Abstract
Patients with end-stage lung disease who are candidates for lung transplantation may acutely decompensate before a donor organ becomes available. In this scenario, extracorporeal life support (ECLS) may be considered as a bridge to transplant or as a bridge to decision. In the current chapter, we review the indications, techniques, and outcomes for bridging to lung transplantation with ECLS.
Keywords: Lung transplantation, extracorporeal membrane oxygenation (ECMO), bridging, extracorporeal life support (ECLS)
Introduction
Lung transplantation is a viable treatment option for patients with end-stage lung disease. This is especially true for patients who present with a rapid decline in respiratory status, requiring advanced airway support. These patients have a high rate of in-hospital mortality, and their candidacy for lung transplantation should be urgently assessed (1-3). Unfortunately, donor lungs are scarce, so bridging strategies may be necessary for survival.
The implementation of the Lung Allocation Score and Eurotransplant high-urgency status has significantly improved the likelihood that an organ will be available. However, despite this improvement, the mortality rate for waitlist patients with acute end-stage exacerbations remains as high as 50% (4,5). The waitlist time for such individuals (median of 12 days) is heavily influenced by blood type, body size, and antibodies. An urgent exacerbation is most likely to develop in patients with idiopathic pulmonary fibrosis or cystic fibrosis, but it can also develop in patients with pulmonary hypertension, bronchiolitis obliterans syndrome, or chronic obstructive pulmonary disease.
In general, patients who present with acute exacerbations require aggressive noninvasive or invasive ventilation strategies. These patients either are already on the transplant list or require an emergent evaluation. Expeditiously deciding whether a patient is an appropriate lung transplant candidate is critical. If the patient is not improving or is worsening, then extracorporeal membrane oxygenation (ECMO) may be considered as a bridge to transplant or to decision. The decision to initiate ECMO should involve a multidisciplinary team to consider reasonable endpoints, cannulation strategies, management goals, and expected outcomes.
Indications
Any patient with refractory hypoxemia or hypercapnia despite optimal ventilatory support and adjunctive medical management is a potential candidate for ECMO (Table 1). Strategies often used to avoid ECMO include mechanical ventilation with 100% oxygen, positive end-expiratory pressure, inhaled nitric oxide, inotropes, paralytics, steroids, and prone positioning. It is important to balance the risks of these interventions with the risks of ECMO. The need for ECMO should be anticipated so that it is placed electively rather than emergently, whenever possible.
Table 1. Indications and contraindications for ECMO bridging.
| Indications |
| Refractory hypoxemia or hypercarbia or right heart failure despite optimal medical management in a patient who is a potential candidate for lung transplantation |
| Absolute contraindications |
| Ineligibility for lung transplantation according to standard criteria |
| Irreversible end-organ damage affecting multiple organs |
| Sepsis and bacteremia |
| Contraindications to systemic anticoagulation |
| Uncontrolled metastatic disease |
| Other terminal illness that is not otherwise treatable with a lung transplant |
| Acute intracerebral hemorrhage or stroke |
| Relative contraindications |
| Age >65 years |
| Limitations in vascular access |
| Obesity (BMI >30) |
| Frailty |
| Allosensitization with prolonged waitlist time |
| Prolonged ventilatory support |
ECMO, extracorporeal membrane oxygenation; BMI, body mass index.
The indication for ECMO is determined by the patient’s candidacy for lung transplantation. If the patient is clearly not a candidate and has an irreversible process, then ECMO should be avoided. If the patient is already on the waitlist and irreversible end-organ damage or other conditions that would preclude him or her from remaining on the list have not developed, then ECMO is certainly indicated (6). The more challenging scenarios involve those patients who are somewhere in between, such as a patient who is not yet cleared for transplantation, or if it is unclear whether a patient’s critical illness is reversible. In such cases, ECMO can be used as a bridge to decision. It is also important to consider the institution’s resources and willingness to absorb the financial and regulatory risks involved in a potentially adverse outcome. It can be helpful for institutions to partner with larger referral centers in their region that have accumulated experience with ECMO bridging.
Contraindications
Although contraindications may vary from program to program, several contraindications have been well established (Table 1). Absolute contraindications for ECMO bridging include the following: ineligibility for transplant according to standard criteria, irreversible end-organ damage affecting multiple organs, sepsis and bacteremia, contraindications to systemic anticoagulation, uncontrolled metastatic disease or another terminal illness that is not otherwise treatable with a lung transplant, and acute intracerebral hemorrhage or stroke.
