Abstract
Background
The preoperative immune-nutritional status has been shown to predict the postoperative prognosis in various types of cancer; however, the prognostic significance of the controlling nutritional status (CONUT) score in resected lung squamous cell carcinoma (SCC) has yet to be elucidated.
Methods
A total of 108 patients with resected lung SCC were analyzed for their clinicopathological factors, including the CONUT score, which can be calculated from the serum albumin, total cholesterol, and total peripheral lymphocyte count. The patients were divided into two groups: CONUT low (0 or 1) or high (≥2).
Results
Among 108 patients, 76 (70.4%) were CONUT low, while 32 (29.6%) were CONUT high. No significant association between the CONUT score and the clinicopathological factors was found. Patients with CONUT high exhibited significantly shorter disease-free and overall survivals (DFS and OS) than those with CONUT low (P=0.016 and P=0.006, respectively). Multivariate analyses showed that the CONUT score [hazard ratio (HR): 1.902, 95% confidence interval (CI): 1.045–3.373, P=0.036], age (HR: 2.286, 95% CI: 1.246–4.304, P=0.007), pathological stage (HR: 2.527, 95% CI: 1.391–4.644, P=0.002), and lymphatic invasion (HR: 2.321, 95% CI: 1.110–4.493, P=0.027) were independent prognostic factors for the DFS. Furthermore, in a multivariate analysis, the CONUT score (HR: 1.909, 95% CI: 0.902–3.860, P=0.081), age (HR: 2.455, 95% CI: 1.208–5.178, P=0.013), pathological stage (HR: 2.488, 95% CI: 1.201–5.306, P=0.014), and lymphatic invasion (HR: 3.409, 95% CI: 1.532–7.240, P=0.004) were shown to be independent prognostic factors for the OS.
Conclusions
The current study showed that the CONUT score was an independent prognostic factor for the DFS and OS in patients with resected lung SCC.
Keywords: Lung squamous cell carcinoma (SCC), controlling nutritional status (CONUT) score, surgical resection, postoperative prognosis
Introduction
Lung cancer is an extremely devastating neoplasm, and its clinical outcome remains poor (1). Subtypes of lung cancer can be mainly divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Among patients with NSCLC, adenocarcinoma is separated from squamous cell carcinoma (SCC), because the biological behaviors and treatment strategies differ by subtype (2,3). Although surgical resection is the main treatment option for patients with stage I, II, and IIIA NSCLC, its clinical course is still not satisfactory (4). Therefore, the identification of biomarkers in patients with SCC would help predict the prognosis and individualize therapy based on the stratification risks. Furthermore, we feel that such biomarkers should not only be useful, but also simple and inexpensive to obtain.
Recently, the immune-nutritional status, such as the prognostic nutritional index (PNI) calculated from serum albumin and the total peripheral lymphocyte count, has been shown to be associated with the postoperative outcomes in various types of cancer, including lung cancer (5-9). For example, a low PNI was reported to be associated with an increased risk of postoperative complications and a poor survival in colorectal cancer patients (8). With regard to lung cancer, there have been several reports showing the prognostic significance of PNI in not only operable but also non-operable patients (6,7,9).
Thus, the significance of PNI has been gradually clarified, and another nutritional index, the controlling nutritional status (CONUT) score, has also been advocated. The CONUT score is calculated from the serum albumin concentration, total peripheral lymphocyte count, and total cholesterol concentration (10). Recently, it was reported that a high CONUT score was associated with a poor postoperative survival in patients with colorectal and esophageal cancer (11-13). Compared with the PNI, the CONUT score appears to be more accurate in evaluating the nutritional status because it includes the serum albumin and cholesterol concentration, while the PNI includes only albumin. The serum albumin concentration can be easily influenced by not only the nutritional status but also by changes in the body fluid volume and inflammation (14). Therefore, adding cholesterol can reduce these influences on the serum albumin, which may lead to the more accurate evaluation of the nutritional status. Furthermore, cholesterol has been reported to correlate with the progression of cancer (15). Therefore, we focused on the CONUT score.
In the current study, we evaluated the relationship between the CONUT score and clinicopathological features, and assessed the impact of the CONUT score on the postoperative survival, including the disease-free and overall survivals (DFS and OS), in patients with resected lung SCC.
