Abstract
Background
Aimed to identify the benefit population from continuation of epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs), this study investigated the efficacy of continuation of EGFR-TKIs plus chemotherapy beyond the response evaluation criteria in solid tumors-progressive disease (RECIST-PD) according to different progression modes and T790M mutational status.
Methods
From November 2009 to July 2015, 630 patients with advanced non-small cell lung cancer (NSCLC) receiving gefitinib as initial EGFR-TKI treatment were screened in Shanghai Pulmonary Hospital. A total of 170 patients with documented gradual or dramatic progression after gefitinib treatment who received chemotherapy alone or in combination with gefitinib were included. Post-RECIST-PD progression-free survival (PPFS) between continuation of gefitinib plus chemotherapy and chemotherapy alone was assessed.
Results
The incidence of T790M mutation was 42.9% (63/147) in patients who got acquired resistance in this study. Median PPFS was 4.0 months [95% confidence interval (CI), 3.1–4.9 months] in the chemotherapy group and 5.0 months (95% CI, 3.6–6.4 months) in the combination group with a borderline statistical significance (P=0.071). Continuation of gefitinib plus chemotherapy resulted in a significant improvement in PPFS compared with chemotherapy alone in patients with EGFRT790M-negative tumors [median PPFS: 6.6 vs. 3.5 months, hazard ratio (HR) 0.50, 95% CI, 0.29–0.88; P=0.011], especially in pemetrexed-based chemotherapy (HR 0.45, 95% CI, 0.24–0.86; P=0.011). PPFS was similar in patients with EGFRT790M-positive tumors (median PPFS: 5.0 vs. 5.5 months, HR 0.80, 95% CI, 0.40–1.61; P=0.520) or EGFRT790M-unknown tumors (median PPFS: 2.0 vs. 3.0 months, HR 1.40, 95% CI, 0.69–2.81; P=0.323).
Conclusions
Our study showed that continuous gefitinib plus chemotherapy, especially pemetrexed-based therapy, significantly improved PPFS in patients with EGFRT790M-negative tumors as compared with chemotherapy alone, suggesting that this subtype of patients may derive clinical benefit from continuation of gefitinib treatment beyond progression.
Keywords: Non-small-cell lung cancer (NSCLC), epidermal growth factor receptor (EGFR), EGFR tyrosine kinase inhibitor (EGFR-TKI), acquired resistance, continuation
Introduction
With the identification of epidermal growth factor receptor (EGFR) mutations that correlate with the efficacy of EGFR tyrosine kinase inhibitors (EGFR-TKIs) (1,2), EGFR-TKIs have been the standard of care for patients with non-small cell lung cancer (NSCLC) harboring activating EGFR mutations. Findings from several randomized controlled trials have demonstrated that EGFR-TKIs significantly improve objective response rate (ORR) and prolong progression-free survival (PFS) along with better quality of life as compared with standard chemotherapeutic regimens (3-9).
Disappointingly, acquired resistance inevitably develops (10). The dominant mechanism of acquired resistance to EGFR-TKIs is the secondary T790M mutation in EGFR exon 20, accounting for approximately 50% of EGFR-TKI resistance (11). Recently, third-generation EGFR-TKIs, namely AZD9291 and CO1686 have shown dramatic response in patients who get acquired resistance driving by secondary T790M mutations (12,13). Other mechanisms include MET amplification, HER2 amplification, PIK3CA mutations, BRAF mutations and epithelial mesenchymal transformation (EMT) (11,14-17). To date, standard management strategies for patients with certain mechanisms of acquired resistance have not yet been well defined and are being investigated in preclinical models or clinical trials.
It has been hypothesized that not all metastatic tumors share the same mechanism of resistance and some of the tumor cells may be sensitive to EGFR-TKIs at the time of progression (18). Indeed, several retrospective studies have observed a survival benefit from continuation of EGFR-TKIs beyond progression (19-24). ASPARATION, an open-label, single-arm, phase 2 study, has recently demonstrated that continuing erlotinib therapy beyond progression result in a 3.1-month improvement in PFS (25). However, in IMPRESS, a randomized phase 3 study, continuation of gefitinib in combination with platinum-based doublet chemotherapy failed to improve survival outcomes compared with chemotherapy alone after disease progression (26).
Clinical observations have suggested that clinical modes of EGFR-TKIs failure may be heterogeneous, including local disease progression, gradual progression and dramatic progression (20). Furthermore, patients with local disease progression or gradual progression may derive clinical benefit from continuing EGFR-TKIs (20). However, IMPRESS and ASPIRATION did not classify the different progression modes or molecular mechanisms of acquired resistance. Thus, we performed the present study to investigate the efficacy of continuation of EGFR-TKIs together with chemotherapy at the time of progression according to different progression subtypes (gradual progression vs. dramatic progression) or resistance mechanisms (T790M-dependent vs. T790M-independent), aiming at identifying patients who may derive clinical benefit from continuation of EGFR-TKIs.
Methods
Patient and study design
Patients with advanced or recurrent stage IV NSCLC who developed acquired resistance to EGFR-TKIs were enrolled in this study. The criteria of acquired resistance to EGFR-TKIs were defined by Jackman et al. (10). Only patients with documented gradual or dramatic progression after gefitinib treatment who received cytotoxic chemotherapy alone or in combination with gefitinib were included and patients with local progression who received EGFR-TKIs plus local therapy were excluded. The definition of clinical modes of acquired resistance to EGFR-TKIs was proposed by Yang et al. as previously described (20,27). In brief, local disease progression was defined as solitary extracranial lesion or limitation in intracranial lesions (covered by a radiation field), gradual progression was defined as minor increment of tumor burden and dramatic progression was defined as rapid increment of tumor burden or with significant clinical symptoms. Tumor assessments were conducted every 6–8 weeks in accordance with the response evaluation criteria in solid tumors (RECIST) (version 1.1). This study was approved by the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University, Shanghai, China, and all participants signed an informed consent (Institutional Review Board No. K17-140).