Relative contraindications for ECMO bridging include the following: age greater than 65 years (because of impaired physiologic reserve), limitations in vascular access, obesity (body mass index >30), frailty, prolonged ventilatory support (i.e., >7 days), and allosensitization with prolonged anticipated waitlist time. Of note, prior lung transplantation is not, in and of itself, a contraindication for ECMO support (7).
In every case in which ECMO bridging is considered, each center will need to weigh the opinions of a multidisciplinary team consisting of a surgical ECMO specialist, lung transplant pulmonologist and surgeon, and critical care physician. Additional consultants should be included depending on the affected organ systems. Input from a physical therapist may also be helpful. Patients who have decompensated to the point that rehabilitation after ECMO is nearly impossible are unlikely to benefit from ECMO. Ambulatory ECMO is helpful for determining a patient’s potential for rehabilitation after ECMO (8). Family member wishes and advanced directives are also critical to consider. End-organ dysfunction including renal, liver, or myocardial dysfunction is worrisome if it is unrelated to the patient’s primary lung disease. For instance, hypoxemia and secondary pulmonary hypertension may improve with ECMO and transplantation, whereas fixed right ventricular dysfunction or fixed renal dysfunction will not. Patients who have been on the ventilator with aggressive support for greater than 7 days are also poor candidates for ECMO, underscoring the importance of anticipating ECMO support early. Resolving these issues in each instance can be difficult, and the consensus of the multidisciplinary group should be followed.
Technical considerations
Veno-venous (VV) and veno-arterial (VA) arrangements are used in ECMO support, both of which deliver blood from the patient to the ECMO oxygenator (outflow), and then from the oxygenator to the patient (inflow) (Figure 1).
Figure 1.
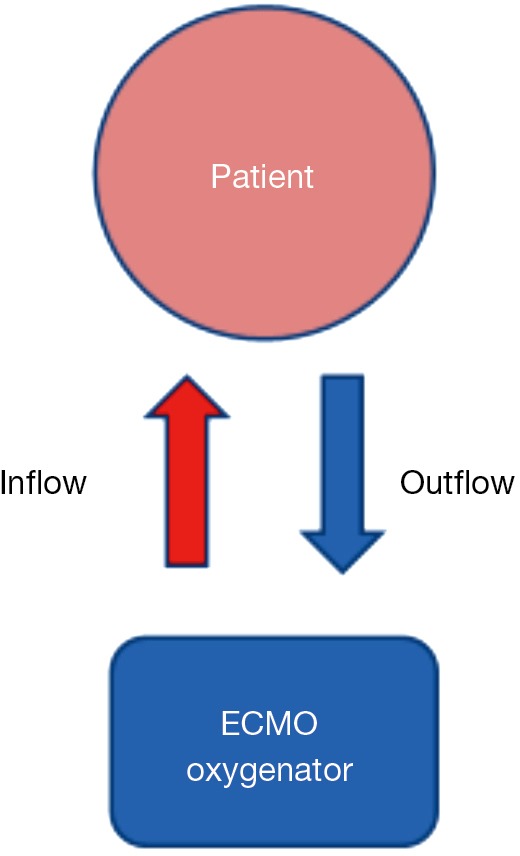
A common strategy for ECMO cannulation involving the use of femoral cannulation for the outflow (deoxygenated blood) and the femoral vein, internal jugular vein, or subclavian vein for the inflow (oxygenated blood) (Fem-IJ/SCV VV ECMO). ECMO, extracorporeal membrane oxygenation.
In VV ECMO, the oxygenated blood goes to the right side of the heart and is pumped through the lungs to the left side of the heart, and finally out to the brain and body. In VA ECMO, oxygenated blood goes directly into the arterial circulation, thereby bypassing the pulmonary circulation.
VV ECMO support
VV ECMO is required for patients with severe lung disease who cannot oxygenate or remove CO2 despite maximal ventilatory support. End-organ dysfunction may develop in these patients, as well as refractory acidosis or worsening pulmonary hypertension. Hemodynamic instability ensues, adding further insult to injury. Therefore, it is best to consider VV ECMO before these adverse events occur. A few fundamental requirements are as follows:
Venous anatomy suitable for the cannulation strategy (venous Doppler is used to confirm that the right internal jugular vein or right subclavian vein is open);
Normal heart function (determined by using echocardiography);
No contraindication to anticoagulation;
Reversible disease process;
Lack of significant resistance to pulmonary arterial flow (it can be less successful for patients with pulmonary hypertension or pulmonary fibrosis).