Methods
Study patients
From January 2003 and December 2012, 108 patients with lung SCC who underwent surgery at the Department of Surgery and Science, Graduate School of Medical Sciences, Kyushu University were included in this study. The clinicopathological features, including age at surgery, gender, smoking history, body mass index, CONUT score, pathologic tumor-node-metastasis (TNM) stage (seventh edition of the lung cancer staging system), tumor differentiation (seventh edition of the General Rule for Clinical and Pathological Record of Lung Cancer, Japan), pleural or lymphovascular invasion, and surgical procedure, were examined. Systemic dissection of the hilar and mediastinal lymph nodes was performed at the same time as pulmonary lobectomy. After surgery, routine check-ups, including a physical examination, blood tests, and chest X-ray, were performed at 3-month intervals for the first 3 years and at 6-month intervals thereafter. Computed tomography was performed twice a year for the first 3 years and then at least annually thereafter. Clinical information and follow-up data were obtained from the medical records. This study was approved by our institutional review board (the Number of the Ethic Approval: 27-261).
CONUT score
The serum albumin concentration, total peripheral lymphocyte count and total cholesterol concentration were used to obtain the CONUT score. The preoperative blood samples obtained within one month prior to surgical resection were used to calculate the CONUT score. The scoring system of CONUT is normal (0–1), mild (2–4), moderate (5–8), and severe (9–12; Table 1). The cut-off value of the CONUT score was determined using a receiver operating characteristic (ROC) curve, and the OS was used as the state variable. As shown in Figure S1, the optimal CONUT cut-off value was found to be 2, with an area under the curve, sensitivity, and specificity of 0.58997, 0.7857, and 0.3846, respectively. Therefore, 108 patients with resected lung SCC were assigned to CONUT low (0, 1) and CONUT high (≥2) groups.
Table 1. The CONUT scoring system.
| Parameters | Normal | Mild | Moderate | Severe |
|---|---|---|---|---|
| Serum albumin (g/mL) | ≥3.5 | 3.0–3.4 | 2.5–2.9 | <2.50 |
| Score | 0 | 2 | 4 | 6 |
| Total lymphocyte count | ≥1,600 | 1,200–1,599 | 800–1,199 | <800 |
| Score | 0 | 1 | 2 | 3 |
| Total cholesterol (mg/dL) | ≥180 | 140–179 | 100–139 | <100 |
| Score | 0 | 1 | 2 | 3 |
| Total score | 0–1 | 2–4 | 5–8 | 9–12 |
| Dysnutritional states | Normal | Mild | Moderate | Severe |
CONUT, controlling nutritional status.
Statistical analyses
The associations between the CONUT score and patient characteristics were analysed using Fisher’s exact test. The OS was defined as the time from the initial surgery until death from any cause, while the DFS was defined as the time from the initial surgery until recurrence. The Kaplan-Meier method was used to estimate the survival probabilities. The curves of the two groups were statistically compared using the log-rank test. The prognostic factors for the DFS and OS were assessed using the logistic regression model with backward elimination method. All of the statistical analyses were conducted using the JMP software program, version 12 (SAS Institute, Cary, NC, USA). P values of <0.05 in Fisher’s exact test were considered to indicate statistically significant differences. In the logistic regression model, the level of statistical significance was set as less than 0.1.
Results
Clinicopathological characteristics of the patients
Table 2 shows the clinicopathological characteristics of the patients included in the present study. The median age of the patients was 71 years (range: 45–89 years), and the number of male and female patients was 96 (88.9%) and 12 (11.1%), respectively. Seventy-five patients (69.4%) had a history of smoking. The numbers of patients with BMI ≥20 and <20 were 47 (43.5%) and 61 (56.5%), respectively. The frequencies of patients with a CONUT score of 0, 1, 2, 3, 4, 5, 6, 7, and 8 were 46 (42.6%), 30 (27.8%), 14 (13.0%), 12 (11.1%), 3 (2.8%), 1 (0.9%), 0 (0.0%), 1 (0.9%), and 1 (0.9%), respectively. As shown in the Materials and Methods section, the CONUT cut-off value was set as 2, and the patients were divided into CONUT low [0, 1; n=76 (70.4%)] and high [≥2; n=32 (29.6%)] groups. The numbers of patients with pathological stage I, II, and III disease were 61 (56.5%), 30 (27.8%), and 17 (15.7%), respectively. Twenty-eight patients (25.9%) underwent sublobar resection, while 80 (74.1%) underwent lobectomy, bilobectomy, or pneumonectomy. As shown in Table 3, Fisher’s exact test revealed no significant association between the CONUT score and the clinicopathological characteristics.
