygen saturation values during 6MWT as a predictor of mortality in patients with non-CF bronchiectasis with a wide range of disease severity. As Lee et al. report, the role of the clinical determinants of the 6MWT in bronchiectasis had been explored (12). But its predict role was not well studied such as BODE index in COPD. Even the FACED or BSI score did not include the parameter of 6WMD (distance) or EID (desaturation). Previous studies focusing on mortality (14-16) did not include the parameters of 6MWT, either. In this study, the 6WMD is lower in non-survival group. Among a larger number of variables examined against mortality, most were not independently predictive and were discarded using a stepwise methodology, to develop a final model that contained only seven covariates. FVC, FEV1, FEV1/FVC, EID (saturation O2% <88%), lowest oxygen saturation during 6MWT, 6MWD, and DSP were significant prognostic factors for survival (with associated P<0.05). And using forward multivariate analysis, only DSP (OR =0.983; 95% CI: 0.974–0.993; P=0.001) was an independent significant factor for survival.
The prognostic value of hypoxemia in chronic obstructive airway disease (8,21) and interstitial lung diseases (10) is well established, but not in patients with non-CF bronchiectasis. Chronic alveolar hypoxia results in sustained pulmonary vasoconstriction and pulmonary vascular remodeling, and subsequent onset and progression of hypoxia-induced pulmonary hypertension (22). Onen et al. found that hypoxemia was more closely correlated with mortality in patients with bronchiectasis (14). Dupont et al. reported that long-term oxygen therapy was an independent factor for survival of patients with bronchiectasis after the first admission to an intensive care unit (23). The results of the present study showed higher resting oxygen saturation under room air conditions among the survivors, although this difference did not reach statistical significance. In the current study, two patients received long-term oxygen therapy, but neither survived.
Another novel finding of this study is that a decrease in saturation during 6MWT was a very common phenomenon among patients with non-CF bronchiectasis. EID of 88% or less was observed in 77.8% and 36.7% of surviving and non-surviving patients, respectively. EID during 6MWT reportedly has prognostic value for patients with COPD (24) or idiopathic interstitial pneumonia (10). However, there is a lack of data for patients with bronchiectasis. The current study is the first to report that EID during 6MWT is more common in non-survivors, although this was not a significant factor after multivariate analysis. In patients with limited airflow, Mak et al. showed that the level of EID during 6MWT was related to the severity of impaired lung function (25). However, impairment of lung function could not be accurately predicted by any single lung function test (25). In the present study, only DSP, the product of the final distance walked in meters and the lowest room air oxygen saturation during 6MWT, accurately predicted mortality among patients with non-CF bronchiectasis after multivariate linear regression analysis.
The application of DSP in patients with a variety of chronic lung diseases has been reported (13,20,26). Lettieri et al. found that DSP provides a novel measurement with slightly better accuracy than other parameters to predict survival of patients with idiopathic pulmonary fibrosis (13). In patients with sarcoidosis, Bourbonnais et al. reported that DSP is an easily calculated clinical parameter to predict health-related quality of life over time and for intervention (26). The results of the present study showed that both walking distance and the lowest oxygen saturation during 6MWT were independently predictive of outcome in non-CF bronchiectasis. Combining both parameters into a composite index, DSP provided more accurate prognostic information than either of the component parts. As with other composites, this index is less reliable than any single component and therefore may be more reliable overall. In ROC analysis, it appeared that a DSP breakpoint of 280 m% had the greatest discriminatory power with regards to mortality. This index, which is simple to use in clinical practice can provide important information that could change investigation protocols and identify patients who require more intensive management.
There were some limitations to this study. First, the number of patients was limited and all were recruited from a single hospital, which may limit the generalizability of the study results. Second, evolutionary variables, such as the number of exacerbations or hospitalizations, were not included in the analysis. Third, important transversal variables related to non-CF bronchiectasis, such as pulmonary hypertension or cardiovascular disorders and quality of life, were not included in the analysis because the limited number of patients did not allow for the inclusion of more variables in the factorial analysis. Fourth, we did not have the complete parameter to calculate the BSI or FACED, mostly due to lack of their previous exacerbations, hospitalization history and mMRC records. It is difficult to re-collect those parameters in BSI or FACED. Finally, the impact of non-tuberculosis mycobacterial colonization or infection of these patients was not studied. Therefore, larger, multicenter studies are needed with larger numbers of patients to corroborate our results.
Conclusions
DSP represents an easy-to-use measure to predict survival of patients with non-CF bronchiectasis. Patients with a DSP below 280 m% are at a significantly increased risk of death. The simplicity, ease of calculation, and availability of 6MWT in a variety of clinical settings underscores the potential utility of DSP as a tool for evaluation of patients with non-CF bronchiectasis. The results of this study may also help to define useful subgroups for the assessment of treatment regimes in much needed randomized controlled trials to help advance non-CF bronchiectasis treatment towards a stronger evidence base.
Acknowledgements
Funding: This study was supported by research grants from the Chang Gung Medical Foundation, Chang Gung University (CMRP370791) and the National Science Council of Taiwan (NSC 100-2314-B-182-046 and MOST 103-2314-B-182A).
