Abstract
Living-donor lobar lung transplantation (LDLLT) was developed to deal with the severe shortage of brain dead door for patients who would not survive the long waiting period. In standard LDLLT, right and left lower lobes removed from two healthy donors are implanted into a recipient after right and left pneumonectomies using cardiopulmonary bypass (CPB). The number of LDLLT has decreased in the USA due to the recent change in allocation system for cadaveric donor lungs. For the past several years, most of the reports on LDLLT have been from Japan, where the average waiting time for a cadaveric lung is exceeding 800 days. LDLLT has been performed both for adult and pediatric patients suffering from various end-stage lung diseases including restrictive, obstructive, vascular and infectious lung diseases. Since only two lobes are implanted, size matching is a very important issue. Functional size matching by measuring donor pulmonary function and anatomical size matching by three-dimensional computed tomography (3D-CT) volumetry are very useful. For oversize graft, we have employed several techniques, including single lobe transplantation, delayed chest closure, downsizing the graft, and middle lobe transplantation. In cases of undersize mismatch, native upper lobe sparing transplant or right-left inverted transplant was performed. The 5-, 10- and 15-year survivals were 80.8%, 72.6% and 61.7%, respectively. There was no difference in survival between standard LDLLT and non-standard LDLLT such as single, sparing and inverted transplant. All donors have been discharged without any restrictions. LDLLT is a viable option for very ill patients who would not survive a long waiting time for cadaveric lungs. We have successfully developed various surgical techniques to overcome size mismatching with favorable outcome.
Keywords: Lung transplantation, cadaveric lung transplantation (CLT), living-donor lobar lung transplantation (LDLLT), size matching
Introduction
To deal with the brain-dead donor shortage, living-donor lobar lung transplantation (LDLLT) was first developed in the USA as an alternative modality for very sick patients who would not survive a waiting time for cadaveric lung transplantation (CLT) (1). In a standard LDLLT, the right and left lower lobes from two healthy donors are implanted into the recipient after right and left pneumonectomies (Figure 1). In the beginning, the procedure was applied to pediatric or small adult patients with cystic fibrosis (CF) (2). It is now well known that LDLLT can be applied to various indications including restrictive, obstructive, infectious and vascular diseases (3-7). Although LDLLT was developed in the USA, its use has decreased there due to the recent change in lung allocation system. For the past several years, most of the reports on LDLLT have been from Japan, where the average waiting time for a cadaveric lung is exceeding 800 days. Besides Japanese experience, England (8), Brazil (9) and China (10) have reported their small practices. We have reported that bilateral LDLLT provides equal or better survival than conventional CLT (11).
Figure 1.
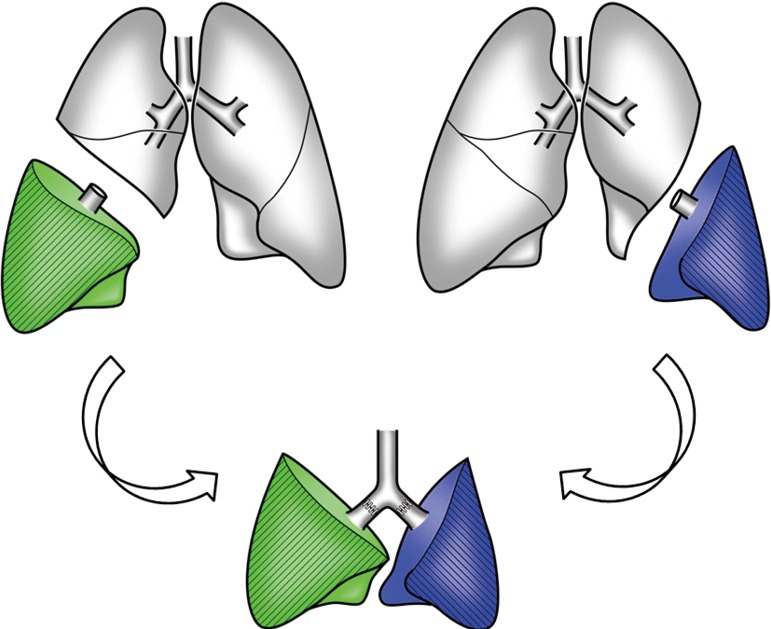
Bilateral living-donor lobar lung transplantation. Right and left lower lobes from two healthy donors are implanted in a recipient in place of whole right and left lungs, respectively.
Recipient selection
Recipient candidates for LDLLT should be less than 65 years old and must meet the criteria for conventional CLT. Our policy has been to limit LDLLT to severely ill patients with rapidly progressive lung disease who would not survive the long waiting time for cadaveric lungs. At the time of transplantation, all of our LDLLT recipients were oxygen dependent and 59% of them were bed bound and 11% of them were on a ventilator at the time of transplantation. It should be also noted that it would not be justified to do two lobectomies from two healthy relatives if the recipient has too many risk factors. It has been debated if LDLLT can be indicated for patients already on a ventilator or requiring re-transplantation. In St. Louis group experience, survival was better after LDLLT than conventional CLT for re-transplantation (12). Perioperative mortality of re-transplantation was only 7.7% after LDLLT versus 42.3% after CLT. USC group reported that ventilator dependency and re-transplantation were significant risk factors of death in their 123 LDLLTs (13). In our experience, all 14 patients on a ventilator for as long as 7 months underwent successful LDLLT.