Table 2. Clinicopathological characteristics of patients with resected lung SCC.
| Factors | Value or number of patients (n=108) |
|---|---|
| Age (years) | |
| Median | 71 |
| Range | 45–89 |
| Sex | |
| Female | 12 |
| Male | 96 |
| Smoking status | |
| Never-smoker | 33 |
| Smoker | 75 |
| BMI (kg/m2) | |
| ≥20 | 47 |
| <20 | 61 |
| CONUT | |
| 0 | 46 |
| 1 | 30 |
| 2 | 14 |
| 3 | 12 |
| 4 | 3 |
| 5 | 1 |
| 6 | 0 |
| 7 | 1 |
| 8 | 1 |
| Pathological T status | |
| 1 | 37 |
| 2 | 52 |
| 3 | 13 |
| 4 | 6 |
| Pathological N status | |
| 0 | 79 |
| 1 | 20 |
| 2 | 9 |
| 3 | 0 |
| Pathological stage | |
| I | 61 |
| II | 30 |
| III | 17 |
| G | |
| 1 | 15 |
| 2 | 71 |
| 3 | 22 |
| PL | |
| Negative | 65 |
| Positive | 43 |
| LY | |
| Negative | 92 |
| Positive | 16 |
| V | |
| Negative | 59 |
| Positive | 49 |
| Surgical procedure | |
| Wedge resection | 18 |
| Segmentectomy | 10 |
| Lobectomy | 69 |
| Bilobectomy | 6 |
| Pneumonectomy | 5 |
| Adjuvant chemotherapy | |
| None | 93 |
| UFT | 6 |
| Platinum-doublet | 9 |
SCC, squamous cell carcinoma; BMI, body mass index; CONUT, controlling nutrition status; PL, pleural invasion; LY, lymphatic invasion; V, vascular invasion; UFT: tegafur-uracil.
Table 3. Association between the CONUT score and clinicopathological factors in patients with resected SCC.
| Factors | N | CONUT score | P value | |
|---|---|---|---|---|
| Low (n=76) | High (n=32) | |||
| Age (years) | 0.525 | |||
| <70 | 47 | 35 | 12 | |
| ≥70 | 61 | 41 | 20 | |
| Sex | 0.747 | |||
| Female | 12 | 8 | 4 | |
| Male | 96 | 68 | 28 | |
| Smoking history | 1.000 | |||
| Never-smoker | 33 | 23 | 10 | |
| Smoker | 75 | 53 | 22 | |
| BMI (kg/m2) | 0.094 | |||
| ≥20 | 47 | 29 | 18 | |
| <20 | 61 | 47 | 14 | |
| Pathological T status | 0.267 | |||
| 1 | 37 | 29 | 8 | |
| ≥2 | 71 | 47 | 24 | |
| Pathological N status | 0.153 | |||
| 0 | 79 | 59 | 20 | |
| ≥1 | 29 | 17 | 12 | |
| Pathological stage | 0.209 | |||
| I | 61 | 46 | 15 | |
| ≥II | 47 | 30 | 17 | |
| G | 0.765 | |||
| 1 | 15 | 10 | 5 | |
| 2, 3 | 93 | 66 | 27 | |
| PL | 0.391 | |||
| Negative | 65 | 48 | 17 | |
| Positive | 43 | 28 | 15 | |
| LY | 0.554 | |||
| Negative | 92 | 66 | 26 | |
| Positive | 16 | 10 | 6 | |
| V | 0.673 | |||
| Negative | 59 | 43 | 16 | |
| Positive | 49 | 33 | 16 | |
| Surgical procedure | ||||
| ≥ Lobectomy | 80 | 57 | 23 | |
| Sublobar resection | 28 | 19 | 9 | |
| Adjuvant chemotherapy | 0.136 | |||
| None | 93 | 68 | 25 | |
| Administered | 15 | 8 | 7 | |
CONUT, controlling nutrition status; SCC, squamous cell carcinoma; BMI, body mass index; PL, pleural invasion; LY, lymphatic invasion; V, vascular invasion.