Ethical Statement: The study protocol was approved by the Institutional Review Board of Chang Gung Medical Foundation (approval no. 97-1105A3) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Fuschillo S, De Felice A, Balzano G. Mucosal inflammation in idiopathic bronchiectasis:cellular and molecular mechanisms. Eur Respir J 2008;31:396-406. 10.1183/09031936.00069007 [DOI] [PubMed] [Google Scholar]
- 2.Reiff DB, Wells AU, Carr DH, et al. CT findings in bronchiectasis: limited value in distinguishing between idiopathic and specific types. AJR Am J Roentgenol 1995;165:261-7. 10.2214/ajr.165.2.7618537 [DOI] [PubMed] [Google Scholar]
- 3.Ooi GC, Khong PL, Chan-Yeung M, et al. High-resolution CT quantification of bronchiectasis: clinical and functional correlation. Radiology 2002;225:663-72. 10.1148/radiol.2253011575 [DOI] [PubMed] [Google Scholar]
- 4.Pasteur MC, Bilton D, Hill AT, et al. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010;65 Suppl 1:i1-58. 10.1136/thx.2010.136119 [DOI] [PubMed] [Google Scholar]
- 5.McShane PJ, Naureckas ET, Tino G., et al. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2013;188:647-56. 10.1164/rccm.201303-0411CI [DOI] [PubMed] [Google Scholar]
- 6.Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014;189:576-85. 10.1164/rccm.201309-1575OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-García Má , de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014;43:1357-67. 10.1183/09031936.00026313 [DOI] [PubMed] [Google Scholar]
- 8.Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis 2011;6:199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 2000;161:487-92. 10.1164/ajrccm.161.2.9906015 [DOI] [PubMed] [Google Scholar]
- 10.Lama VN, Flaherty KR, Toews GB, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003;168:1084-90. 10.1164/rccm.200302-219OC [DOI] [PubMed] [Google Scholar]
- 11.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 12.Lee AL, Button BM, Ellis S, et al. Clinical determinants of the 6-Minute Walk Test in bronchiectasis. Respir Med 2009;103:780-5. 10.1016/j.rmed.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 13.Lettieri CJ, Nathan SD, Browning RF, et al. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med 2006;100:1734-41. 10.1016/j.rmed.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 14.Onen ZP, Gulbay BE, Sen E, et al. Analysis of the factors related to mortality in patients with bronchiectasis. Respir Med 2007;101:1390-7. 10.1016/j.rmed.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 15.Loebinger MR, Wells AU, Hansell DM, et al. Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J 2009;34:843-9. 10.1183/09031936.00003709 [DOI] [PubMed] [Google Scholar]
- 16.Goeminne PC, Nawrot TS, Ruttens D, et al. Mortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysis. Respir Med 2014;108:287-96. 10.1016/j.rmed.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 17.Tsang KW, Tipoe GL. Bronchiectasis: not an orphan disease in the East. Int J Tuberc Lung Dis 2004;8:691-702. [PubMed] [Google Scholar]
- 18.Hallstrand TS, Boitano LJ, Johnson WC, et al. The timed walk test as a measure of severity and survival in idiopathic pulmonary fibrosis. Eur Respir J 2005;25:96-103. 10.1183/09031936.04.00137203 [DOI] [PubMed] [Google Scholar]
- 19.Eaton T, Young P, Milne D, et al. Six-minute walk, maximal exercise tests: reproducibility in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 2005;171:1150-7. 10.1164/rccm.200405-578OC [DOI] [PubMed] [Google Scholar]
- 20.Alhamad EH, Shaik SA, Idrees MM, et al. Outcome measures of the 6 minute walk test: relationships with physiologic and computed tomography findings in patients with sarcoidosis. BMC Pulm Med 2010;10:42. 10.1186/1471-2466-10-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casanova C, Cote C, Marin JM, et al. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest 2008;134:746-52. 10.1378/chest.08-0520 [DOI] [PubMed] [Google Scholar]
- 22.Ball MK, Waypa GB, Mungai PT, et al. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1α. Am J Respir Crit Care Med 2014;189:314-24. 10.1164/rccm.201302-0302OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupont M, Gacouin A, Lena H, et al. Survival of patients with bronchiectasis after the first ICU stay for respiratory failure. Chest 2004;125:1815-20. 10.1378/chest.125.5.1815 [DOI] [PubMed] [Google Scholar]
- 24.Takigawa N, Tada A, Soda R, et al. Distance and oxygen desaturation in 6-min walk test predict prognosis in COPD patients. Respir Med 2007;101:561-7. 10.1016/j.rmed.2006.06.017 [DOI] [PubMed] [Google Scholar]
- 25.Mak VH, Bugler JR, Roberts CM, et al. Effect of arterial oxygen desaturation on six minute walk distance, perceived effort, and perceived breathlessness in patients with airflow limitation. Thorax 1993;48:33-8. 10.1136/thx.48.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourbonnais JM, Malaisamy S, Dalal BD, et al. Distance saturation product predicts health-related quality of life among sarcoidosis patients. Health Qual Life Outcomes 2012;10:67. 10.1186/1477-7525-10-67 [DOI] [PMC free article] [PubMed] [Google Scholar]