CF is the most frequent indication for LDLLT in USA. This is because only two lobar grafts are implanted and CF patients are usually small in body size. CF is very rarely seen in Japan where the distribution of diagnoses is quite unique as compared with USA (14). Various lung diseases including restrictive, obstructive, infectious and vascular lung diseases have been accepted for LDLLT candidate in our experience. Among them three major indications were, interstitial pneumonia, bronchiolitis obliterans, and pulmonary hypertension. Majority of the patients with interstitial pneumonia were on systemic steroid therapy (6). Major cause of bronchiolitis obliterans was graft-versus-host disease after hematopoietic stem cell transplantation for hematologic disorders such as leukemia (15). High dose epoprostenol therapy had been already given to patients with idiopathic pulmonary arterial hypertension (7). Although knowledge of the survival predictors in each disease is helpful, ultimately the timing of LDLLT must be decided base on the unique situation of each patient.
Donor selection
Table 1 summarizes the eligibility criteria for living lobar lung donation at Kyoto University. We have accepted only immediate family members (relatives within the third degree or a spouse) in our institution. However, extended family members and unrelated individuals have been accepted in other non-Japanese institutions (16). It should be prohibited to extract more than one lobe from one donor.
Table 1. The eligibility criteria for living lung donation (Kyoto University).
| Medical criteria |
| Age 20–60 years |
| ABO blood type compatible with recipient |
| Relatives within the third degree or a spouse |
| No significant past medical history |
| No recent viral infection |
| No significant abnormalities on echocardiogram and electrocardiogram |
| No significant ipsilateral pulmonary pathology on computed tomography |
| Arterial oxygen tension ≥80 mmHg (room air) |
| Forced vital capacity, forced expiratory volume in one second ≥85% of predicted |
| No previous ipsilateral thoracic surgery |
| No active tobacco smoking |
| Social and ethical criteria |
| No significant mental disorders proved by a psychiatrist |
| No ethical issues or concerns about donor motivation |
It is very important to confirm that potential donors are competent, willing to donate without psychologic pressure from the others. They should be medically and psychosocially suitable. We inform them about the risks and benefits as a donor, and also inform them about the risks, benefits, other possible treatment option of the recipient. We interview potential donors at least 3 times to provide them what is called “cooling-off” period.
Regarding preoperative workup, posterior-anterior and lateral chest X-ray, enhanced high-resolution computed tomography (CT) of the chest, pulmonary function tests, arterial blood gases, electrocardiogram, and Doppler echocardiogram are performed. Three-dimensional multidetector CT angiography is created for the confirmation of the pulmonary arterial and venous anatomy (Figure 2) (17). The completeness of inter pulmonary fissures is carefully evaluated by high-resolution CT. Although human leukocyte antigens (HLA) matching is not required for donor selection, we perform a prospective cross-match to rule-out the presence of anti-HLA antibodies.
Figure 2.
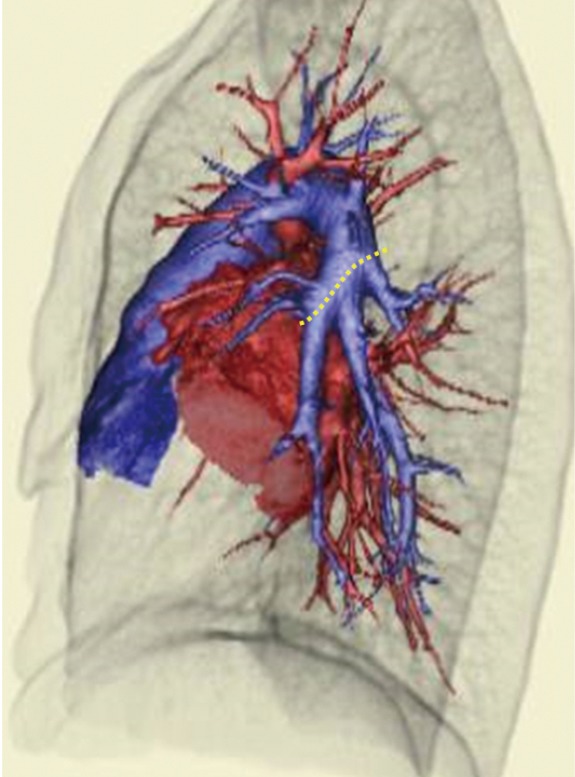
Three-dimensional computed tomography angiography in a left donor. A yellow-dotted line shows the planned cutting oblique line of the pulmonary artery, thus to preserve ligula branches.
We usually select the larger donor with better vital capacity for the right-side donor and select the other for the left-side donor.
Size matching
Because only two lobes are implanted in standard LDLLT, it is very important to evaluate size matching between the donor and recipient. We often implant relatively small grafts in LDLLT in which only two lobar grafts are implanted. However, excessively small grafts may result in lung edema with high pulmonary vascular resistance (18). Dead chest cavity may cause persistent air leakage and empyema. Overexpansion of the donor grafts may lead to obstructive physiology by small airway closure (19).
For small children under the height of 100 cm, the adult lower lobe is usually too big. It may not be possible to close the chest after implanting excessively oversized grafts, because chest closure could increase airway resistance, atelectasis and hemodynamic instability (20).
Functional size matching
We mainly use the forced vital capacity (FVC) size matching to evaluate undersized grafts. Pulmonary function can be measured in living-donor but not in cadaveric-donor. It allows us to perform more precise functional matching in LDLLT. For “functional size matching”, we rely on graft FVC (4,21). We have made a formula for estimating the graft FVC based on the donor’s measured FVC and the number of pulmonary segments implanted. Total FVC of the two grafts can be calculated by the following equation given that the right lower lobe consists of 5 segments, the left lower lobe of 4 and the whole lung of 19.