DFS and OS according to the CONUT score
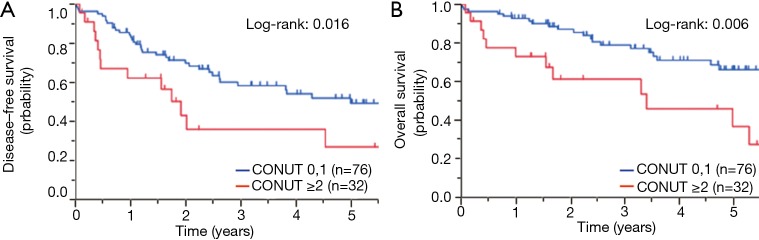
As shown in Figure 1A, patients with CONUT high exhibited significantly shorter DFS than those with CONUT low: the 5-year DFS in patients with CONUT low and high was 51.9% and 27.3%, respectively (P=0.016). The OS in patients with CONUT high was significantly poorer than that in those with CONUT low, with the 5-year OS in CONUT low and high groups being 67.1% and 38.7%, respectively (P=0.006; Figure 1B).
Figure 1.
Kaplan-Meier curves according to the controlling nutritional status (CONUT) score. The (A) DFS and (B) OS were significantly worse in patients with CONUT high (≥2) than in those with CONUT low (0, 1; P=0.016 and P=0.006, respectively).
Univariate and multivariate analyses for the DFS according to the CONUT score
A univariate analysis showed that the CONUT score [hazard ratio (HR): 2.070, 95% confidence interval (CI): 1.147–3.638, P=0.017], pathological tumor stage (HR: 1.670, 95% CI: 0.098–3.258, P=0.098), pathological nodal stage (HR: 1.698, 95% CI: 0.931–2.995, P=0.083), pathological stage (HR: 2.272, 95% CI: 1.304–4.011, P=0.004), lymphatic invasion (HR: 2.347, 95% CI: 1.142–4.433, P=0.022), and vessel invasion (HR: 1.648, 95% CI: 0.950–2.887, P=0.076) were significantly associated with the DFS (Table 4). These significant associations were retained in a multivariate analysis: the CONUT score (HR: 1.902, 95% CI: 1.045–3.373, P=0.036), age (HR: 2.286, 95% CI: 1.246–4.304, P=0.007), pathological stage (HR: 2.527, 95% CI: 1.391–4.644, P=0.002), and lymphatic invasion (HR: 2.321, 95% CI: 1.110–4.493, P=0.027; Table 4).
Table 4. Univariate and multivariate analyses for the association between the DFS and the clinicopathological factors.
| Factors | N | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Age (years) | ||||||||
| <70 | 47 | 1.000 | 1.000 | |||||
| ≥70 | 61 | 1.613 | 0.913–2.919 | 0.100 | 2.286 | 1.246–4.304 | 0.007 | |
| Sex | ||||||||
| Female | 12 | 1.000 | ||||||
| Male | 96 | 1.102 | 0.507–2.889 | 0.821 | ||||
| Smoking history | ||||||||
| Never-smoker | 33 | 1.000 | ||||||
| Smoker | 75 | 1.359 | 0.746–2.621 | 0.324 | ||||
| BMI (kg/m2) | ||||||||
| ≥20 | 47 | 1.000 | ||||||
| <20 | 61 | 0.790 | 0.452–1.377 | 0.404 | ||||
| CONUT | ||||||||
| Low | 76 | 1.000 | 1.000 | |||||
| High | 32 | 2.070 | 1.147–3.638 | 0.017 | 1.902 | 1.045–3.373 | 0.036 | |
| Pathological T status | ||||||||
| 1 | 37 | 1.000 | ||||||
| ≥2 | 71 | 1.670 | 0.098–3.258 | 0.098 | ||||
| Pathological N status | ||||||||
| 0 | 79 | 1.000 | ||||||
| ≥1 | 29 | 1.698 | 0.931–2.995 | 0.083 | ||||
| Pathological stage | ||||||||
| I | 61 | 1.000 | 1.000 | |||||
| ≥II | 47 | 2.272 | 1.304–4.011 | 0.004 | 2.527 | 1.391–4.644 | 0.002 | |
| G | ||||||||
| 1 | 15 | 1.000 | ||||||
| 2, 3 | 93 | 1.185 | 0.544–3.107 | 0.691 | ||||
| PL | ||||||||
| Negative | 65 | 1.000 | ||||||
| Positive | 43 | 1.564 | 0.896–2.713 | 0.115 | ||||
| LY | ||||||||
| Negative | 92 | 1.000 | 1.000 | |||||
| Positive | 16 | 2.347 | 1.142–4.433 | 0.022 | 2.321 | 1.110–4.493 | 0.027 | |
| V | ||||||||
| Negative | 59 | 1.000 | ||||||
| Positive | 49 | 1.648 | 0.950–2.887 | 0.076 | ||||
| Surgical procedure | ||||||||
| ≥ Lobectomy | 80 | 1.000 | ||||||
| Sublobar resection | 28 | 0.948 | 0.474–1.761 | 0.871 | ||||
| Adjuvant chemotherapy | ||||||||
| None | 93 | 1.000 | ||||||
| Administered | 15 | 1.047 | 0.454–2.121 | 0.906 | ||||
CONUT, controlling nutrition status; PL, pleural invasion; LY, lymphatic invasion; V, vascular invasion.