A common strategy is to use femoral cannulation for the outflow (deoxygenated blood), and the femoral vein, internal jugular vein, or subclavian vein for the inflow (oxygenated blood) (Fem-IJ/SCV VV ECMO) (Figure 1). For this procedure, the patient’s neck and groin are fully prepped and draped. The veins are accessed with a large-bore introducer needle by using ultrasound guidance, and 100 to 200 units/kg of heparin are administered. A long J-wire is advanced through the femoral needle to the level of the right atrium, which is confirmed by using transesophageal echocardiography (TEE).
A series of dilators are passed over the wire before the femoral venous cannula (typically 22–26 F) is advanced to the level of the inferior vena cava (IVC). Importantly, the wire must not be looped; TEE can be used to help confirm this. The femoral cannula is much longer than the inflow cannula, which is typically a shorter arterial-type cannula (14–16 F). This cannula is advanced over a separate wire into the subclavian or internal jugular vein to the level of the superior vena cava (SVC). TEE is used to document these relative positions. The opposite femoral vein can be used if needed, but the risk of recirculation is greater.
The cannulas are clamped, carefully deaired, and connected to the ECMO circuit. Clamps are released, and ECMO is initiated. A chest radiograph is used to verify the location of the cannulas. Importantly, adequate separation between the two cannulas must be present; if not, recirculation can occur if the upper inflow cannula flows right into the lower outflow cannula. Oxygenation will be very poor in this situation, and the cannulas should be adjusted accordingly. If there is any concern for resistance across the pulmonary vasculature, a pulmonary vasodilator should be started. Another option is a right-sided Tandem with an oxygenator. The oxygenator on the ECMO circuit oxygenates the blood while the sweep feature removes CO2. Typical PaO2 levels just beyond the circuit are in the 400 to 450 mmHg range, whereas values in the periphery will range from 80 to 150 mmHg. Often, ventilator support is still required to maintain target oxygenation.
The advantage of this cannulation strategy is that it can be done at the bedside, if needed, or in the operating room. It is relatively straightforward for most cardiothoracic or general surgeons because they are familiar with the percutaneous wire technique. This is particularly useful when placing cannulas emergently at an outside facility (9). Also, the oxygenation tends to be excellent and highly predictable.
The downside of using this cannulation strategy is that femoral or IVC complications can be lethal. In an emergency, TEE may not be available, increasing the risk of vascular complications. Care must be taken to ensure the smooth passage of the femoral venous cannula because it can kink at the level of the subcutaneous tissue. The incisions are often small and can be closed with a deep single purse string suture (nonabsorbable) through the muscle, followed by an external pressure hold for 30 minutes. Alternatively, a femoral cutdown allows exposure to the vein if it is not identified percutaneously. Another important downside of using this approach is that the patient is immobile and cannot move with the groin and neck cannula in place.
The three-stage Avalon venous cannula (MAQUET Cardiovascular, LLC, Wayne, NJ) allows the patient to ambulate and is becoming popular for VV ECMO support (10) (Figure 2). The technique for insertion is similar to that described above, except that the right internal jugular vein is most often used for access. The cannula can be large (27–31 F) and should be inserted with the use of fluoroscopy and TEE guidance. The internal jugular vein is accessed, and a wire is advanced into the infrahepatic IVC under fluoroscopy. If the wire does not traverse the IVC, a sheath is inserted, and a glide catheter and wire can be used to traverse the right atrial-IVC junction. The glide wire is then exchanged for a heavier wire (i.e., Amplatz or Lunderquist), and the Avalon venous cannula is advanced over the wire. The cannula is positioned as such that the inflow limb is towards the patient’s neck, aligning the inflow port with the tricuspid valve (Figure 2) (11).
Figure 2.