Clinical characteristics and treatment strategies were collected from patient medical records including survival data, clinical modes of resistance, tumor EGFR mutational status at baseline and disease progression. At the time of development of acquired resistance, re-biopsy samples were obtained from either primary sites or metastasis sites, whichever available.
We mainly compared post-RECIST-progressive disease (PD) PFS (PPFS) between continuation of EGFR-TKIs in combination with chemotherapy and chemotherapy alone in patients who developed acquired resistance to gefitinib according to clinical modes of acquired resistance (gradual vs. dramatic progression) and mechanism of acquired resistance (T790M-dependent vs. T790M-independent). Other objectives included ORR and disease control rate (DCR).
EGFR mutation analysis
EGFR gene mutations in exon 18–21 of the tyrosine kinase domain were analyzed in EGFR-TKI-naïve or TKI-resistant samples by using a polymerase chain reaction (PCR)-based method, the Scorpion Amplification Refractory Mutation System (ARMS) as previously described (27,28).
Treatment
After the development of acquired resistance, subsequent treatment regimens depended on the physicians’ discretion according to patients’ age, Eastern Cooperative Oncology Group (ECOG) performance status (PS), lines of initial gefitinib treatment, toxicities of previous therapy and/or the patient’s insurance. Patients were given platinum plus cytotoxic drug (pemetrexed, docetaxel, paclitaxel or gemcitabine) with or without gefitinib when receiving first-line gefitinib. Meanwhile, for patients receiving subsequent lines of gefitinib, single cytotoxic drug with or without gefitinib could be prescribed.
Statistical analysis
PPFS was defined as from the date of acquired resistance to post-RECIST-PD or death, whichever came first. All categorical variables were analyzed using the χ2 test or Fisher’ exact test. All time-to-event outcomes were estimated using the Kaplan-Meier method and were compared among patient groups using the log-rank test or the Cox proportional hazards model. All statistical analyses were carried out by using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). A two-side P value of less than 0.05 (P<0.05) was considered statistically significant.
Results
Patient demographics and clinical characteristics
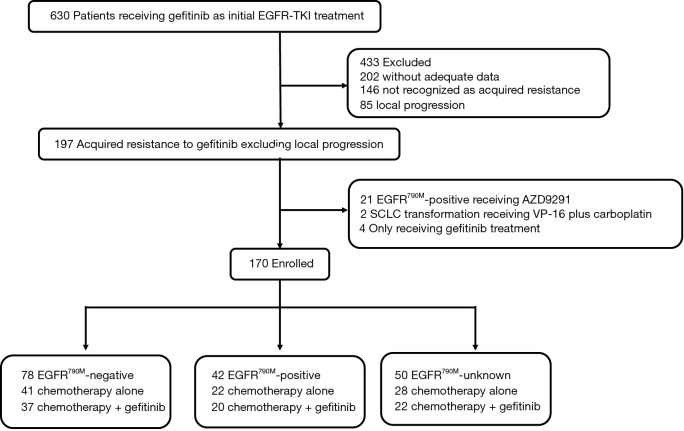
From November 2009 to July 2015, 630 patients with full followed-up data receiving gefitinib as initial EGFR-TKI treatment were screened in our institute. Among them, 197 patients developed documented acquired resistance to gefitinib and patients with local progression were excluded. Of those, 170 patients who met the inclusion criteria with adequate PPFS data were enrolled in the current study (Figure 1). The demographics and clinical characteristics of enrolled 170 patients are presented in Table 1. Briefly, 25.3% of patients were older than 65 years old, 41.8% were male, 78.2% were never-smokers, 92.9% had adenocarcinoma histologic subtypes, 94.7% had ECOG PS 0 or 1, 81.2% had stage IV disease, 27.6% had brain metastasis at baseline. 86.4% of patients harbored EGFR-sensitive mutations (including 48.2% harbored exon 19 deletions and 38.2% harbored exon 21 L858R mutations), 4.7% with rare mutations (G719X, L861Q, and S768I et al.), 4.7% with wild type EGFR mutations, and 4.1% with unknown mutational status. 58.2% of patients received gefitinib as first-line therapy, 54.7% responded to initial gefitinib treatment and 51.8% achieved clinical benefit of at least 10 months. When acquired resistance developed, 40.6% of patients had gradual progression and 74.6% (147/197) received re-biopsy to identify potentially resistance mechanism. Of those who received re-biopsy, the incidence of secondary T790M mutations was 42.9% (63/147) and 66.7% (42/63) patients with secondary T790M mutations were enrolled in the study. Two (1.4%) patients had small cell lung cancer (SCLC) transformation. In the light of the investigators’ discretion, 46.5% received continuation of gefitinib in combination with chemotherapy as post-RECIST PD therapeutic regimens. Briefly, in chemotherapy alone group, 66 (72.5%) patients received pemetrexed-based therapy, 25 (27.5%) patients received non-pemetrexed-based therapy (13 received taxel-based therapy, 9 received gemcitabine-based therapy, 3 received vinorelbine-based therapy); in combination group, 61 (77.2%) patients received pemetrexed-based therapy, 18 (22.8%) patients received non-pemetrexed-based therapy (9 received taxel-based therapy, 5 received gemcitabine-based therapy, 4 received vinorelbine-based therapy).
