Abstract
Background
Esophageal cancer is the eighth most common cancer worldwide. The prognosis of esophageal cancer patients is dismal, especially those with distant organ metastasis. However, there are few studies describing the patterns of distant metastasis in esophageal cancer systematically.
Methods
We gathered the data from Surveillance Epidemiology and End Results (SEER) database between 2010 and 2013. Categorical variables were analyzed by the Pearson Chi square test, and continuous variables were analyzed by the two-sample t test. Survival estimation and comparison among different variables were performed using Kaplan-Meier method. A multivariable logistic regression model was used to calculate odds ratios (OR) for sex, age, anatomical site, and histological type on specific metastases. Proportional hazards regression model was conducted to obtain adjusted hazard ratio (HRs) for different predictors of overall survival.
Results
A total of 9,934 patients were eligible. Liver was the most common metastatic site in the patients of esophageal cancer and followed by lung, bone and brain. Some clinical features, including age, sex, histology type and histologic grade were independent risk factors for different sites of metastasis. Younger age, poorer differentiation, adenoma type and more metastatic sites might lead to poorer prognosis.
Conclusions
Our findings revealed the patterns of metastasis in esophageal cancer, which could help clinicians to identify patients with metastasis and provide proper treatment.
Keywords: Esophageal cancer, metastatic pattern, Surveillance Epidemiology and End Results (SEER) program
Introduction
Esophageal cancer is the eighth most common cancer worldwide, with 455,800 new cases estimated in 2012, and the sixth leading cause of death from cancer with 400,200 deaths (1). The prognosis of esophageal cancer patients is dismal, especially those with distant organ metastases (2). Poor outcomes in patients with esophageal cancer are related to the propensity for metastases, even when tumors are superficial (2,3) and lack of effective treatment modality for those with distant metastasis (4). However, there are few studies describing the patterns of distant metastasis in esophageal cancer systematically. In this study, we described demographic and clinical characteristics and overall survival in patients with distant metastatic esophageal cancer using Surveillance Epidemiology and End Results (SEER) database from 2010 to 2013.
Methods
Study population
The SEER program is the largest publicly available cancer database, which collects information on cancer incidence and survival from 17 population-based cancer registries covering approximately 28% of the United States population. We hypothesize that SEER is a good database from which to analyze the distant metastasis pattern for esophageal cancer. However, SEER does not include any information on the location of metastases until 2010. As a result, we can only analyze the data between 2010 and 2013.
The criteria defined for inclusion in this study were primary histologically confirmed esophageal cancer and diagnosed between 2010 and 2013. We excluded a total of 3,222 patients (24%) from those diagnosed with esophageal cancer in the SEER database (n=13,156) mainly because of unknown histologic grade (n=2,680) or pathology type (n=467) of tumor, lack of racial information (n=45) or unstaged tumors (n=1). Patients with ‘blanks’ metastatic site (n=319) should also be excluded. A total of 9,934 patients with esophageal cancer matching the specified criteria were included in the final sample for this analysis (Figure 1).
Figure 1.
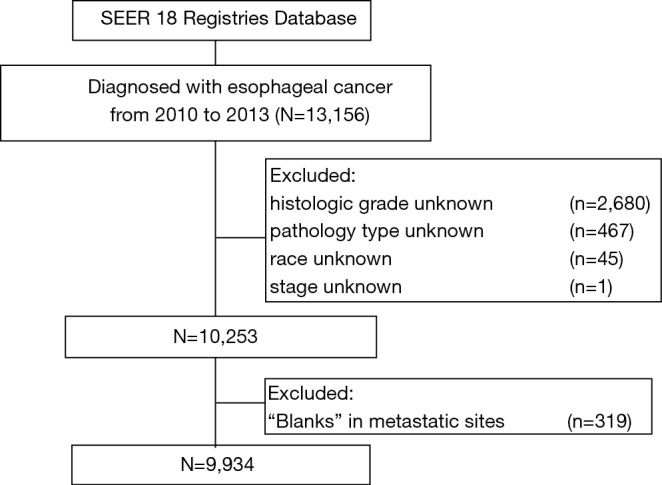
A flowchart of patient selection from the SEER database
Statistical analysis
Demographic of patients and tumor characteristics were summarized with descriptive statistics. Categorical variables were analyzed by the Pearson Chi square test, and continuous variables were analyzed by the two-sample t test.