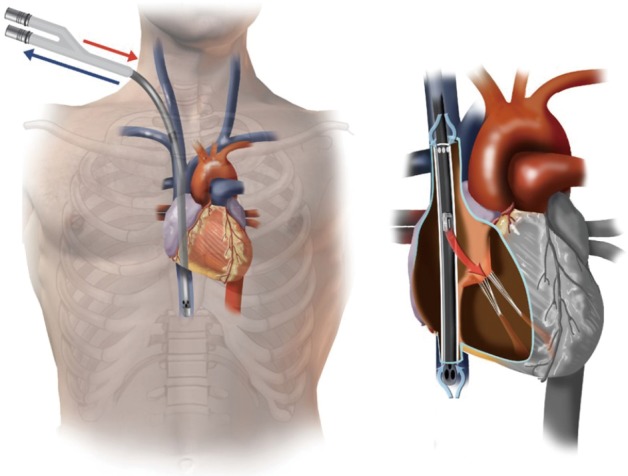
Avalon cannula for veno-venous extracorporeal membrane oxygenation. The Avalon cannula removes blood through a proximal and distal port in the SVC and IVC. Oxygenated blood returns to the body through the middle inflow port oriented towards the tricuspid valve. Original figure by Hirose and colleagues (11), originally published by BioMed Central. Used with permission. SVC, superior vena cava; IVC, inferior vena cava.
Flow through the tricuspid valve is confirmed by using TEE. To prevent recirculation, it is important to ensure that the SVC and IVC inflow ports are in their respective locations. The advantage of this approach is that the patient has the ability to mobilize. The disadvantage is that peripheral oxygenation is not always predictable and highly depends on the degree of pulmonary vascular resistance. Also, vascular complications such as IVC or right ventricular perforation can be catastrophic (11). It is best to have a practitioner with experience performing this technique under fluoroscopy.
VA ECMO support
Patients with indications for ECMO support who also have elevated pulmonary vascular resistance or cardiac dysfunction will require VA ECMO support. VA ECMO can be achieved through a variety of configurations. Essentially, an artery is used to deliver oxygenated blood (inflow) to the body, bypassing the pulmonary circulation, while a vein is used to deliver deoxygenated blood (outflow) to the ECMO oxygenator. Mobile ECMO can be achieved by placing a shorter percutaneous venous outflow cannula (22–24 F) in the internal jugular or subclavian vein to achieve the so-called “sports model”. The arterial cannula is placed directly into the axillary artery (12), and the patient is prepped and draped. A 6-cm incision is made under the clavicle, and proximal and distal control of the axillary artery is achieved. After heparin is administered, the vessel is clamped, and an 8-mm polyester graft is sewn to the axillary artery and tunneled through the subcutaneous tissue to a small counter incision. The graft is connected to the ECMO tubing by using a 1/4 by 3/8-inch adapter and is secured with heavy ties and banding ties. The incision is closed with absorbable sutures. The key advantage to this technique is patient mobility. However, the main disadvantage is the risk of limb hyperperfusion, which has been reported in up to 25% of cases but can be reduced by starting with lower ECMO flows (13).
In cases of severe primary or secondary pulmonary hypertension, other strategies are also available that take advantage of elevated right-sided pressures. The pumpless lung assist device involves a connection between the pulmonary artery and left atrium to bypass the lungs and provide oxygenation, decarboxylation, and right ventricular unloading (14,15). Also, a balloon septostomy has been used successfully in patients with VV ECMO support, allowing oxygenated blood to pass into the systemic circulation (16).
Central ECMO is achieved through a median sternotomy. A purse string is placed directly on the aorta with pledgeted sutures and also in the right atrium. After a small stab incision is made in the aorta, an arterial cannula (20–22 F) is inserted that is deaired and secured to the ECMO circuit. Likewise, a plastic venous cannula is inserted through the right atrium and secured. These cannulas are often externalized through counter incisions in the upper abdomen to allow for sternal closure. Central ECMO is used in cases in which the axillary artery is too small (i.e., <6–8 mm) or is insufficient to provide oxygenated blood to the periphery. The advantages of central ECMO are excellent oxygenation, low risk of stroke, and no risk of limb complication. The downsides are its invasiveness and lack of patient mobility, which can be addressed by externalizing the cannulas. A small right lateral thoracotomy can also be used to limit invasiveness, although it can be technically more challenging to perform.