Univariate and multivariate analyses for the OS according to the CONUT score
The CONUT score (HR: 2.378, 95% CI: 1.171–4.699, P=0.018), age (HR: 1.852, 95% CI: 0.933–3.834, P=0.079), lymph node metastasis (HR: 2.022, 95% CI: 1.010–3.956, P=0.047), pathological stage (HR: 2.688, 95% CI: 1.362–5.513, P=0.004), pleural invasion (HR: 1.857, 95% CI: 0.951–3.641, P=0.069) and lymphatic invasion (HR: 3.533, 95% CI: 1.643–7.172, P=0.002) were shown to be significantly associated with the OS in a univariate analysis (Table 5). A multivariate analysis showed that the CONUT score (HR: 1.909, 95% CI: 0.902–3.860, P=0.081) as well as age (HR: 2.455, 95% CI: 1.208–5.178, P=0.013), pathological stage (HR: 2.488, 95% CI: 1.201–5.306, P=0.014), and lymphatic invasion (HR: 3.409, 95% CI: 1.532–7.240, P=0.004) were independently associated with the OS (Table 5).
Table 5. Univariate and multivariate analyses for the association between the OS and the clinicopathological factors.
| Factors | N | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Age (years) | ||||||||
| <70 | 47 | 1.000 | 1.000 | |||||
| ≥70 | 61 | 1.852 | 0.933–3.834 | 0.079 | 2.455 | 1.208–5.178 | 0.013 | |
| Sex | ||||||||
| Female | 12 | 1.000 | ||||||
| Male | 96 | 1.570 | 0.560–6.548 | 0.428 | ||||
| Smoking history | ||||||||
| Never-smoker | 33 | 1.000 | ||||||
| Smoker | 75 | 1.000 | 0.506–2.059 | 0.990 | ||||
| BMI (kg/m2) | ||||||||
| ≥20 | 47 | 1.000 | ||||||
| <20 | 61 | 0.875 | 0.449–1.716 | 0.695 | ||||
| CONUT | ||||||||
| Low | 76 | 1.000 | 1.000 | |||||
| High | 32 | 2.378 | 1.171–4.699 | 0.018 | 1.909 | 0.920–3.860 | 0.081 | |
| Pathological T status | ||||||||
| 1 | 37 | 1.000 | ||||||
| ≥2 | 71 | 1.685 | 0.819–3.810 | 0.161 | ||||
| Pathological N status | ||||||||
| 0 | 79 | 1.000 | ||||||
| ≥1 | 29 | 2.022 | 1.010–3.956 | 0.047 | ||||
| Pathological stage | ||||||||
| I | 61 | 1.000 | 1.000 | |||||
| ≥II | 47 | 2.688 | 1.362–5.513 | 0.004 | 2.488 | 1.201–5.306 | 0.014 | |
| G | ||||||||
| 1 | 15 | 1.000 | ||||||
| 2, 3 | 93 | 1.065 | 0.420–3.589 | 0.905 | ||||
| PL | ||||||||
| Negative | 65 | 1.000 | ||||||
| Positive | 43 | 1.857 | 0.951–3.641 | 0.069 | ||||
| LY | ||||||||
| Negative | 92 | 1.000 | 1.000 | |||||
| Positive | 16 | 3.533 | 1.643–7.172 | 0.002 | 3.409 | 1.532–7.240 | 0.004 | |
| V | ||||||||
| Negative | 59 | 1.000 | ||||||
| Positive | 49 | 1.423 | 0.732–2.803 | 0.298 | ||||
| Surgical procedure | ||||||||
| ≥ Lobectomy | 80 | 1.000 | ||||||
| Sublobar resection | 28 | 1.061 | 0.447–2.255 | 0.886 | ||||
| Adjuvant chemotherapy | ||||||||
| None | 93 | 1.000 | ||||||
| Administered | 15 | 1.049 | 0.390–2.380 | 1.049 | ||||
CONUT, controlling nutrition status; PL, pleural invasion; LY, lymphatic invasion; V, vascular invasion.