Overall survival (OS) was defined as the period from diagnosis to death as a result of any cause. Survival estimation and comparison among different variables were performed using Kaplan-Meier method.
A multivariable logistic regression model was used to calculate odds ratios (OR) for sex, age, anatomical site, and histological type on specific metastases. Proportional hazards regression model was conducted to obtain adjusted hazard ratio (HRs) for different predictors of overall survival.
A two-sided P value of <05 was considered statistically significant. All statistical analyses were performed using SPSS 19.0 (SPSS, Chicago, IL, USA), and the survival curves were drawn with GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA).
Results
Patient characteristics
The study group consisted of 9,934 patients with esophageal cancer diagnosed from 2010 to 2013, including 7,979 men (80.3%) and 1,955 women (19.7%). According to UICC TNM classification 7th edition, 32.7% (n=3,245) of the patients were stage IV (Figure 2). Esophageal adenocarcinoma (EAC) was almost twice (61.4% vs. 32.6%) than esophageal squamous cell carcinoma (ESCC) when diagnosed.
Figure 2.
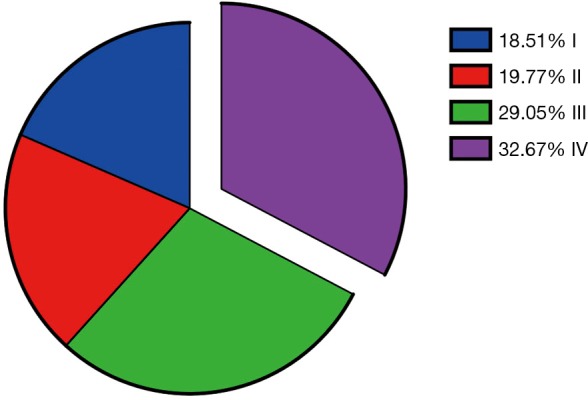
Proportions of different stages in selected patients
Clinical features of metastases were as follows (Table1).
Table 1. Clinical characteristics of patients.
| Features | Liver metastasis, n (%) | P | Lung metastasis, n (%) | P | Bone metastasis, n (%) | P | Brain metastasis, n (%) | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | No | Yes | |||||
| Sex | <0.001 | 0.048 | <0.001 | 0.233 | ||||||||
| Women | 1,755 (89.8) | 200 (10.2) | 1,787 (91.4) | 168 (8.6) | 1,847 (94.5) | 108 (5.5) | 1,930 (98.7) | 25 (1.3) | ||||
| Men | 6,633 (83.1) | 1,346 (16.9) | 7,180 (90.0) | 799 (10.0) | 7,321 (91.8) | 658 (8.2) | 7,847 (98.3) | 132 (1.7) | ||||
| Age | <0.001 | 0.760 | <0.001 | <0.001 | ||||||||
| Mean | 67.05 | 64.88 | 66.73 | 66.61 | 66.88 | 64.72 | 66.78 | 62.64 | ||||
| Race | 0.001 | <0.001 | 0.270 | 0.351 | ||||||||
| Caucasian | 7,100 (83.9) | 1,367 (16.1) | 7,691 (90.8) | 776 (9.2) | 7,799 (92.1) | 668 (7.9) | 8,327 (98.3) | 140 (1.7) | ||||
| African American | 890 (87.5) | 127 (12.5) | 884 (86.7) | 133 (13.1) | 948 (93.2) | 69 (6.8) | 1,006 (98.9) | 11 (1.1) | ||||
| Asian | 398 (88.4) | 52 (11.6) | 392 (87.1) | 58 (12.9) | 421 (93.6) | 29 (6.4) | 444 (98.7) | 6 (1.3) | ||||
| Histology | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| ESCC | 2,952 (91.1) | 288 (8.9) | 2,872 (88.6) | 368 (11.4) | 3,058 (94.4) | 182 (5.6) | 3,222 (99.4) | 18 (0.6) | ||||
| EAC | 4,922 (80.7) | 1,176 (19.3) | 5,542 (90.9) | 556 (9.1) | 5,576 (91.4) | 522 (8.6) | 5,965 (97.8) | 133 (2.2) | ||||
| Others | 514 (86.2) | 82 (13.8) | 553 (92.8) | 43 (7.2) | 534 (89.6) | 62 (10.4) | 590 (99.0) | 6 (1.6) | ||||
| Grade | <0.001 | <0.001 | <0.001 | 0.077 | ||||||||
| 1 | 593 (93.4) | 42 (6.6) | 604 (95.1) | 31 (4.9) | 612 (96.4) | 23 (3.6) | 629 (99.1) | 6 (0.9) | ||||
| 2 | 3,595 (87.2) | 530 (12.8) | 3,751 (90.9) | 374 (9.1) | 3,887 (94.2) | 238 (5.8) | 4,069 (98.6) | 56 (1.4) | ||||
| 3–4 | 4,200 (81.2) | 974 (18.8) | 4,612 (89.1) | 562 (10.9) | 4,669 (90.2) | 505 (9.8) | 5,079 (98.2) | 95 (1.8) | ||||
ESCC, esophageal squamous cell carcinoma; EAC, esophageal adenocarcinoma.