Finally, femoral VA ECMO is a common arrangement for percutaneous placement, as described above for VV ECMO. The difference is that, for femoral VA ECMO, the inflow arterial cannula is inserted percutaneously or through an open cutdown into the common femoral artery. A cutdown is often required for closure of the arterial defect. The arterial cannula is typically 16 to 19 F, depending on the size of the femoral artery; the venous cannula is similar to that described above for femoral VV ECMO (12). The main advantage of femoral VA ECMO is the ease of placement. A disadvantage is that upper-body oxygenation will be compromised if the patient’s cardiac function is good. The reason for this is that the poorly oxygenated blood from the left atrium and ventricle will be pumped out around the aortic arch. The femoral artery’s contribution to oxygenation in this case will be limited to the lower half of the body, and a “watershed” will form at the level of the descending aorta—a condition often referred to as Harlequin syndrome (17). This phenomenon is the reason why femoral VA ECMO is typically indicated only for patients in cardiogenic shock. One way to avoid this around this is to consider placing an additional inflow arterial cannula in the internal jugular or subclavian vein in a so-called “VVA” configuration (18). Other disadvantages of femoral VA ECMO include femoral artery complications such as bleeding or dissection, as well as patient immobility (17). Also, distal femoral perfusion cannulas are often used to prevent distal limb ischemia. If the femoral VA cannulas are in the same limb and a large venous cannula is used, venous congestion could lead to severe compartment syndrome.
In summary, VV ECMO is used for additional oxygenation and can be placed peripherally or through a 3-port system (Avalon) for mobility. VA ECMO is used in patients with elevated pulmonary vascular resistance, cardiac dysfunction, and pulmonary hypertension. The advantages and disadvantages of either method should be carefully considered, ideally through a team approach involving the surgeon, pulmonologist, and intensivist, as well as the patient and family. Moreover, other extracorporeal life support (ECLS) technologies are available for achieving specific goals in terms of decarboxylation, oxygenation, and hemodynamic support. For instance, CO2 removal can be accomplished with the use of extracorporeal CO2 removal (i.e., ECCO2R). In addition, two types of Novalung (Novalung GmbH, Hechingen, Germany) configurations are available: the peripheral Novalung, which is an VA configuration that relies on the patient’s cardiac output but allows decarboxylation and partial oxygenation; and the pulmonary artery to left atrium Novalung (i.e., PA-LA Novalung), which is a configuration that bypasses the lungs, relies on the patient’s cardiac output, and provides decarboxylation and oxygenation. Table 2 [adapted from (19) by Reeb and colleagues] summarizes these various ECLS technologies and their respective uses.
Table 2. Summary of various ECLS technologies and their respective uses.
| ECLS technique | Oxygenation | Decarboxylation | Circulatory support |
|---|---|---|---|
| VV ECMO | Central organs | Yes | No |
| Peripheral VA ECMO | Peripheral organs | Yes | Yes |
| Central VA ECMO | Central organs | Yes | Yes |
| ECCO2R | No | Yes | No |
| Peripheral Novalung* | No | Yes | No |
| PA-LA Novalung* | Yes | Yes | RV remodeling |
*, Novalung GmbH, Hechingen, Germany. Adapted from the original and used with permission (19). ECLS, extracorporeal life support; VV, veno-venous; ECMO, extracorporeal membrane oxygenation; VA, veno-arterial; ECCO2R, extracorporeal carbon dioxide removal; PA-LA, pulmonary artery to left atrium.
Management of ECMO
ECMO in patients waiting for a lung transplant is managed according to standard ECMO practices. Generally, in the rare event that recovery may occur, the lungs are allowed to rest with minimal tidal volume (typically <6 cc/kg) and positive end-expiratory pressure (5–10 mmHg). The lungs should be assessed periodically for recovery. ECMO support is weaned with optimal tidal volume ventilation while blood gasses and oxygen saturation are assessed. It is preferable—although not always feasible—for the patient to remain extubated. Noninvasive ventilation strategies may be used for this (5,20-23). If the patient is intubated, an early tracheostomy should be considered to allow participation in conditioning programs.
Lab values are checked every 4 h to ensure that coagulation is optimized. Standard hemoglobin thresholds are not uniformly agreed upon. In general, enough hemoglobin is needed to maintain good distal organ perfusion; we employ a threshold of 8 gm/dL before transfusion. The danger of overtransfusing a patient is that antibodies develop. The danger of undertransfusing is that end-organ dysfunction can be exacerbated.