Total FVC of the two grafts = measured FVC of the right donor ×5/19 + measured FVC of the left donor ×4/19.
Our acceptable lower threshold of the total FVC of the two grafts is 45% of the predicted FVC of the recipient (calculated based on height, age, and sex). We think that the ratio should be more than 50% for recipients with pulmonary vascular diseases such as pulmonary hypertension.
Total FVC of the two grafts/predicted FVC of the recipient >0.45–0.50.
Anatomical size matching
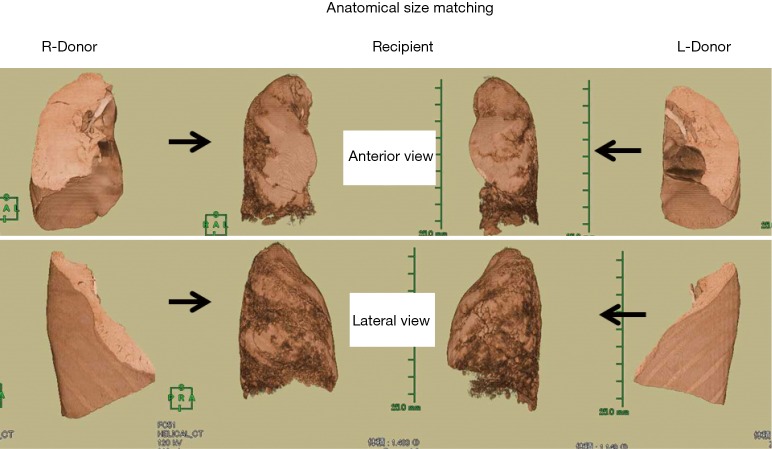
We mainly use the volumetric size matching to evaluate oversized grafts. For “anatomical size matching”, three-dimensional CT (3D-CT) volumetry is performed both for the donor and the recipient (22). CT images are obtained using a multi-detector CT scanner during a single respiratory pause at the end of maximum inspiratory effort. Contiguous 0.5-mm slices, reconstructed using a standard lung reconstruction algorithm, are used for volumetric analysis and the entire CT image is exported to a workstation (AZE Virtual Place Lexus; AZE Co., Ltd., Tokyo, Japan) for 3D-CT volumetry (Figure 3). Using automated segmentation, the volumes of each lung and the graft lobes are calculated automatically. The upper and lower thresholds of anatomical size matching have not been precisely determined yet. We have accepted a wide range of volume ratios between the donor’s lower lobe graft and the corresponding recipient’s chest cavity. The upper threshold of the volume ratio appears to be about 200% based on 3D-CT size matching.
Figure 3.
Anatomical size matching for the donor grafts and the recipient thorax using three-dimensional volumetry. The recipient was an adult male with pulmonary fibrosis. His right and left hemithorax was 1,483 and 1,149 mL, respectively. The right donor was a male whose right lower lobe was 1,637 mL. The left donor was a female whose left lower lobe was 716 mL. The right graft was oversize (110%) and the left graft was undersize (62.3%). Uneventful bilateral living-donor lobar lung transplantation was performed.
Surgical technique
Performing bilateral LDLLT requires a lot of man power for three surgical teams and a back-table team. We communicate each other closely to identify appropriate timing for graft extraction to minimize graft ischemic time. We usually bring the recipient and the right-side donor to operating room (OR) at the same time followed by bringing the left-side donor 30 min later.
Donor lobectomy
Because heparin is used in the donor, we usually place an epidural catheter the day before the operation for postoperative pain management. Donors are intubated with a left-sided double lumen endotracheal tube under general anesthesia. With lateral decubitus position, a posterolateral thoracotomy is made though the 5th intercostal space. At first, interlobar fissures are divided by linear stapling devices. It is important to open pericardium near the inferior pulmonary vein circumferentially. In the interlobar fissure, pulmonary artery branches are to be dissected carefully. It is important to define the anatomy of the pulmonary artery branches to middle lobe and the lower lobe in the right-side donor. In the left-side donor, the anatomy of the pulmonary arteries to the lingular segment should be carefully evaluated. When the branches of middle lobe artery and lingular artery are small, they could be ligated and sacrificed. When such branches are large, we try to preserve them by arterioplasty using autopericardial patch (23,24).
When the recipient’s pneumonectomy is nearly completed, intravenous prostaglandin E1 is administered with a dose of decreasing a systolic blood pressure by 10 to 20 mmHg. Then, 5,000 units of heparin and 500 mg of methylprednisolone are given intravenously. We place a vascular clamp on the interlobar pulmonary artery followed by placing another vascular clamp on the inferior pulmonary vein intrapericardially. The pulmonary vein, the pulmonary artery and bronchus are divided in this order. Vascular stamps are sutured with 5-0 polypropylene running suture. The bronchus is closed with 4-0 polypropylene interrupted sutures and covered with pedicled pericardial fat tissue.
At the back table, the lobar grafts are flushed with preservation solution (ET-Kyoto solution in our institution) both antegradely and retrogradely. We ventilate the lobar grafts gently with room air throughout the flush.
Recipient implantation
When a recipient is very unstable, we dissect right femoral vessels under local anesthesia just in case for urgent vascular access. Adult recipients are intubated with a left-side double lumen endotracheal tube under general anesthesia. For children and small adults, a single lumen endotracheal tube is used. Both chest cavities are entered through the 4th intercostal space by means of “clamshell” incision. To facilitate postoperative sternal fixation, the sternum is notched at the level of transection.