Metastasis pattern
According to the database, we had metastatic information related to liver, lung, bone and brain metastases. Patients who had metastasis to at least one of the four sites accounted for 77.3% (2,507/3,245) of stage IV diseases. Proportions of metastases to the four sites above were presented in Figure 3. In the patients of all-stage esophageal cancer, liver (15.6%) was the most common metastatic site among the four sites followed by lung (9.7%) and bone (7.7%). Metastasis to brain (1.6%) was the least among the four sites. In all stage IV patients, the proportion of liver, lung, bone and brain metastasis was 47.6%, 29.8%, 23.6%, and 4.8%, respectively. The sum was over 100% due to multiple metastases in some patients.
Figure 3.
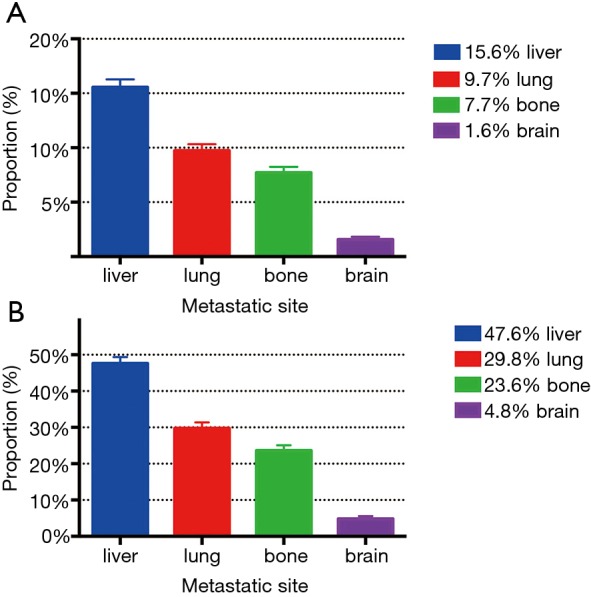
Proportions of metastasis to different sites in all-stage esophageal cancer patients (A) and stage IV patients (B).
We found that histology type was one of the independent factors for metastatic diseases (Table 2). ESCC had a higher incidence rate of lung metastasis (11.4% vs. 9.1% (EAC), P<0.001), while EAC had a higher incidence rate of liver (19.3% vs. 8.9%, P<0.001), bone (8.6% vs. 5.6%, P<0.001) and brain (2.2% vs. 0.6%, P<0.001) metastasis than ESCC (Table 1). The characteristics of lung metastasis might be different from other sites.
Table 2. Multivariate logistic regression model for odds of specific metastases in esophageal cancer.