Bleeding is a major complication with ECMO. Coagulation factors should be checked regularly and corrected as needed with additional products (21). Platelet levels should be kept at >50,000, the international normalized ratio at <1.8, and fibrinogen at >200 mg/dL. Heparin is used to maintain the activated clotting time between 160 and 200. Diuresis with medications and/or continuous renal replacement therapy is important to maintain a euvolemic status. ECMO is best managed by an intensive critical care team and an ECMO specialist, who is usually a perfusionist and/or a respiratory therapist trained in ECMO. This team, along with the bedside nurse, will make minute-by-minute adjustments on the basis of established center-specific protocols. The team works closely with pulmonologists and transplant surgeons to ensure that the global objective of maintaining end-organ oxygen delivery and possible pulmonary recovery is achieved.
Physical deconditioning is common with ECMO. To prevent this, physical therapy is critical. Paralytic agents and excessive steroids should be avoided. Every effort should be made to perform active range of motion exercises with the patient. Ambulatory ECMO is helpful for conditioning and for assessing a patient’s potential for recovery after transplantation (23). Ko and colleagues (24) showed that multiple physical therapy sessions including ambulatory ECMO are safe with the use of a mobilization screening protocol.
The goals of patient care should be reviewed regularly with the team and the patient’s family. A palliative care consult is important early in the course of ECMO support. In general, if the patient is stable or improving, then ECMO can be reasonably continued. However, progressive worsening should prompt a discussion about the possible withdrawal of support. While there are no specific time limits on ECMO support, 14 days is typically the upper limit of support time before worsening end-organ status is observed. In the rare case of recovery, weaning trials can be used to assess the ability of the patient to separate from ECMO (20).
Outcomes
During the last decade, the outcomes of ECMO bridging have gradually improved. A report on the US trends in bridging outcomes by Hayanga and colleagues (25) showed that in 2000–2002, the 1-year survival rate after ECMO bridging was 25%, which was increased to 74% in 2009–2011. This may be because of improvements in circuit design, better management, or better patient selection (20). In their study, patients older than 35 years and those with cystic fibrosis or other diagnoses did worse. Patients who were bridged had a higher risk of dialysis-dependent renal failure. In every case, patients who were bridged did worse than those who were not bridged, but the gap in 1-year survival narrowed by the 2009–2011 era (74% vs. 86%). This difference in survival, however, must be considered in the context that patients who are not bridged have a 100% mortality rate without a transplant.
Because old age increases the risk of perioperative mortality, older patients should be approached with caution. The report by Hayanga and colleagues (25) showed that age greater than 35 years was an independent risk factor in patients who were bridged. Nonetheless, a follow-up case report and literature review described a successful transplantation for a 70-year old patient who was bridged with conscious sedation and no mechanical ventilation (26). This highlights the importance of patient selection, the optimization of the bridging strategy, and the careful weighing of competing risk factors, rather than having a strict cutoff. In general, any patient older than 65 years should have very few or no additional risk factors to be considered for bridging to transplantation.
Transplant volume may also be an important factor in determining outcomes for bridging. In a United Network for Organ Sharing (UNOS) review, Hayanga and colleagues (27) showed an adjusted hazard ratio for mortality of 2.74 for patients who were bridged to lung transplantation with ECMO in a low-volume center (i.e., 1–5 transplants/year) versus a high-volume center (i.e., >15 transplants/year). This is an important consideration and suggests that transportation from a low-volume center to a higher-volume center may be wise, from both a risk and quality standpoint.
In 2012, a study by Lang and colleagues (28) in Vienna, Austria showed a 90% success rate for patients who were bridged to transplantation but a 24% rate of in-hospital mortality after transplantation; median bridging time was 5.5 days (range, 1–63 days). Patients who were bridged and survived the initial 3-month period after transplantation had a 5-year survival rate that was equivalent to that of patients who were not bridged (63% vs. 72%, P=0.33). This again emphasizes the importance of selecting patients who are most likely to tolerate the perioperative insult of ECMO. It also underscores the significance of optimizing patients on ECMO and knowing when the patient is making a turn for the worse and may no longer be a good candidate.