We at first perform pleural and hilar dissections as much as possible before heparinization, which would reduce blood loss. Regarding intraoperative circulatory support, we have utilized extracorporeal membrane oxygenation (ECMO) instead of conventional cardiopulmonary bypass (CPB) since 2012. We try to minimize heparin administration and aim to maintain activated clotting time between 180 to 200 seconds. Two drainage cannulas are placed, one to the right atrium via the right femoral vein, the other to the superior vena cava via the right appendage. These two drainage cannulas are connected by a Y connector. The ascending aorta is also cannulated for blood feeding.
After right pneumonectomy, the right lower lobe graft is implanted. The bronchus, the pulmonary vein, and the pulmonary artery are anastomosed in this order. The bronchial anastomosis is performed with a running 4-0 polydioxanone suture for membranous portion and with simple interrupted sutures for cartilaginous portion. When the bronchial size is similar between the recipient main bronchus and the donor lobar bronchus, we use end-to-end anastomosis. Telescoping technique is used when the discrepancy in bronchial size is obvious. No bronchial wrapping is employed. The pulmonary venous anastomosis is performed between the donor inferior pulmonary vein and the recipient upper pulmonary vein using a running 6-0 polypropylene suture. The venous suture is left untied for the subsequent deair procedure at the time of reperfusion. Lastly, the pulmonary arterial anastomosis is conducted in an end-to-end fashion using a running 6-0 polypropylene suture. Just before completing the right graft implantation, 500 mg of methylprednisolone is given intravenously and nitric oxide inhalation is initiated at 20 ppm. Antegrade reperfusion of the graft is done by releasing the vascular clamp on the pulmonary artery. We discard preservation solution and some amount of blood through the untied venous anastomosis to ensure the deair. After the right graft is reperfused and ventilated, ECMO flow is gradually decreased to about 70% of the full flow to maintain adequate blood flow to the first implanted right graft.
Left pneumonectomy and left graft implantation are conducted in the same manner. After the both lungs are reperfused and ventilated, ECMO is gradually weaned to the level of 10% of the full flow. When the blood gas and hemodynamics are satisfactory, ECMO is removed.
Strategies for size mismatch in LDLLT
Oversized graft
An adult lobe could be too big for a small child. We have employed several compensatory techniques such as single lobe transplantation with or without contralateral pneumonectomy, delayed chest closure, downsizing the graft, and implanting middle lobe. Single LDLLT is also indicated when only one living-donor is found in the family. We previously reported acceptable results after single LDLLT for very sick patients. However, when two donors were available, bilateral LDLLT provided better outcome (25).
Undersized graft
For large adults, two lower lobe grafts may be too small. We have developed two transplant procedures, native upper lobe sparing LDLLT (26) and right-left inverted LDLLT (27).
Native upper lobe sparing LDLLT (Figure 4) is indicated when the total graft FVC is less than 60% of the recipient’s predicted FVC. The recipient lung should not be infected and the interlobar fissure should be well developed. Ideally, the native upper lobes are less impaired than the lower lobes as seen on high-resolution CT or are better perfused on perfusion scintigraphy. The surgical procedure of native upper lobe sparing transplant is similar to that of standard LDLLT except that the bronchus is anastomosed distally to the second carina, the pulmonary vein to the lower pulmonary vein and the pulmonary artery to the interlobar artery in the fissure.
Figure 4.
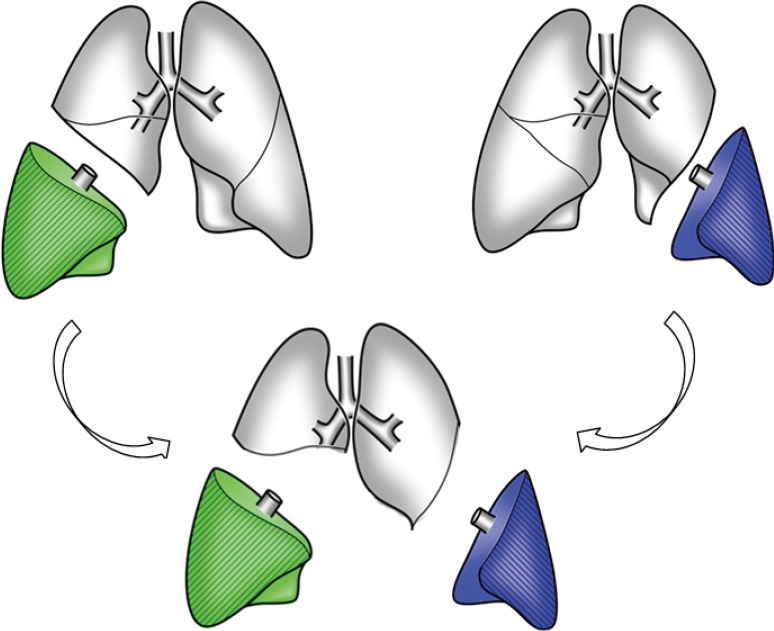
Native upper lobe sparing living-donor lobar lung transplantation. Bilobectomy and left lower lobectomy are performed in the recipient, and lower lobar grafts are implanted.