| Features | Liver metastasis | Lung metastasis | Bone metastasis | Brain metastasis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | P | OR | P | OR | P | OR | P | ||||
| Sex | <0.001 | 0.007 | 0.022 | 0.479 | |||||||
| Women | 1 | 1 | 1 | 1 | |||||||
| Men | 1.353 | 1.282 | 1.289 | 0.852 | |||||||
| Age | 0.985 | <0.001 | 1.000 | 0.892 | 0.985 | <0.001 | 0.971 | <0.001 | |||
| Race | 0.299 | 0.007 | 0.845 | 0.720 | |||||||
| Caucasian | 1 | 1 | 1 | 1 | |||||||
| African American | 1.167 | 1.354 | 1.054 | 1.253 | |||||||
| Asian | 0.927 | 1.355 | 0.924 | 1.259 | |||||||
| Histology | <0.001 | 0.001 | 0.001 | <0.001 | |||||||
| ESCC | 1 | 1 | 1 | 1 | |||||||
| EAC | 2.294 | 0.802 | 1.424 | 4.246 | |||||||
| Others | 1.304 | 0.558 | 1.489 | 1.740 | |||||||
| Grade | <0.001 | <0.001 | <0.001 | 0.115 | |||||||
| 1 | 1 | 1 | 1 | 1 | |||||||
| 2 | 2.177 | 1.908 | 1.661 | 1.507 | |||||||
| 3–4 | 3.324 | 2.451 | 2.804 | 1.971 | |||||||
ESCC, esophageal squamous cell carcinoma; EAC, esophageal adenocarcinoma; OR, odds ratio
As for sex, men had a higher incidence rate than women in terms of most of metastatic sites [liver (16.9% vs. 10.2%, P<0.001), lung, (10.0% vs. 8.6%, P=0.048) and bone (8.2% vs. 5.5%, P<0.001)] (Tables 1,2). For most of the metastatic sites (liver, bone and brain), we found that there was a significant difference (P<0.001) in age between patients with and without metastasis and age was also an independent risk factor for metastatic diseases above. Younger patients were more likely to suffer from metastatic diseases (Table 1).
Besides, histologic grade of tumor was also an independent risk factor for metastatic disease (Table 2). In most of the metastatic sites, it could be seen that poorer differentiation was independently associated with higher incidence of metastasis (Table 1).
Survival
Survival in metastatic esophageal cancer was shown in Figure 4. Among the four sites of distant metastases, there was no significant difference in OS (P=0.078) (Figure 4A), irrespective of number of metastatic sites. However, we found a decrease in OS with the increase of number of metastatic sites. The median OS of one, two, three and four metastatic sites were 5.0, 4.0, 3.0 and 2.0 months, respectively and there was a significant difference (P<0.001) (Figure 4B). Among stage IV patients, there were also significant differences in OS between different histologic grades (P<0.001) (Figure 5A) and histology types (P=0.012) (Figure 5B). However, OS had no significant difference between men and women (P=0.455) (Figure 5C).
Figure 4.
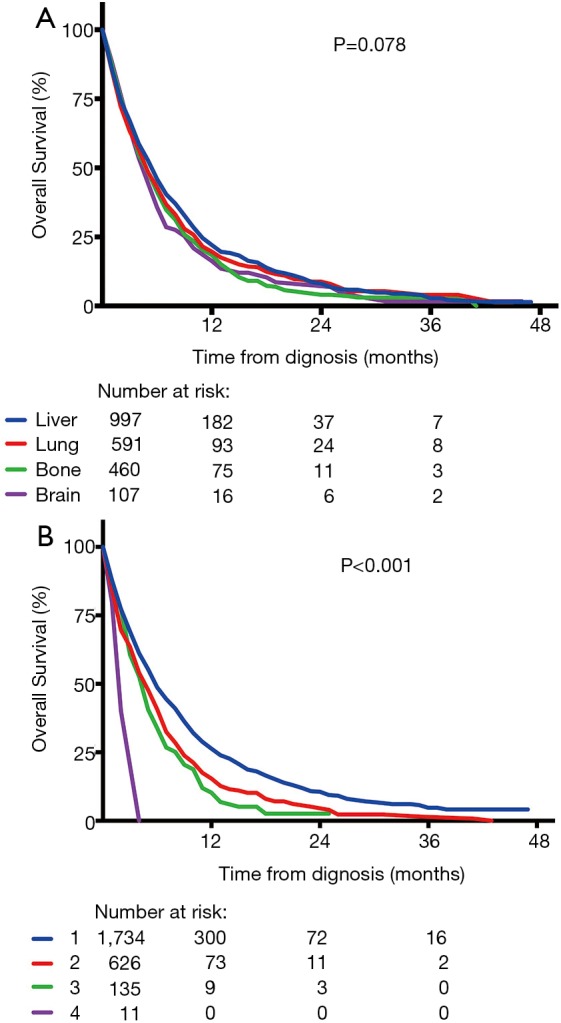
Survival curves of overall survival (OS) for (A) specific metastatic site (P=0.078) and (B) different number of metastatic sites (P<0.001).