Discussion
Various types of blood sample-derived biomarkers that can simply and inexpensively predict the postoperative survival in cancer patients have been advocated, and such biomarkers include systemic inflammatory response indicators, such as neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios, and immune-nutritional status, such as PNI and CONUT (5-9,11-13,16). With regard to the significance of the CONUT score in patients with cancer, there are several reports that describe the prognostic impact of the CONUT score on the preoperative prognosis in patients with colorectal and esophageal cancer (11-13). Furthermore, the CONUT score was reported to be associated with adverse events by radiotherapy for head and neck cancer, morbidity after esophagectomy, and surgical site infection after colorectal surgery (13,17). However, there have been no reports regarding the significance of the CONUT score in patients with lung cancer. In the current article, we described the prognostic significance of the CONUT score in patients with surgically resected lung SCC: patients with CONUT high exhibited a significantly shorter DFS and OS than those with CONUT low (P=0.016 and 0.006, respectively). In addition, multivariate analyses showed that the CONUT score were independently associated with the DFS and OS. Thus, this is the first report to describe the prognostic impact of the preoperative CONUT score in patients with SCC who underwent surgery.
The CONUT score, which can be calculated from the serum albumin concentration, total peripheral lymphocyte count and total cholesterol concentration, was originally advocated for the early detection and continuous control of hospital undernutrition in 2003 and was reported to be significantly associated with the Subjective Global Assessment (SGA), which is another nutritional index, with the kappa index being 0.488 (P=0.034) (10). Although SGA is simple, inexpensive, and not time-consuming, it demands a degree of skill and experience in light of its nature as a “subjective” assessment. In contrast, since the CONUT score is derived based solely on the results of blood samples, physicians can simply, objectively, and continuously assess the nutritional status of the patient over the course of the treatment. Furthermore, as in the current study, it allows for the retrospective examination of the associations between the score and clinical outcomes, which is one of its advantages. Therefore, we performed a retrospective analysis of the CONUT score.
Our results showing the prognostic impact of the score on the prognosis of lung SCC patients appears to have clinical significances in several respects. First, the CONUT score might be useful for predicting the prognosis and personalizing therapy. Patients with a high CONUT score should be more intensively followed up than those with a low CONUT score. Furthermore, nutritional intervention prior to surgical resection of the lung SCC might improve the surgical outcomes in patients with a high CONUT score. A number of reports have suggested that nutritional intervention might improve the treatment outcomes and reduce the postoperative hospital stay in several types of cancer, including esophageal and head/neck cancer (18,19). For example, perioperative arginine-containing enteral nutrition led to the improvement of the long term OS and DFS in malnourished patients with head and neck cancer (18). Another report showed that, when cisplatin-based chemotherapy was administered, short-term exogenous ghrelin intervention was able to reduce the rates of adverse events via the stimulation of food intake (19). Furthermore, as demonstrated by Polanski et al., a strong relationship between the quality of life and weight loss shows the importance of dietary management in patients with lung cancer (20). Given these findings, future studies are warranted to investigate the significance of preoperative nutritional intervention in patients with resected lung SCC. Furthermore, our data emphasize the importance of the preoperative assessment of the nutritional status, surgical resection, and postoperative follow-up by a multidisciplinary team consisting of surgeons, nurses, and nutritionists.