In right-left inverted LDLLT (Figure 5), the donor right lower lobe (5 segments) is inverted and implanted into the recipient’s left chest cavity instead of the donor left lower lobe (4 segments). It is usually indicated when total graft FVC is less than 60% of the recipient’s predicted FVC or when donor’s left lower lobectomy would be technically difficult due to interlobar pulmonary artery anatomy. The technical details have been described previously. At the time of left pneumonectomy in the recipient, upper and lower bronchi are stapled separately. After rotating the right lower lobe graft from its anatomic position to 180° about its superior-inferior axis, the graft is placed in the recipient’s left chest cavity. The bronchus is anastomosed to the recipient’s left upper bronchus and the left lower bronchial stamp is left closed. The pulmonary artery anastomosis is performed behind the bronchus. The donor pulmonary vein is anastomosed to the recipient’s left upper pulmonary vein or occasionally to the recipient’s left appendage.
Figure 5.
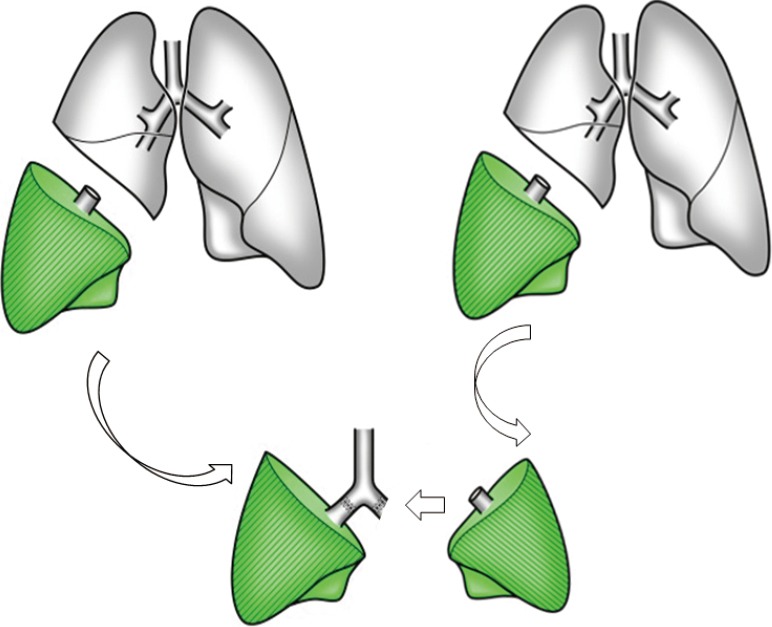
Right-to-left inverted living-donor lobar lung transplantation. The donor right lower lobe (5 segments) is inverted and implanted into the recipient’s left chest cavity instead of the donor left lower lobe (4 segments).
Nearly half of our LDLLTs were performed as non-standard LDLLT using single, sparing or inverted techniques. Most of those patients would not have been accepted if the aforementioned new techniques were not used. The survival after non-standard LDLLT were similar to survival rates after standard LDLLT (28).
Postoperative management
Meticulous postoperative management is required in ICU. We usually keep the patient intubated for more than 3 days to maintain optimal expansion of the implanted lobar grafts. Pressure-limited ventilation is used and maximal ventilation pressure is kept less than 25 cmH2O. Fiberoptic bronchoscopy is performed twice a day when intubated to assess bronchial anastomoses and to clean retained secretions by suction. Aggressive bedside rehabilitation is initiated as soon as possible.
Triple drug therapy with cyclosporine (CSA) or tacrolimus (FK), mycophenolate mofetil (MMF), and corticosteroids are used for postoperative immunosuppression. We do not use induction cytolytic therapy. Methylprednisolone (125 mg) is administered intravenously during the first 3 days. We give all other immunosuppressants via the nasal tube inserted to the proximal jejunum to protect renal function.
We do not perform routine transbronchial lung biopsy for rejection monitoring. It is because the risk of pneumothorax and bleeding after transbronchial lung biopsy may be greater after LDLLT. Acute rejection can be judged on the basis of careful monitoring radiographic and clinical findings. It is unique that acute rejection is usually seen as unilateral pulmonary infiltration on chest radiographs and CT after LDLLT because two lobes are donated by different donors. Clinical findings of early acute rejection include dyspnea, low grade fever, leukocytosis, hypoxemia. When acute rejection is suspected, we administer a trial bolus dose of methylprednisolone 500 mg and carefully observe various clinical signs. If the patient improves after the first dose of methylprednisolone, two additional daily bolus doses are given.
Outcome of living-donors
Donors outcome is as important as that of the recipient. All our donors have returned to their previous life styles. However, long-term outcomes of donors have not been well documented because the donor follow-up is discontinued after 1 year.
Perioperative complications in living donors
According to previous reports, no perioperative death has been reported although relatively high morbidity after lobectomy has been described (29,30). Morbidity rates ranged from 20% to 60%. Report in 2006 from the Vancouver Forum Lung Group identified approximately 550 living lung donors (16). About 5% of live-donors have experienced complications requiring surgical or bronchoscopic intervention. Three technical differences between living donor lobectomy and standard lobectomy may explain the relatively high morbidity: (I) the pericardiotomy around the inferior pulmonary vein may lead to arrhythmias and pericarditis; (II) the division of the right lower lobe bronchus in an oblique fashion may increase the risk for bronchial fistula and stenosis; (III) heparin administration may result in perioperative bleeding.
We recently reported on our experience in donor lobectomy (31). Post-operative complications before discharge were seen in 20%. We also reported that health related quality of life and dyspnea of the living-donors deteriorated postoperatively in a prospective study (32).
Pulmonary function of living donors
As lung is not a regenerative organ, donors lose their pulmonary function permanently. We prospectively performed pulmonary function test 3, 6, and 12 months after donor lobectomy (33). Both FVC and forced expiratory volume in one second (FEV1) recovered up to about 90% of the preoperative value 1 year after donor lobectomy.