Figure 5.
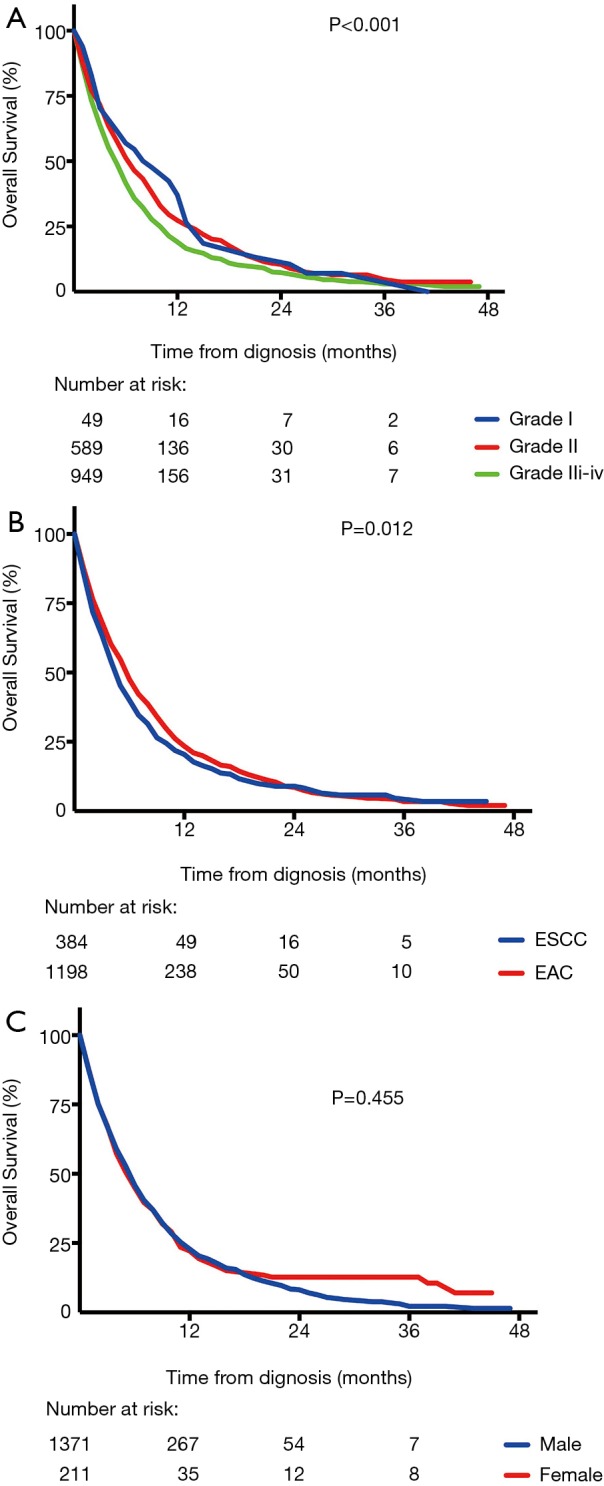
Survival curves of overall survival (OS) for (A) different histologic grades (P<0.001); (B) different histology type (P=0.012); (C) women and men (P=0.455).
According to the result of multivariate analysis, age, histology type and number of metastatic sites were independent prognostic factors of overall survival and histologic grade tended to be another prognostic factor (Table 3). Patients with younger age, poorer differentiation, adenoma type and more metastatic sites were more likely to decrease life expectancy.
Table 3. Multivariate analysis for survival in stage IV esophageal cancer.
| Features | HR | 95% CI | P |
|---|---|---|---|
| Age | 1.016 | 1.012 to 1.020 | <0.001 |
| Histologic grade | <0.001 | ||
| 1 | 1 (reference) | ||
| 2 | 0.996 | 0.768 to 1.291 | |
| 3-4 | 1.253 | 0.970 to 1.618 | |
| Histology type | 0.016 | ||
| ESCC | 1 (reference) | ||
| EAC | 0.879 | 0.795 to 0.971 | |
| Others | 1.033 | 0.849 to 1.257 | |
| Number of metastatic sites | <0.001 | ||
| 1 | 1 (reference) | ||
| 2 | 1.272 | 1.150 to 1.406 | |
| 3 | 1.491 | 1.232 to 1.805 | |
| 4 | 3.190 | 1.758 to 5.787 | |
ESCC, esophageal squamous cell carcinoma; EAC, esophageal adenocarcinoma; HR, hazard ratio.