In a review of 26 cases of bridging to transplantation, Weig and colleagues (29) showed a success rate that was lower than that reported by the Vienna group (50% vs. 90%). Median time on ECMO was 33 days (range, 17–55 days). No notable differences were observed between patients who survived to transplantation and those who did not. In addition, Weig and colleagues (29) studied several potential risk factors and found that patients who did not survive lung transplantation after bridging had higher bilirubin levels, pulmonary artery pressures, and sequential organ failure assessment (SOFA) scores than did the surviving patients. A bilirubin level >3 mg/dL and a SOFA score >9 predicted a uniformly fatal outcome. Again, these are high-risk features that need to be carefully considered before committing to transplantation and when considering ECMO as a bridge to transplantation.
A study by Crotti and colleagues (30) showed a successful bridging rate of 68%. Time on ECMO was an independent factor in predicting survival after transplantation, with patients who underwent transplantation after less than 14 days of ECMO having a 100% 1-year survival rate and patients who were on ECMO for more than 14 days before transplantation having a 50% 1-year survival rate. Mean SOFA scores from the initiation of ECMO to the end of ECMO went from 5.6 to 6.7 in the early group and from 5.2 to 9.7 in the late group. The patients on noninvasive ventilation before transplantation had a 20% mortality rate while on the waitlist and a 60% 1-year survival rate after transplantation, whereas patients requiring intubation before transplantation had a 40% mortality rate while on the waitlist and a 47% 1-year survival rate after transplantation.
Mason and colleagues (31) showed a successful bridging rate of 74%. They observed that patients who were bridged had a significantly longer hospital stay, greater coagulopathy, and higher rates of dialysis and tracheostomy. Despite this, they saw no difference in 3-year survival rates between patients who were bridged with ECMO and those who were not. Several important complications resulted in death while patients awaited transplantation on ECMO, including renal failure (21%), sepsis (16%), diffuse intravascular coagulopathy (10%), anoxic brain injury (5%), and multisystem organ failure (5%). Patient morbidities after transplantation were also significant and included open chest management (50%), continuation of ECMO (21%), and reoperation for bleeding (29%).
In 2013, 11 centers in France combined data from 36 patients who were bridged with ECMO into a registry report. Their cumulative success with bridging was 83%; however, only 56% of patients were discharged from the hospital (32). Furthermore, only 47% of patients who were bridged were living at 17-month follow-up. Cystic fibrosis patients had the best survival, with a 56% survival rate at 3 years from the initiation of ECMO. This was contrary to the report by Hayanga and colleagues (25). Toyoda and colleagues (33) reported a 77% success rate in 31 patients who were bridged with ECMO. The median duration of ECMO was 91 h. They noted significantly higher rates of PGD3 requiring ECMO support (54% vs. 6%) and a longer median hospital stay (46 vs. 27 days) in the bridged group than in the non-bridged group. Despite this, no significant difference was observed in the 2-year survival rate after transplantation, regardless of preoperative ECMO status (74% for both preoperative ECMO and no ECMO).
Collaud and colleagues (7) performed a literature review and pooled analyses to assess the role of ECMO bridging in retransplantation. They found that the 1-year overall survival rate was 48%. The intertransplant interval was a significant factor affecting survival in these patients. For the subgroup of patients with an intertransplant interval of >2 years and who were bridged on awake ECMO (ambulatory, communicating, low-vent requirements), the 1-year survival rate was 67%.
Another study performed at Zurich University Hospital in Switzerland, showed an 86% successful bridging rate. Intensive care unit and ventilation times were significantly longer in patients bridged to transplantation than in controls who were not bridged (34). The rates of PGD3 and mortality at 2 years were also higher for bridged patients. For a subgroup of patients bridged on awake ECMO, all of them were living at a median follow-up time of 10.8 months. Similarly, Lang and colleagues (28) showed that patients bridged with awake ECMO had a 2-year survival rate of 60%, compared with a 2-year survival rate of 29% for patients bridged with ventilation, sedation +/− ECMO. Thus, evidence indicates that awake ECMO is a good prognostic indicator for patients who can tolerate it.
Biscotti and colleagues (4) reported their 9-year experience at Columbia Presbyterian and described a 55% success rate when bridging with ECMO. They identified several factors by using univariate analysis that predicted the likelihood of whether a patient will survive to transplantation. A higher percentage of inotrope or vasopressor use was noted in the non-survival group. In addition, a higher simplified acute physiology II score and a lower rate of ambulation were found in the group that was not successfully bridged. Consistent with the French experience described above, patients with cystic fibrosis had the most favorable prognosis for surviving to transplantation. Also, the need for renal replacement therapy was higher in the group of patients who did not receive a transplant. Cystic fibrosis patients had the best rate of survival after transplantation, whereas patients with interstitial lung disease had the worst rate of survival after transplantation.