Outcome of LDLLT recipient
The USC group reported their long-term outcome on 123 LDLLT recipients including 39 children (13). Re-transplantation and mechanical ventilation were found to be risk factors for perioperative mortality. One-, 3-, and 5-year survival rate was 70%, 54%, and 45%, respectively. St. Louis group demonstrated similar results in 38 pediatric recipients receiving LDLLT (34).
As of June 2017, the author has performed 124 LDLLTs (47 at Okayama University and 77 at Kyoto University). There were 79 females and 45 males with ages ranging from 6 to 64 years (average 33.9 years). Twenty-nine of the patients were children and 95 were adults.
Recipient’s diagnoses were listed in Table 2. Interstitial pneumonia, bronchiolitis obliterans, and pulmonary hypertension were the three major indications. All our patients were very sick and were depending on oxygen inhalation preoperatively. Seventy-five patients (61%) were bed bound and 14 (11%) were on a ventilator.
Table 2. Diagnoses for living-donor lobar lung transplantation.
| Diagnoses | Number |
|---|---|
| Interstitial pneumonia | 48 |
| Bronchiolitis obliterans | 35 |
| Pulmonary hypertension | 22 |
| Bronchiectasis | 6 |
| Lymphangioleiomyomatosis | 4 |
| Re-transplantation | 4 |
| Cystic fibrosis | 1 |
| Emphysema | 1 |
| Others | 3 |
| Total | 124 |
Bilateral LDLLT was performed in 108 patients and single LDLLT was performed in 16 small patients. There were 8 early deaths, for a hospital mortality of 6.5%. The causes of hospital death were graft failure due to excessive small grafts in 3, infection in 2, acute rejection in 1, heart failure in 1 and multi-organ failure in 1. There were 20 late deaths during a follow-up period of 2–225 months. The causes of late death were chronic lung allograft dysfunction (CLAD) in 8, malignancy in 7 including PTLD in 3, sepsis in 2, encephalitis in 1, and unknown cause in 2. The 5-, 10- and 15-year survivals were 80.8%, 72.6% and 61.7%, respectively (Figure 6).
Figure 6.
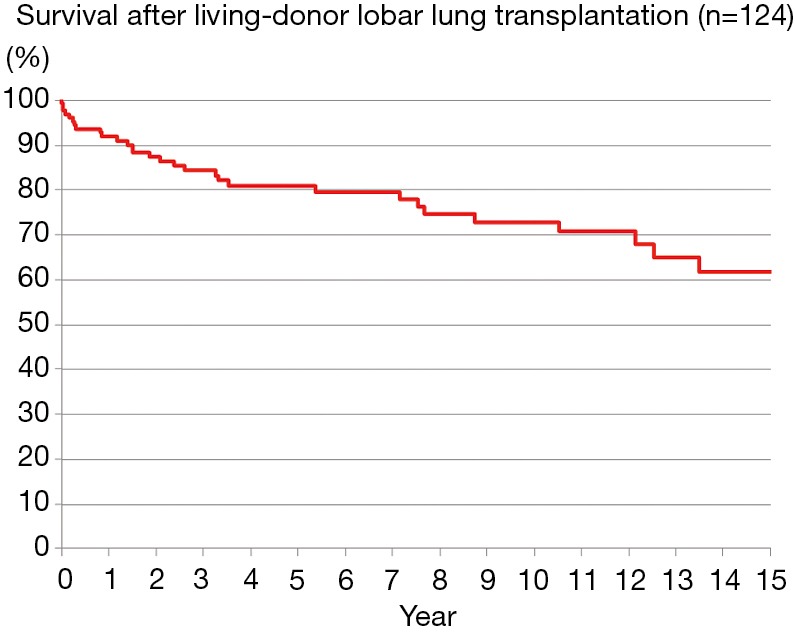
Survival after living-donor lobar lung transplantation (n=124). The 5-, 10- and 15-year survivals were 80.8%, 72.6% and 61.7%, respectively.
Comparison with CLT
There are some advantages and disadvantages of LDLLT compared to CLT as summarized in Table 3. The graft ischemic time for LDLLT is significantly shorter than CLT. Although relatively small grafts are implanted, primary graft failure seems to be less frequently encountered after LDLLT because of “small but perfect grafts”.
Table 3. Comparison between LDLLT and CLT.
| Variables | LDLLT | CLT |
|---|---|---|
| Waiting time | Short | Long |
| Schedule | Controllable | Uncontrollable |
| Ischemic time | Short | Long |
| Graft size | Small | Full |
| Primary graft failure | Infrequent | 10–20% |
| Infection transmitted from graft | Infrequent | Frequent |
| Number of teams | 3 | 2 |
| Bronchial complication | Rare | 5% |
| Chronic rejection | Often unilateral | Major cause of death |
LDLLT, living-donor lobar lung transplantation; CLT, cadaveric lung transplantation.
The incidence of bronchial complications after CLT has been reported to be about 5%. Although we have accepted patients with high-dose systemic corticosteroid therapy in LDLLT, excellent bronchial healing was obtained in most of the recipients. Various factors including short donor bronchus, relatively high blood flow into the small grafts, normal lung parenchyma with short ischemic time, are contributors of better oxygen supply to the donor bronchus and excellent bronchial healing in LDLLT (35). In our LDLLT experience at Kyoto University, airway complications developed in 4.7% and stenosis of the segmental bronchus was characteristic (36).