Figure 1.
Patient flow diagram. EGFR-TKIs, epidermal growth factor receptor-tyrosine kinase inhibitors; SCLC, small cell lung cancer.
Table 1. Patient demographics and clinical characteristics.
| Characteristics | All patients (n=170) | Post RECIST-PD regimens | P | |
|---|---|---|---|---|
| Chemo alone (n=91) | Chemo + gefitinib (n=79) | |||
| Age, median (range), No. (%) (years) | 59 [30–84] | 0.286 | ||
| ≥65 | 43 (25.3) | 20 (22.0) | 23 (29.1) | |
| <65 | 127 (74.7) | 71 (78.0) | 56 (70.9) | |
| Gender, No. (%) | 0.029 | |||
| Male | 71 (41.8) | 45 (49.5) | 26 (32.9) | |
| Female | 99 (58.2) | 46 (50.5) | 53 (67.1) | |
| Smoking status, No. (%) | 0.234 | |||
| Never | 133 (78.2) | 68 (74.7) | 65 (82.3) | |
| Former/current | 37 (21.8) | 23 (25.3) | 14 (17.1) | |
| EGFR mutation, No. (%) | 0.806 | |||
| Exon 19 deletion | 82 (48.2) | 41 (45.1) | 31 (39.2) | |
| Exon 21 L858R mutation | 65 (38.2) | 29 (31.9) | 31 (39.2) | |
| Other (exon 18/20) | 8 (4.7) | 5 (5.5) | 3 (3.8) | |
| Wild type | 8 (4.7) | 5 (5.5) | 3 (3.8) | |
| Unknown | 7 (4.1) | 11 (12.1) | 11 (13.9) | |
| Histologic subtype, No. (%) | 0.729 | |||
| Adenocarcinoma | 158 (92.9) | 84 (92.3) | 74 (93.7) | |
| Non-adenocarcinoma | 12 (7.2) | 7 (7.7) | 5 (6.3) | |
| ECOG PS, No. (%) | 0.472 | |||
| 0 | 1 (0.6) | 0 (0.0) | 1 (1.3) | |
| 1 | 160 (94.1) | 87 (95.6) | 73 (92.4) | |
| ≥2 | 9 (5.3) | 4 (4.4) | 5 (6.4) | |
| Disease stage, No. (%) | 0.664 | |||
| IIIB | 9 (5.3) | 4 (4.4) | 5 (6.3) | |
| IV | 138 (81.2) | 73 (80.2) | 65 (82.3) | |
| Recurrent | 23 (13.5) | 14 (15.4) | 9 (11.4) | |
| Brain metastasis at baseline | 0.277 | |||
| Yes | 47 (27.6) | 22 (24.2) | 25 (31.6) | |
| No | 123 (72.4) | 69 (75.8) | 54 (68.4) | |
| Previous response to gefitinib | 0.707 | |||
| Partial response | 93 (54.7) | 51 (56.0) | 42 (53.2) | |
| Stable disease | 77 (45.3) | 40 (44.0) | 37 (46.8) | |
| Time to progression after initial gefitinib treatment (months) | 0.206 | |||
| ≤10 | 82 (48.2) | 48 (52.7) | 34 (43.0) | |
| >10 | 88 (51.8) | 43 (47.3) | 45 (57.0) | |
| Lines of initial gefitinib treatment | 0.085 | |||
| 1 | 99 (58.2) | 54 (59.3) | 45 (57.0) | |
| 2 | 50 (29.4) | 22 (24.2) | 28 (35.4) | |
| ≥3 | 21 (12.4) | 15 (16.5) | 6 (7.6) | |
| Progression mode, No. (%) | 0.122 | |||
| Gradual | 69 (40.6) | 32 (35.2) | 37 (46.8) | |
| Dramatic | 101 (59.4) | 59 (64.8) | 42 (53.2) | |
| T790M status, No. (%) | 0.917 | |||
| Negative | 78 (45.9) | 41 (45.1) | 37 (46.8) | |
| Positive | 42 (24.7) | 22 (24.2) | 20 (25.3) | |
| Unknown | 50 (29.4) | 28 (30.8) | 22 (27.8) | |
| Rebiopsy sites, No. (%) | 0.454 | |||
| Primary site | 74 (43.5) | 42 (46.2) | 32 (40.5) | |
| Metastasis site | 46 (27.1) | 21 (23.1) | 25 (31.6) | |
| Not Performed | 50 (29.4) | 28 (30.8) | 22 (27.8) | |
EGFR, epidermal growth factor receptor; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR-TKIs, EGFR tyrosine kinase inhibitors; Post-RECIST PD, post-Response Evaluation Criteria in Solid Tumors-progressive disease.
Efficacy
At the time of data cutoff (December 1, 2015), median duration of follow-up for the PPFS was 9 months [interquartile range (IQR), 4.6–15.3 months]. Median PPFS in the whole population was 4.5 months [95% confidence interval (CI), 3.7–5.3 months]. There was a borderline statistical significance in PPFS between the chemotherapy alone group and the combination group [hazard ratio (HR) 0.72, 95% CI, 0.50–1.03; P=0.071]. Median PPFS was 4.0 months (95% CI, 3.1–4.9 months) in the chemotherapy group and 5.0 months (95% CI, 3.6–6.4 months) in the combination group.