Several limitations associated with the present study warrant mention. First, this is single-institution retrospective study that investigated 108 patients with resected lung SCC. Although lung adenocarcinoma is the most frequent histology in Japan, SCC is the second in frequency (21). Furthermore, there are many countries in which SCC is the most common histology of lung cancer (22). Therefore, the results of the current study may have some significance in clinical settings. Further studies should be performed to investigate the significance of the CONUT score in a larger subset of patients, including not only SCC but also adenocarcinoma patients. Third, due to the nature of a retrospective study, the current study could not include the factors that could impact the inflammation and nutritional status, such as medications and other medical conditions. Furthermore, not all information on weight loss and the performance status at the time of diagnosis were available. These points should also be clarified in a future study.
In conclusion, the current study showed that the CONUT score was an independent prognostic factor for the DFS and OS in patients with resected lung SCC. This study provides the first evidence of the prognostic impact of the CONUT score in such a population, although the findings should be validated in a larger study prospectively conducted by a multidisciplinary team consisting of not only surgeons but also nutritionists.
Figure S1.
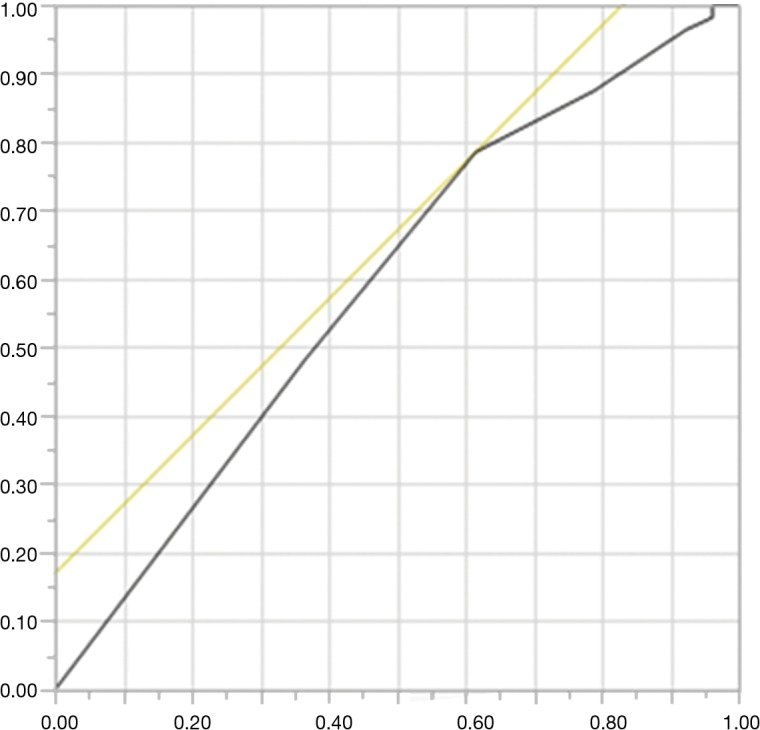
A ROC curve for the CONUT. The continuous variables CONUT and OS were used as the test and state variables, respectively. The optimal CONUT cut-off value was found to be 2, with an area under curve, sensitivity, and specificity being 0.58997, 0.7857, and 0.3846, respectively. CONUT, controlling nutrition status; ROC, receiver operating characteristic; OS, overall survival.
Acknowledgements
We thank Brian Quinn for his critical comments on the manuscript.