It is well known that bronchiolitis obliterans syndrome (BOS) is the major unsolved problem after CLT. We and the USC group reported less BOS incidence after LDLLT. It may be related to the shorter ischemic time in LDLLT. Interestingly, most of LDLLT recipients developed unilateral BOS and their FEV1 decline stopped within 9 months. Transplanting two different donor grafts appears to be a great benefit to the recipient because contra-lateral unaffected lung may function as a reservoir when BOS occurs unilaterally (37,38).
Acknowledgements
None.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Starnes VA, Lewiston NJ, Luikart H, et al. Current trends in lung transplantation. Lobar transplantation and expanded use of single lungs. J Thorac Cardiovasc Surg 1992;104:1060-5; discussion 1065-6. [PubMed] [Google Scholar]
- 2.Starnes VA, Barr ML, Cohen RG, et al. Living-donor lobar lung transplantation experience: intermediate results. J Thorac Cardiovasc Surg 1996;112:1284-90; discussion 1290-1. 10.1016/S0022-5223(96)70142-3 [DOI] [PubMed] [Google Scholar]
- 3.Starnes VA, Barr ML, Schenkel FA, et al. Experience with living-donor lobar transplantation for indications other than cystic fibrosis. J Thorac Cardiovasc Surg 1997;114:917-21; discussion 921-2. 10.1016/S0022-5223(97)70005-9 [DOI] [PubMed] [Google Scholar]
- 4.Date H, Aoe M, Nagahiro I, et al. Living-donor lobar lung transplantation for various lung diseases. J Thorac Cardiovasc Surg 2003;126:476-81. 10.1016/S0022-5223(03)00235-6 [DOI] [PubMed] [Google Scholar]
- 5.Date H, Aoe M, Sano Y, et al. Improved survival after living-donor lobar lung transplantation. J Thorac Cardiovasc Surg 2004;128:933-40. 10.1016/j.jtcvs.2004.07.032 [DOI] [PubMed] [Google Scholar]
- 6.Date H, Tanimoto Y, Goto K, et al. A new treatment strategy for advanced idiopathic interstitial pneumonia: living-donor lobar lung transplantation. Chest 2005;128:1364-70. 10.1378/chest.128.3.1364 [DOI] [PubMed] [Google Scholar]
- 7.Date H, Kusano KF, Matsubara H, et al. Living-donor lobar lung transplantation for pulmonary arterial hypertension after failure of epoprostenol therapy. J Am Coll Cardiol 2007;50:523-7. 10.1016/j.jacc.2007.03.054 [DOI] [PubMed] [Google Scholar]
- 8.Mohite PN, Popov AF, Yacoub MH, et al. Live related donor lobar lung transplantation recipients surviving well over a decade: still an option in times of advanced donor management. J Cardiothorac Surg 2013;8:37. 10.1186/1749-8090-8-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camargo SM, Camargo Jde J, Schio SM, et al. Complications related to lobectomy in living lobar lung transplant donors. J Bras Pneumol 2008;34:256-63. 10.1590/S1806-37132008000500003 [DOI] [PubMed] [Google Scholar]
- 10.Chen QK, Jiang GN, Ding JA, et al. First successful bilateral living-donor lobar lung transplantation in China. Chin Med J (Engl) 2010;123:1477-8. [PubMed] [Google Scholar]
- 11.Date H, Sato M, Aoyama A, et al. Living-donor lobar lung transplantation provides similar survival to cadaveric lung transplantation even for very ill patients†. Eur J Cardiothorac Surg 2015;47:967-72; discussion 972-3. 10.1093/ejcts/ezu350 [DOI] [PubMed] [Google Scholar]
- 12.Kozower BD, Sweet SC, de la Morena M, et al. Living donor lobar grafts improve pediatric lung retransplantation survival. J Thorac Cardiovasc Surg 2006;131:1142-7. 10.1016/j.jtcvs.2005.08.074 [DOI] [PubMed] [Google Scholar]
- 13.Starnes VA, Bowdish ME, Woo MS, et al. A decade of living lobar lung transplantation: recipient outcomes. J Thorac Cardiovasc Surg 2004;127:114-22. 10.1016/j.jtcvs.2003.07.042 [DOI] [PubMed] [Google Scholar]
- 14.Date H. Current status and problems of lung transplantation in Japan. J Thorac Dis 2016;8:S631-6. 10.21037/jtd.2016.06.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen F, Yamane M, Inoue M, et al. Less maintenance immunosuppression in lung transplantation following hematopoietic stem cell transplantation from the same living donor. Am J Transplant 2011;11:1509-16. 10.1111/j.1600-6143.2011.03591.x [DOI] [PubMed] [Google Scholar]
- 16.Barr ML, Belghiti J, Villamil FG, et al. A report of the Vancouver Forum on the care of the live organ donor: lung, liver, pancreas, and intestine data and medical guidelines. Transplantation 2006;81:1373-85. 10.1097/01.tp.0000216825.56841.cd [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Fujinaga T, Shoji T, et al. Short-term outcome in living donors for lung transplantation: the role of preoperative computer tomographic evaluations of fissures and vascular anatomy. Transpl Int 2012;25:732-8. 10.1111/j.1432-2277.2012.01444.x [DOI] [PubMed] [Google Scholar]
- 18.Fujita T, Date H, Ueda K, et al. Experimental study on size matching in a canine living-donor lobar lung transplant model. J Thorac Cardiovasc Surg 2002;123:104-9. 10.1067/mtc.2002.117280 [DOI] [PubMed] [Google Scholar]