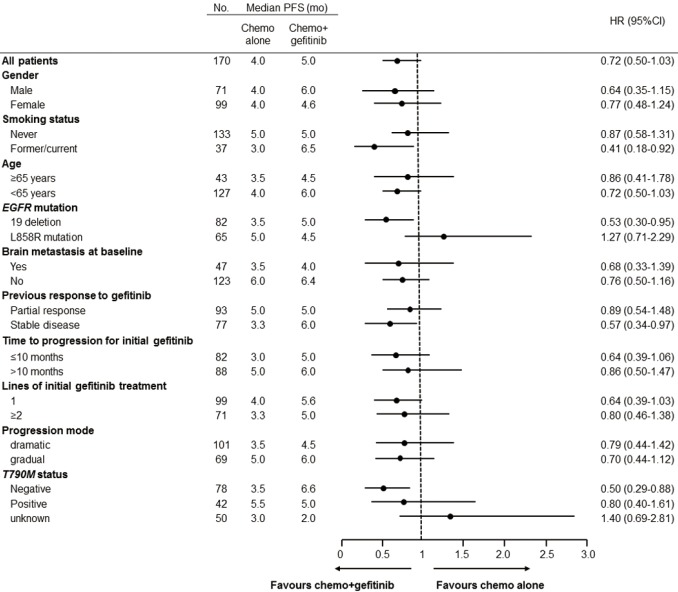
We further conducted subgroup analyses according to clinical and molecular characteristics between treatments (Figure 2). Findings from subgroup analyses of PPFS were generally in line with the overall result. It was noteworthy that, in patients with EGFRT790M-negative mutations, continuation of gefitinib plus chemotherapy resulted in a significant improvement in PPFS compared with chemotherapy alone (HR 0.50, 95% CI, 0.29–0.88; P=0.011) (Figure 3). Median PPFS was 6.6 months (95% CI, 6.0–7.2 months) in the combination group and 3.5 months (95% CI, 2.6–4.4 months) in the chemotherapy alone group. However, this survival benefit was only observed in pemetrexed-based chemotherapy (HR 0.45, 95% CI, 0.24–0.86; P=0.011) but not in non-pemetrexed-based chemotherapy (HR 0.72, 95% CI, 0.20–2.57; P=0.603) (Figure 4). Moreover, there was no statistically significant difference in PPFS between treatments in patients with EGFRT790M-positive mutations (HR 0.80, 95% CI, 0.40–1.61; P=0.520) or unknown T790M mutational status (HR 1.40, 95% CI, 0.69–2.81; P=0.323) (Figure 2).
Figure 2.
Forest plot of progression-free survival subgroup analyses.
Figure 3.
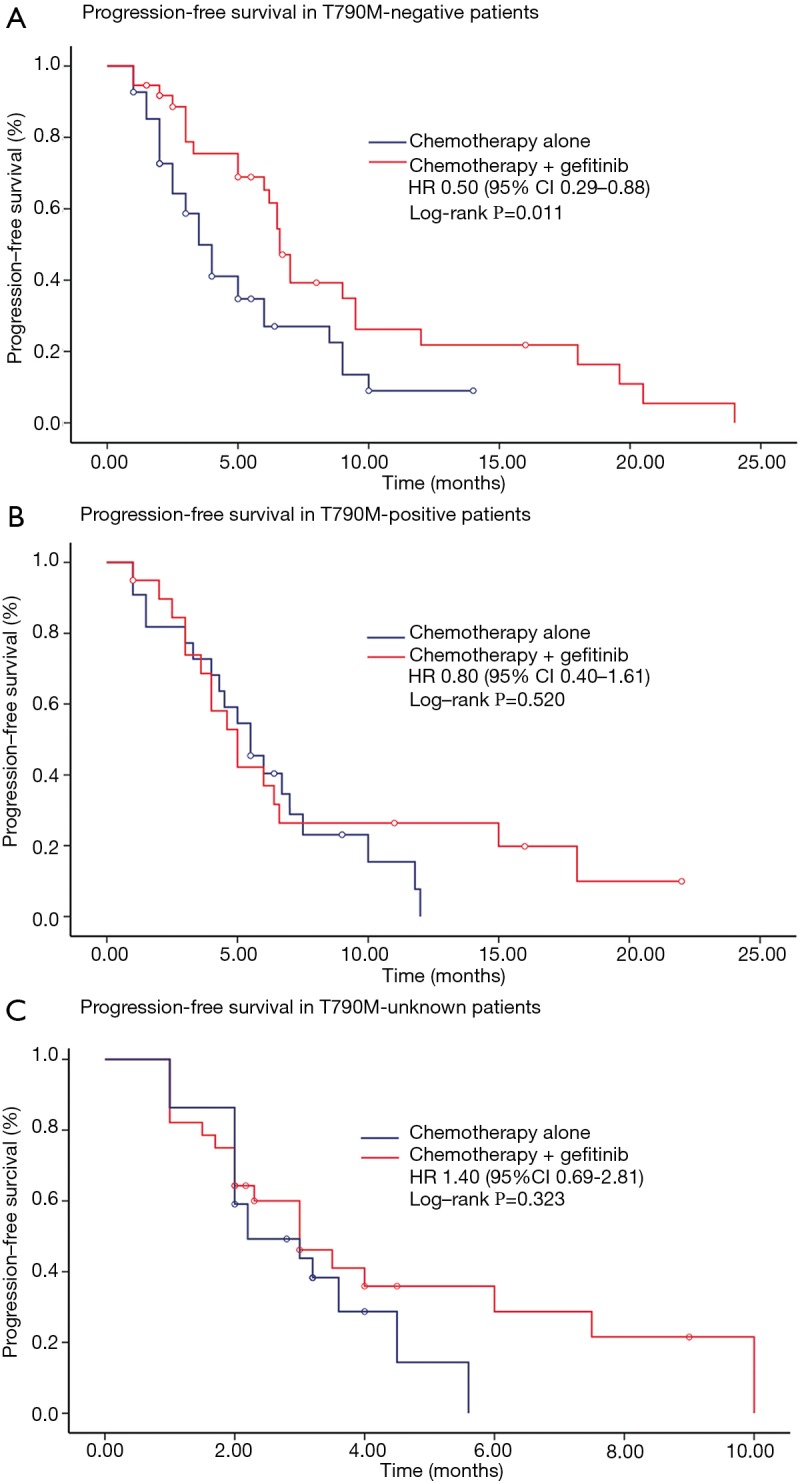
Kaplan-Meier survival curves of progression-free survival between chemotherapy alone and chemotherapy plus gefitinib (A) in patients with EGFRT790M-negative mutations (3.5 vs. 6.6 months, P=0.011), (B) in patients with EGFRT790M-positive mutations (5.5 vs. 5.0 months, P=0.520) and (C) in patients with unknown T790M mutational status (3.0 vs. 2.0 months, P=0.323).