Discussion
In this paper, we investigated patterns of distant organ metastases in esophageal cancer and presented results from SEER database. The relation between metastatic sites and clinical characteristics, including sex, age, race, histology type, histologic grade, was also discussed in this article, which would provide references for diagnosis and treatment.
With the development of diagnostic techniques, we found more metastatic diseases than ever before. From SEER database, the proportion of metastasis was growing over the past 20 years and it was 43.3% in patients without any prior treatment between 2009 and 2013. In our study, liver was the most common metastatic site among four sites, followed by lung, bone and brain, and the proportions were in consistent with autopsy findings.(5) In autopsy findings, lung (31%), liver (23%) and bone (13%) were also the most common sites and brain metastasis was still rare (less than 5%). Proportions of metastases from autopsy findings were much higher than our study due to the inclusion of recurrence and metastasis after treatment when autopsy, but the distribution pattern of metastatic sites was similar.
We found some relations between clinical characteristics and metastatic sites. Younger patients were more likely to suffer from metastatic diseases and had poorer prognosis, which was confirmed by an autopsy study. Barz H and Barz D (6) have demonstrated that the frequency of metastases from 5,708 cancers of various types, confirmed on autopsies, gradually decreases among patients beyond the age of 60. Cases without metastases have been found three times more in elderly people than in younger people and the sclerosis of capillaries may be an important factor for the reduction of distant organ metastases in elderly people.
Moreover, histologic grade might be another risk factor for metastatic diseases in our study. Quantities of studies have indicated that histologic grade should be regarded as a strong predictor of survival and survival always decreases monotonically according to histologic grade (7,8). The relation between histologic grade, metastatic disease and survival may result from the biological behavior of tumor, which still needs further research.
Prognosis of metastatic esophageal cancer is always dismal, and 5-year relative survival is 4.5% (9), which is much lower when compared with other high-incidence metastatic cancer, like breast cancer (26.3%) (10) and colorectal cancer (13.5%) (11). The main reasons are malignant biological behaviors and lack of effective systemic treatment. The objective response rates of 5-fluorouracil/cisplatin and paclitaxel/cisplatin, recommended regimens from NCCN guideline, are only 30–40% (12,13), which has limited influence on survival. No effective targeted therapy has been applied in metastatic esophageal cancer except for trastuzumab in HER2-neu overexpressing metastatic adenocarcinoma (14), which is rare among all patients (15).
In our study, there was no significant difference among four metastatic sites. However, OS was related to number of metastatic sites. Hellman and Weichselbaum (16) have proposed that a state of oligometastases and patients with oligometastases may benefit from local aggressive therapy and prolong their survival (17).
Accordingly, early diagnosis is extremely important. Five-year relative survival are 41.3% and 22.8% for localized and regional esophageal cancer (9), respectively, much more than metastatic esophageal cancer. Even when oligometastases, diagnosis as early as possible still could provide a chance for further treatment and better quality of life.
Knowledge of metastatic patterns may be useful in making clinical decisions, including early diagnosis and treatment. Evaluation when diagnosis should be systemic and targeted. Since liver and lung are the most common sites, regular imaging recommended by NCCN guideline (18), such as computed tomography (CT) with contrast for chest or abdomen, should be maintained and imaging of other sites should be applied when patients are with high risk factors for specific site of metastasis.
Diagnosis of metastasis is not only for staging, but also for further treatment. Nowadays, varieties of treatment modalities for oligometastases, including stereotactic body radiotherapy (SBRT), radiofrequency ablation and surgery, have been applied and proved to be effective (17). Our findings provide information on the patterns of distant metastases in esophageal cancer so that patients with metastatic esophageal cancer can be diagnosed and treated properly.
To our knowledge, this is the first SEER based study focusing on the metastatic pattern of esophageal cancer. However, there are still some obvious limitations due to the retrospective background of the study. First of all, it is necessary to mention that the database of the study only provides the data from 2010 to 2013 due to lack of enough information. Moreover, we only have information on metastasis to liver, lung, bone and brain, which may lead to an underestimation of other sites of metastasis. However, the four sites of metastasis could account for more than 70% of stage IV esophageal cancer both in our study and in autopsy findings (5). Furthermore, all information on metastasis is from their first diagnosis and we lack the following information, including treatment modalities and progression or remission after treatment. At last, we do not have the details about metastasis, like sizes, exact metastatic lesion quantity in one specific organ.