Ethical Statement: This study was approved by our institutional review board (the Number of the Ethic Approval: 27-261).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. 10.1038/nature13385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. 10.1038/nature11404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawabata N, Fujii Y, Asamura H, et al. Lung cancer in Japan: analysis of lung cancer registry cases resected in 2004. Nihon Kokyuki Gakkai Zasshi 2011;49:327-42. [PubMed] [Google Scholar]
- 5.Sakurai K, Tamura T, Toyokawa T, et al. Low Preoperative Prognostic Nutritional Index Predicts Poor Survival Post-gastrectomy in Elderly Patients with Gastric Cancer. Ann Surg Oncol 2016;23:3669-76. 10.1245/s10434-016-5272-6 [DOI] [PubMed] [Google Scholar]
- 6.Shimizu K, Okita R, Saisho S, et al. Preoperative neutrophil/lymphocyte ratio and prognostic nutritional index predict survival in patients with non-small cell lung cancer. World J Surg Oncol 2015;13:291. 10.1186/s12957-015-0710-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoji F, Morodomi Y, Akamine T, et al. Predictive impact for postoperative recurrence using the preoperative prognostic nutritional index in pathological stage I non-small cell lung cancer. Lung Cancer 2016;98:15-21. 10.1016/j.lungcan.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 8.Tokunaga R, Sakamoto Y, Nakagawa S, et al. Prognostic Nutritional Index Predicts Severe Complications, Recurrence, and Poor Prognosis in Patients With Colorectal Cancer Undergoing Primary Tumor Resection. Dis Colon Rectum 2015;58:1048-57. 10.1097/DCR.0000000000000458 [DOI] [PubMed] [Google Scholar]
- 9.Sheng J, Yang YP, Ma YX, et al. Low Prognostic Nutritional Index Correlates with Worse Survival in Patients with Advanced NSCLC following EGFR-TKIs. PLoS One 2016;11:e0147226. 10.1371/journal.pone.0147226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005;20:38-45. [PubMed] [Google Scholar]
- 11.Iseki Y, Shibutani M, Maeda K, et al. Impact of the Preoperative Controlling Nutritional Status (CONUT) Score on the Survival after Curative Surgery for Colorectal Cancer. PLoS One 2015;10:e0132488. 10.1371/journal.pone.0132488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokunaga R, Sakamoto Y, Nakagawa S, et al. CONUT: a novel independent predictive score for colorectal cancer patients undergoing potentially curative resection. Int J Colorectal Dis 2017;32:99-106. 10.1007/s00384-016-2668-5 [DOI] [PubMed] [Google Scholar]
- 13.Yoshida N, Baba Y, Shigaki H, et al. Preoperative Nutritional Assessment by Controlling Nutritional Status (CONUT) is Useful to estimate Postoperative Morbidity After Esophagectomy for Esophageal Cancer. World J Surg 2016;40:1910-7. 10.1007/s00268-016-3549-3 [DOI] [PubMed] [Google Scholar]
- 14.de Ulibarri Perez JI, Fernandez G, Rodriguez Salvanes F, et al. Nutritional screening; control of clinical undernutrition with analytical parameters. Nutr Hosp 2014;29:797-811. [DOI] [PubMed] [Google Scholar]
- 15.Cengiz O, Kocer B, Surmeli S, et al. Are pretreatment serum albumin and cholesterol levels prognostic tools in patients with colorectal carcinoma? Med Sci Monit 2006;12:CR240-7. [PubMed] [Google Scholar]
- 16.Sanchez-Salcedo P, de-Torres JP, Martinez-Urbistondo D, et al. The neutrophil to lymphocyte and platelet to lymphocyte ratios as biomarkers for lung cancer development. Lung Cancer 2016;97:28-34. 10.1016/j.lungcan.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 17.Kono T, Sakamoto K, Shinden S, et al. Pre-therapeutic nutritional assessment for predicting severe adverse events in patients with head and neck cancer treated by radiotherapy. Clin Nutr 2016. [Epub ahead of print]. 10.1016/j.clnu.2016.10.021 [DOI] [PubMed] [Google Scholar]
- 18.Buijs N, van Bokhorst-de van der Schueren MA, Langius JA, et al. Perioperative arginine-supplemented nutrition in malnourished patients with head and neck cancer improves long-term survival. Am J Clin Nutr 2010;92:1151-6. 10.3945/ajcn.2010.29532 [DOI] [PubMed] [Google Scholar]
- 19.Hiura Y, Takiguchi S, Yamamoto K, et al. Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients: a prospective, randomized, placebo-controlled phase 2 study. Cancer 2012;118:4785-94. 10.1002/cncr.27430 [DOI] [PubMed] [Google Scholar]
- 20.Polański J, Jankowska-Polańska B, Uchmanowicz I, et al. Malnutrition and Quality of Life in Patients with Non-Small-Cell Lung Cancer. Adv Exp Med Biol 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21.Fukui T, Taniguchi T, Kawaguchi K, et al. Comparisons of the clinicopathological features and survival outcomes between lung cancer patients with adenocarcinoma and squamous cell carcinoma. Gen Thorac Cardiovasc Surg 2015;63:507-13. 10.1007/s11748-015-0564-5 [DOI] [PubMed] [Google Scholar]
- 22.Davidson MR, Gazdar AF, Clarke BE. The pivotal role of pathology in the management of lung cancer. J Thorac Dis 2013;5 Suppl 5:S463-78. [DOI] [PMC free article] [PubMed] [Google Scholar]