Figure 4.
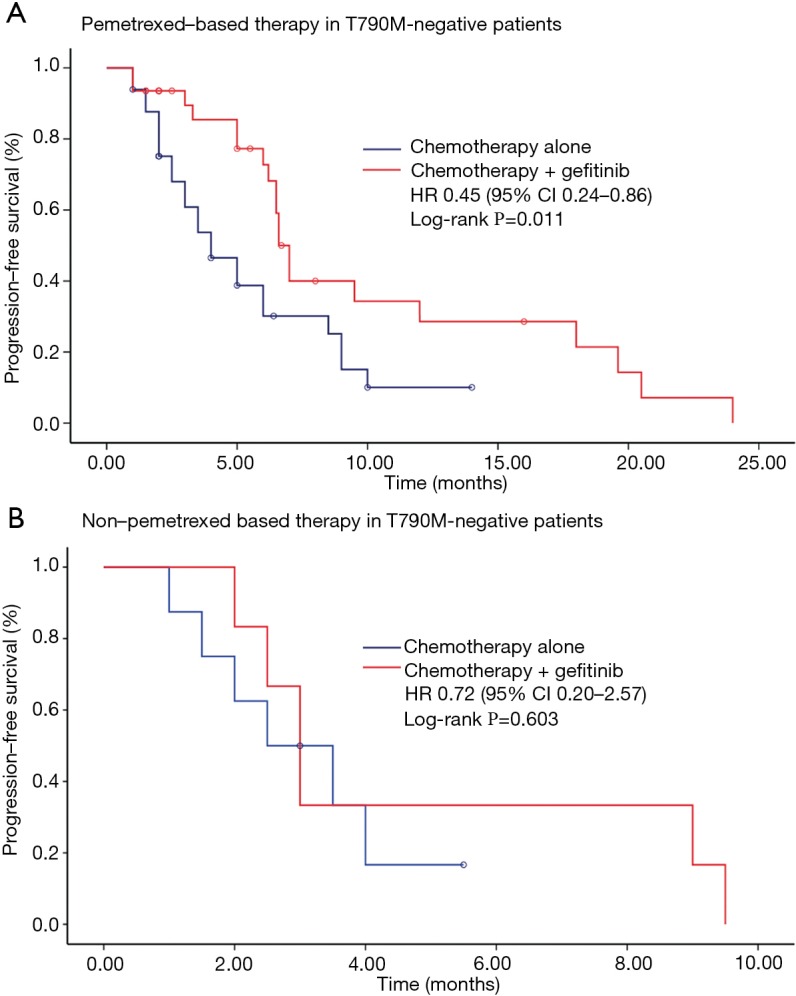
Kaplan-Meier survival curves of progression-free survival between chemotherapy alone and chemotherapy plus gefitinib in patients with EGFRT790M-negative mutations treated with (A) pemetrexed-based therapy (4.0 vs. 6.6 months, P=0.011) and non-pemetrexed-based therapy (2.5 vs. 3.0 months, P=0.603).
Patients with gradual progression showed a tendency to be associated with better survival compared with dramatic progression (median PPFS: 6.0 vs. 3.6 months, HR 0.78, 95% CI, 0.55–1.23; P=0.186). However, there was no significant difference in PPFS between the combination group and the chemotherapy alone group either in patients with gradual progression (6.0 vs. 5.0 months, HR 0.70, 95% CI, 0.44–1.22; P=0.416) or those with dramatic progression (4.5 vs. 3.5 months, HR 0.79, 95% CI, 0.44–1.42; P=0.115). Intriguingly, there was also a statistical survival benefit in patients receiving continuing gefitinib plus chemotherapy with EGFR exon 19 deletion (HR 0.53, 95% CI, 0.30–0.95; P=0.024), former/current smokers (HR 0.41, 95% CI, 0.18–0.92; P=0.019), who achieved a stable disease from initial gefitinib treatment (HR 0.57, 95% CI, 0.34–0.97; P=0.028) (Figure 2).
The overall response rate was higher in patients receiving the combination therapy than those receiving chemotherapy alone [30.4% vs. 19.8%; odds ratio (OR) 0.75, 95% CI, 0.51–1.10], however, the difference was not statistically significant (P=0.110). Furthermore, more patients achieved an objective response in the combination group than those in the chemotherapy alone group in patients with EGFRT790M-negative mutations (35.1% vs. 19.5%, P=0.120), EGFRT790M-positive mutations (25.0% vs. 22.7%, P=0.863) or unknown T790M mutational status (27.3% vs. 17.9%, P=0.425), still the differences were also not statistically significant. In addition, there was no statistical difference between treatments either in the whole population or in subgroups regarding DCR (Table 2).