In conclusion, based on SEER database, we revealed that liver was the most common metastatic site in the patients of esophageal cancer and followed by lung, bone and brain. Some clinical features, including age, sex, histology type and histologic grade were independent risk factors for different sites of metastasis, which may help in surveillance. Prognosis of metastatic esophageal cancer was dismal, and younger age, poorer differentiation, adenoma type and more metastatic sites might lead to poorer prognosis. Our findings put forward the patterns of metastasis in esophageal cancer, which could help clinicians to identify patients with metastasis and deliver proper treatment.
Acknowledgements
Funding: This study was financially supported by grants from the National Natural Science Foundation of China (No. 21172043 and No. 21441010).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. 10.1056/NEJMra035010 [DOI] [PubMed] [Google Scholar]
- 3.Pennathur A, Farkas A, Krasinskas AM, et al. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg 2009;87:1048-54; discussion 54-5. 10.1016/j.athoracsur.2008.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka T, Fujita H, Matono S, et al. Outcomes of multimodality therapy for stage IVB esophageal cancer with distant organ metastasis (M1-Org). Dis Esophagus 2010;23:646-51. 10.1111/j.1442-2050.2010.01069.x [DOI] [PubMed] [Google Scholar]
- 5.Mandard AM, Chasle J, Marnay J, et al. Autopsy findings in 111 cases of esophageal cancer. Cancer 1981;48:329-35. [DOI] [PubMed] [Google Scholar]
- 6.Barz H, Barz D. [Age dependence of metastases. A study of more than 5000 cases of death from cancer]. Arch Geschwulstforsch 1984;54:77-83. [PubMed] [Google Scholar]
- 7.Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1-8. 10.1111/j.1442-2050.2008.00901.x [DOI] [PubMed] [Google Scholar]
- 8.Dickson GH, Singh KK, Escofet X, et al. Validation of a modified GTNM classification in peri-junctional oesophago-gastric carcinoma and its use as a prognostic indicator. Eur J Surg Oncol 2001;27:641-4. 10.1053/ejso.2001.1200 [DOI] [PubMed] [Google Scholar]
- 9.Institute NC. SEER Stat Fact Sheets: Esophageal Cancer. 2016. Avaible online: http://seer.cancer.gov/statfacts/html/esoph.html. Accessed 09.04 2016.
- 10.Institute NC. SEER Stat Fact Sheets: Female Breast Cancer. http://seer.cancer.gov/statfacts/html/breast.html. 2016. http://seer.cancer.gov/statfacts/html/breast.html. Accessed 09.04 2016.
- 11.Institute NC. SEER Stat Fact Sheets: Colon and Rectum Cancer. http://seer.cancer.gov/statfacts/html/breast.html. 2016. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed 09.04 2016.
- 12.Lorenzen S, Schuster T, Porschen R, et al. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2009;20:1667-73. 10.1093/annonc/mdp069 [DOI] [PubMed] [Google Scholar]
- 13.Petrasch S, Welt A, Reinacher A, et al. Chemotherapy with cisplatin and paclitaxel in patients with locally advanced, recurrent or metastatic oesophageal cancer. Br J Cancer 1998;78:511-4. 10.1038/bjc.1998.524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 15.Sauter ER, Keller SM, Erner S, et al. HER-2/neu: a differentiation marker in adenocarcinoma of the esophagus. Cancer Lett 1993;75:41-4. 10.1016/0304-3835(93)90205-N [DOI] [PubMed] [Google Scholar]
- 16.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol 2011;8:378-82. [DOI] [PubMed] [Google Scholar]
- 17.Lo SS, Moffatt-Bruce SD, Dawson LA, et al. The role of local therapy in the management of lung and liver oligometastases. Nat Rev Clin Oncol 2011;8:405-16. 10.1038/nrclinonc.2011.75 [DOI] [PubMed] [Google Scholar]
- 18.Network NCC. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Esophageal and Esophagogastric Junction Cancers. 2016. Available online: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. Accessed 09.14 2016.