- 19.Haddy SM, Bremner RM, Moore-Jefferies EW, et al. Hyperinflation resulting in hemodynamic collapse following living donor lobar transplantation. Anesthesiology 2002;97:1315-7. 10.1097/00000542-200211000-00042 [DOI] [PubMed] [Google Scholar]
- 20.Oto T, Date H, Ueda K, et al. Experimental study of oversized grafts in a canine living-donor lobar lung transplantation model. J Heart Lung Transplant 2001;20:1325-30. 10.1016/S1053-2498(01)00362-X [DOI] [PubMed] [Google Scholar]
- 21.Date H, Aoe M, Nagahiro I, et al. How to predict forced vital capacity after living-donor lobar-lung transplantation. J Heart Lung Transplant 2004;23:547-51. 10.1016/j.healun.2003.07.005 [DOI] [PubMed] [Google Scholar]
- 22.Chen F, Kubo T, Yamada T, et al. Adaptation over a wide range of donor graft lung size discrepancies in living-donor lobar lung transplantation. Am J Transplant 2013;13:1336-42. 10.1111/ajt.12188 [DOI] [PubMed] [Google Scholar]
- 23.Chen F, Miwa S, Bando T, et al. Pulmonary arterioplasty for the remaining arterial stump of the donor and the arterial cuff of the donor graft in living-donor lobar lung transplantation. Eur J Cardiothorac Surg 2012;42:e138-9. 10.1093/ejcts/ezs460 [DOI] [PubMed] [Google Scholar]
- 24.Kayawake H, Chen-Yoshikawa TF, Aoyama A, et al. Excellent outcome of donor lobectomy with various surgical techniques for the interlobar artery. Eur J Cardiothorac Surg 2017;51:279-83. [DOI] [PubMed] [Google Scholar]
- 25.Date H, Shiraishi T, Sugimoto S, et al. Outcome of living-donor lobar lung transplantation using a single donor. J Thorac Cardiovasc Surg 2012;144:710-5. 10.1016/j.jtcvs.2012.05.054 [DOI] [PubMed] [Google Scholar]
- 26.Aoyama A, Chen F, Minakata K, et al. Sparing Native Upper Lobes in Living-Donor Lobar Lung Transplantation: Five Cases From a Single Center. Am J Transplant 2015;15:3202-7. 10.1111/ajt.13357 [DOI] [PubMed] [Google Scholar]
- 27.Chen F, Miyamoto E, Takemoto M, et al. Right and left inverted lobar lung transplantation. Am J Transplant 2015;15:1716-21. 10.1111/ajt.13148 [DOI] [PubMed] [Google Scholar]
- 28.Date H, Aoyama A, Hijiya K, et al. Outcomes of various transplant procedures (single, sparing, inverted) in living-donor lobar lung transplantation. J Thorac Cardiovasc Surg 2017;153:479-86. 10.1016/j.jtcvs.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 29.Bowdish ME, Barr ML, Schenkel FA, et al. A decade of living lobar lung transplantation: perioperative complications after 253 donor lobectomies. Am J Transplant 2004;4:1283-8. 10.1111/j.1600-6143.2004.00514.x [DOI] [PubMed] [Google Scholar]
- 30.Yusen RD, Hong BA, Messersmith EE, et al. Morbidity and mortality of live lung donation: results from the RELIVE study. Am J Transplant 2014;14:1846-52. 10.1111/ajt.12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen F, Yamada T, Sato M, et al. Postoperative pulmonary function and complications in living-donor lobectomy. J Heart Lung Transplant 2015;34:1089-94. 10.1016/j.healun.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 32.Chen F, Oga T, Sakai H, et al. A prospective study analyzing one-year multidimensional outcomes in living lung transplant donors. Am J Transplant 2013;13:3003-9. 10.1111/ajt.12476 [DOI] [PubMed] [Google Scholar]
- 33.Chen F, Fujinaga T, Shoji T, et al. Outcomes and pulmonary function in living lobar lung transplant donors. Transpl Int 2012;25:153-7. 10.1111/j.1432-2277.2011.01401.x [DOI] [PubMed] [Google Scholar]
- 34.Sweet SC. Pediatric living donor lobar lung transplantation. Pediatr Transplant 2006;10:861-8. 10.1111/j.1399-3046.2006.00559.x [DOI] [PubMed] [Google Scholar]
- 35.Toyooka S, Yamane M, Oto T, et al. Bronchial healing after living-donor lobar lung transplantation. Surg Today 2009;39:938-43. 10.1007/s00595-008-4049-3 [DOI] [PubMed] [Google Scholar]
- 36.Miyamoto E, Chen-Yoshikawa TF, Higuchi H, et al. Stenosis of the segmental bronchus is a characteristic airway complication in living-donor lobar lung transplantation. J Heart Lung Transplant 2016;35:389-92. 10.1016/j.healun.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 37.Shinya T, Sato S, Kato K, et al. Assessment of mean transit time in the engrafted lung with 133Xe lung ventilation scintigraphy improves diagnosis of bronchiolitis obliterans syndrome in living-donor lobar lung transplant recipients. Ann Nucl Med 2008;22:31-9. 10.1007/s12149-007-0078-z [DOI] [PubMed] [Google Scholar]
- 38.Miyamoto E, Chen F, Aoyama A, et al. Unilateral chronic lung allograft dysfunction is a characteristic of bilateral living-donor lobar lung transplantation. Eur J Cardiothorac Surg 2015;48:463-9. 10.1093/ejcts/ezu463 [DOI] [PubMed] [Google Scholar]