Table 2. Treatment response according to T790M status.
| Outcome, n (%) | T790M− (n=78) | P | T790M+ (n=42) | P | Unknown (n=50) | P | |||
|---|---|---|---|---|---|---|---|---|---|
| Chemo (n=41) | Chemo + gefitinib (n=37) | Chemo (n=22) | Chemo + gefitinib (n=20) | Chemo (n=28) | Chemo + gefitinib (n=22) | ||||
| DCR | 24 (58.5) | 26 (70.3) | 0.281 | 16 (72.7) | 14 (70.0) | 0.845 | 10 (35.7) | 13 (59.1) | 0.100 |
| ORR | 8 (19.5) | 13 (35.1) | 0.120 | 5 (22.7) | 5 (25.0) | 0.863 | 5 (17.9) | 6 (27.3) | 0.425 |
| PR | 8 (19.5) | 13 (35.1) | – | 5 (22.7) | 5 (25.0) | – | 5 (17.9) | 6 (27.3) | – |
| SD | 16 (39.0) | 13 (35.1) | – | 11 (50.0) | 9 (45.0) | – | 5 (17.9) | 7 (31.8) | – |
| PD | 17 (41.5) | 11 (29.7) | – | 6 (27.3) | 6 (30.0) | – | 18 (64.3) | 9 (40.9) | – |
DCR, disease control rate; ORR, objective response rate; PR, partial response; SD, stable disease; PD, progressive disease; Chemo, chemotherapy; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor.
Toxicity
The most common treatment-related adverse events (AEs) in the combination group were nausea/vomiting (50.6%), diarrhea (41.7%), rash (35.4%), increased ALT/AST (31.6%), fatigue (30.4%), leukopenia (17.7%) and neutropenia (17.7%). Meanwhile, the most common treatment-related AEs in the chemotherapy group were nausea/vomiting (53.8%), fatigue (34.1%), increased ALT/AST (26.3%), rash (19.8%), leukopenia (19.8%), neutropenia (17.6%) and diarrhea (17.6%). No treatment-related death was observed (Table S1).
Discussion
As far as we know, this study is the first one to compare the efficacy of gefitinib plus chemotherapy with chemotherapy alone according to different progression modes and T790M mutational status in patients who get acquired resistance to gefitinib. We found that gefitinib plus chemotherapy failed to improve PPFS compared with chemotherapy alone in the whole population. Notably, we found that gefitinib together with chemotherapy, especially pemetrexed-based therapy, significantly improved PPFS in patients with EGFRT790M-negative mutations at the time of resistance.
IMPRESS was the first randomized trial to compare the efficacy of gefitinib in addition to pemetrexed plus cisplatin with chemotherapy alone in patients who experienced acquired resistance to EGFR-TKIs (26). However, there was no significant difference with respect to PFS between treatments (median PFS: 5.4 vs. 5.4 months; HR 0.86, 95% CI, 0.65–1.13; P=0.27), suggesting that gefitinib plus platinum-based doublet chemotherapy is not recommended for unselected patients with acquired resistance to first-line gefitinib. Findings from our present study also showed that continuing gefitinib plus chemotherapy failed to improve survival compared with chemotherapy alone in unselected patients who developed acquired resistance with a borderline statistical significance (P=0.071). The PPFS of chemotherapy alone in our study was shorter than that in IMPRESS (4.0 vs. 5.4 months), maybe given that in our study, 41.8% (71/170) patients received gefitinib as second- or later-line therapy. Meanwhile, LUX-Lung 5 demonstrated that afatinib plus paclitaxel resulted in a significant improvement in both PFS (median PFS: 5.6 vs. 2.8 months; HR 0.60, 95% CI, 0.43–0.85; P=0.0031) and ORR (32.1% vs. 13.2%; P=0.005) in comparison with single-agent chemotherapy in patients with acquired resistance to erlotinib/gefitinib and afatinib after an initial clinical benefit (≥12 weeks) (29). Moreover, in ASPIRATION, continuing erlotinib extended PFS from 11 to 14.1 months in patients beyond RECIST progression (25).These inconsistent results suggested that, obviously, not all patients were appropriate for continuation of EGFR-TKIs. However, how to guide the decision whether to continue EGFR-TKI treatment and who are the “selected patients” that may benefit from continuation of EGFR-TKIs?
T790M mutation is the dominant mechanism of acquired resistance to EGFR-TKIs and defines a unique subtype of patients who get acquired resistance (11-13). Thus, we further divided enrolled patients into three categories according to T790M mutational status: positive, negative or unknown status, with an effort to investigate whether T790M status could help guide the decision whether to continue EGFR-TKI treatment. In the present study, the incidence of T790M mutation was 42.9% in patients who received re-biopsy, which was consistent with previous studies (21,27,30,31). Intriguingly, we observed that in patients with EGFRT790M-negative tumors, continuation of gefitinib plus chemotherapy resulted in a significant improvement in PPFS compared with chemotherapy alone (median PPFS: 6.6 vs. 3.5 months) but not in patients with EGFRT790M-positive or EGFRT790M-unknown tumors. Notably, post-hoc subgroup analysis of IMPRESS also found that in patients with EGFRT790M-negavive tumors tested via plasma circulating tumor-derived DNA (ctDNA), continuation of gefitinib plus platinum-based doublet chemotherapy tended to prolong survival outcomes (median PFS: 6.7 vs. 5.4 months; HR 0.67, 95% CI, 0.43–1.03, P=0.0745) (32). The extension of PFS by continuing gefitinib in our study was similar to that in IMPRESS (6.6 vs. 6.7 months). Thus, together with post-hoc analysis of IMPRESS, our study suggested that patients with EGFRT790M-negative tumors may be the “selected patients” who may benefit from continuation of gefitinib beyond RECIST progression. Recently, given the tremendous efficacy of AZD9291 in EGFRT790M-positive tumors, this drug has been approved by U.S. Food and Drug Administration for patients with T790M mutations. However, the efficacy of AZD9291 was limited in EGFRT790M-negative tumors, with a response rate of 21% and a median PFS of only 2.8 months (12). Currently, there was unmet need for patients with EGFRT790M-independent resistance mechanisms, the combination treatment strategies for this subtype of patients, constituting up to 50% of patients with acquired resistance, were promising and deserved further investigation.
It was noteworthy that the clinical benefit was only observed in patients receiving pemetrexed-based combination therapy but not in other chemotherapeutic regimens. Interestingly, in cell lines and xenografts models, intermittent combination of pemetrexed and gefitinib delayed the emergence of acquired resistance and prevented the appearance of T790M mutations and EMT (33). In a randomized phase 2 trial, pemetrexed plus gefitinib significantly prolonged PFS from 10.9 to 15.8 months as compared with gefitinib alone (HR 0.68, 95% CI, 0.48–0.96; P=0.029) (34). These results, including findings of our study, suggested a synergistic effect of pemetrexed and gefitinib on delaying acquired resistance. However, the synergistic effect was also observed in other chemotherapeutic regimens and EGFR-TKIs (35,36). Given limited patients in our study receiving other chemotherapeutic regimens, the efficacy of non-pemetrexed-based chemotherapy overcoming acquired resistance therefore needs further investigation.
Findings from previous study showed that patients with gradual progression may benefit from continuing EGFR-TKIs beyond resistance (20). In ASPIRATION, patients with slow progression could benefit from continuation of EGFR-TKI therapy beyond progression (25). Although consistent with the study by Yang et al. (20) that patients with gradual progression showed a tendency to be associated with better survival compared with dramatic progression in our study (median PPFS: 6.0 vs. 3.6 months, P=0.186), there was no significant difference in PPFS between treatments either in patients with gradual progression or those with dramatic progression. With the development of precision medicine, we believed that re-biopsy of resistance samples to define mechanisms of acquired resistance should be preferably considered in routine practice, thereafter selecting accompanying tailored therapies.
Several limitations of the present study should be taken into account. First, due to its retrospective nature, prospective sample size estimation was not applicable and potential selection bias may cause a confounding effect on assessment on survival. Second, except for SCLC transformation, other EGFRT790M-independent mechanisms of resistance were not evaluated and the efficacy of continuation of gefitinib in patients with these mechanisms was unknown. Third, overall survival data was immature and the effect of continuation of gefitinib on overall survival was inconclusive.
Conclusions
In the present study, we found that gefitinib in combination with chemotherapy, especially pemetrexed-based therapy, significantly improved PPFS in patients with EGFRT790M-negative tumors as compared with chemotherapy alone, which highlight the role of T790M mutational status in guiding the subsequent therapeutic decision after the development of acquired resistance of gefitinib.
Table S1. Adverse events.
| Adverse event | Chemotherapy + gefitinib (n=79) | Chemotherapy (n=91) | |||
|---|---|---|---|---|---|
| All grades, n (%) | Grade ≥3, n (%) | All grades, n (%) | Grade ≥3, n (%) | ||
| Nausea/vomiting | 40 (50.6) | 1 (1.3) | 49 (53.8) | 2 (2.2) | |
| Diarrhea | 33 (41.7) | 1 (1.3) | 16 (17.6) | 0 (0) | |
| Rash | 28 (35.4) | 0 (0) | 18 (19.8) | 0 (0) | |
| Increased ALT/AST | 25 (31.6) | 1 (1.3) | 24 (26.3) | 2 (2.2) | |
| Fatigue | 24 (30.4) | 0 (0) | 31 (34.1) | 0 (0) | |
| Leukopenia | 14 (17.7) | 2 (2.5) | 18 (19.8) | 3 (3.3) | |
| Neutropenia | 14 (17.7) | 2 (2.5) | 16 (17.6) | 4 (4.4) | |
| Anemia | 5 (6.3) | 0 (0) | 5 (5.5) | 0 (0) | |
Acknowledgements
Funding: This study was supported in part by grants from key project of Shanghai Municipal Commission of Health and Family Planning (No. 2013zyjb0401), Outstanding Young Doctor Program of Shanghai Municipal Commission of Health and Family Planning (No. XYQ2013097) and Shanghai Sailing Program (16YF1409600).
Ethical Statement: This study was approved by the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University, Shanghai, China, and all participants signed an informed consent (Institutional Review Board No. K17-140).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. 10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. 10.1126/science.1099314 [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 4.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. 10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 5.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. 10.1016/S1470-2045(09)70364-X [DOI] [PubMed] [Google Scholar]
- 6.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 7.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 8.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 9.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. 10.1016/S1470-2045(13)70604-1 [DOI] [PubMed] [Google Scholar]
- 10.Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol 2010;28:357-60. 10.1200/JCO.2009.24.7049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. 10.1126/scitranslmed.3002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. 10.1056/NEJMoa1411817 [DOI] [PubMed] [Google Scholar]
- 13.Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2015;372:1700-9. 10.1056/NEJMoa1413654 [DOI] [PubMed] [Google Scholar]
- 14.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039-43. 10.1126/science.1141478 [DOI] [PubMed] [Google Scholar]
- 15.Ohashi K, Sequist LV, Arcila ME, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A 2012;109:E2127-33. 10.1073/pnas.1203530109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takezawa K, Pirazzoli V, Arcila ME, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov 2012;2:922-33. 10.1158/2159-8290.CD-12-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. 10.1158/1078-0432.CCR-12-2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chmielecki J, Foo J, Oxnard GR, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med 2011;3:90ra59. 10.1126/scitranslmed.3002356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg SB, Oxnard GR, Digumarthy S, et al. Chemotherapy with Erlotinib or chemotherapy alone in advanced non-small cell lung cancer with acquired resistance to EGFR tyrosine kinase inhibitors. Oncologist 2013;18:1214-20. 10.1634/theoncologist.2013-0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JJ, Chen HJ, Yan HH, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer 2013;79:33-9. 10.1016/j.lungcan.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Quan Q, Ding L, et al. Continuation of epidermal growth factor receptor tyrosine kinase inhibitor treatment prolongs disease control in non-small-cell lung cancers with acquired resistance to EGFR tyrosine kinase inhibitors. Oncotarget 2015;6:24904-11. 10.18632/oncotarget.4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishie K, Kawaguchi T, Tamiya A, et al. Epidermal growth factor receptor tyrosine kinase inhibitors beyond progressive disease: a retrospective analysis for Japanese patients with activating EGFR mutations. J Thorac Oncol 2012;7:1722-7. 10.1097/JTO.0b013e31826913f7 [DOI] [PubMed] [Google Scholar]
- 23.Yoshida T, Yoh K, Niho S, et al. RECIST progression patterns during EGFR tyrosine kinase inhibitor treatment of advanced non-small cell lung cancer patients harboring an EGFR mutation. Lung Cancer 2015;90:477-83. 10.1016/j.lungcan.2015.09.025 [DOI] [PubMed] [Google Scholar]
- 24.Li S, Zhou F, Ren S, et al. Response to pemetrexed rechallenge after acquired resistance of EGFR-TKI in a patient with advanced NSCLC. Lung Cancer 2014;84:203-5. 10.1016/j.lungcan.2014.02.010 [DOI] [PubMed] [Google Scholar]
- 25.Park K, Yu CJ, Kim SW, et al. First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer: The ASPIRATION Study. JAMA Oncol 2016;2:305-12. 10.1001/jamaoncol.2015.4921 [DOI] [PubMed] [Google Scholar]
- 26.Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol 2015;16:990-8. 10.1016/S1470-2045(15)00121-7 [DOI] [PubMed] [Google Scholar]
- 27.Li W, Ren S, Li J, et al. T790M mutation is associated with better efficacy of treatment beyond progression with EGFR-TKI in advanced NSCLC patients. Lung Cancer 2014;84:295-300. 10.1016/j.lungcan.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 28.Wu C, Zhao C, Yang Y, et al. High Discrepancy of Driver Mutations in Patients with NSCLC and Synchronous Multiple Lung Ground-Glass Nodules. J Thorac Oncol 2015;10:778-83. 10.1097/JTO.0000000000000487 [DOI] [PubMed] [Google Scholar]
- 29.Schuler M, Yang JC, Park K, et al. Afatinib beyond progression in patients with non-small-cell lung cancer following chemotherapy, erlotinib/gefitinib and afatinib: phase III randomized LUX-Lung 5 trial. Ann Oncol 2016;27:417-23. 10.1093/annonc/mdv597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun JM, Ahn MJ, Choi YL, et al. Clinical implications of T790M mutation in patients with acquired resistance to EGFR tyrosine kinase inhibitors. Lung Cancer 2013;82:294-8. 10.1016/j.lungcan.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 31.Kuiper JL, Heideman DA, Thunnissen E, et al. Incidence of T790M mutation in (sequential) rebiopsies in EGFR-mutated NSCLC-patients. Lung Cancer 2014;85:19-24. 10.1016/j.lungcan.2014.03.016 [DOI] [PubMed] [Google Scholar]
- 32.Soria JC, Kim SW, Wu YL, et al. Gefitinib/Chemotherapy vs Chemotherapy in EGFR MutationPositive NSCLC Resistant to First-Line Gefitinib: IMPRESS T790M Subgroup. WCLC 2015;ORAL17.08.
- 33.La Monica S, Madeddu D, Tiseo M, et al. Combination of Gefitinib and Pemetrexed Prevents the Acquisition of TKI Resistance in NSCLC Cell Lines Carrying EGFR-Activating Mutation. J Thorac Oncol 2016;11:1051-63. 10.1016/j.jtho.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 34.Cheng Y, Murakami H, Yang PC, et al. Randomized Phase II Trial of Gefitinib With and Without Pemetrexed as First-Line Therapy in Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancer With Activating Epidermal Growth Factor Receptor Mutations. J Clin Oncol 2016;34:3258-66. 10.1200/JCO.2016.66.9218 [DOI] [PubMed] [Google Scholar]
- 35.Laurila N, Koivunen JP. EGFR inhibitor and chemotherapy combinations for acquired TKI resistance in EGFR-mutant NSCLC models. Med Oncol 2015;32:205. 10.1007/s12032-015-0627-6 [DOI] [PubMed] [Google Scholar]
- 36.Dal Bello MG, Alama A, Barletta G, et al. Sequential use of vinorelbine followed by gefitinib enhances the antitumor effect in NSCLC cell lines poorly responsive to reversible EGFR tyrosine kinase inhibitors. Int J Cancer 2015;137:2947-58. 10.1002/ijc.29647 [DOI] [PubMed] [Google Scholar]