Table 3 summarizes the favorable and unfavorable traits of patients on ECMO that can be considered to help predict whether an outcome will be successful after transplantation. This is based on the consolidation of the above-referenced data, as well as on our own institutional experience, but it should not be used in isolation to decide who should undergo transplantation, given that the literature in the field is still evolving.
Table 3. Factors that affect post-transplant survival in patients on ECMO support.
| Favorable factors |
| Age <50 years |
| Normal or marginally elevated total bilirubin |
| Normal or mildly elevated pulmonary artery pressures |
| <14-day duration on ECMO |
| Low SOFA score (<6) |
| Non-invasive ventilation |
| Ability to participate in physical therapy (i.e., “awake ECMO”) |
| Unfavorable factors |
| Age >60 years |
| Total bilirubin >3 |
| Severe pulmonary hypertension |
| Prolonged ECMO (>14 days) |
| Prolonged mechanical ventilation |
| Prolonged immobility on ECMO |
| SOFA score >9 |
| Major bleeding, infectious complications, or end-organ complications on ECMO |
| Retransplant with a retransplant interval <1 year |
SOFA, sequential organ failure assessment; ECMO, extracorporeal membrane oxygenation.
Conclusions
There are two fundamental questions that one faces when deciding which patients to place on ECMO: (I) is this patient a good candidate for ECMO? (II) Should this patient be transplanted off of ECMO? For the first question, any patient who is even remotely close to being considered a transplant candidate and who has refractory hypoxemia or hypercapnia should be offered ECMO support. At the minimum, this allows a bridge to decision. For the second question, several considerations have been described in the outcomes section above and in Table 3 that help guide daily multidisciplinary discussions and decisions. There is not a commitment to transplantation just because the patient is on ECMO. Discussions among a multidisciplinary team and the patient’s family should occur daily to weigh the patient’s quality of life and the chance of survival.
Acknowledgements
Editorial assistance was provided by Nicole Stancel, PhD, ELS, of the Texas Heart Institute (Houston, TX).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Blivet S, Philit F, Sab JM, et al. Outcome of patients with idiopathic pulmonary fibrosis admitted to the ICU for respiratory failure. Chest 2001;120:209-12. 10.1378/chest.120.1.209 [DOI] [PubMed] [Google Scholar]
- 2.Mollica C, Paone G, Conti V, et al. Mechanical ventilation in patients with end-stage idiopathic pulmonary fibrosis. Respiration 2010;79:209-15. 10.1159/000225932 [DOI] [PubMed] [Google Scholar]
- 3.Rangappa P, Moran JL. Outcomes of patients admitted to the intensive care unit with idiopathic pulmonary fibrosis. Crit Care Resusc 2009;11:102-9. [PubMed] [Google Scholar]
- 4.Biscotti M, Gannon WD, Agerstrand C, et al. Awake Extracorporeal Membrane Oxygenation as Bridge to Lung Transplantation: A 9-Year Experience. Ann Thorac Surg 2017. 10.1016/j.athoracsur.2016.11.056 [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb J, Warnecke G, Hadem J, et al. Outcome of critically ill lung transplant candidates on invasive respiratory support. Intensive Care Med 2012;38:968-75. 10.1007/s00134-012-2551-y [DOI] [PubMed] [Google Scholar]
- 6.Rajagopal K, Hoeper MM. State of the Art: Bridging to lung transplantation using artificial organ support technologies. J Heart Lung Transplant 2016;35:1385-98. 10.1016/j.healun.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 7.Collaud S, Benden C, Ganter C, et al. Extracorporeal Life Support as Bridge to Lung Retransplantation: A Multicenter Pooled Data Analysis. Ann Thorac Surg 2016;102:1680-6. 10.1016/j.athoracsur.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 8.Biscotti M, Sonett J, Bacchetta M. ECMO as bridge to lung transplant. Thorac Surg Clin 2015;25:17-25. 10.1016/j.thorsurg.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 9.Lee SG, Son BS, Kang PJ, et al. The feasibility of extracorporeal membrane oxygenation support for inter-hospital transport and as a bridge to lung transplantation. Ann Thorac Cardiovasc Surg 2014;20:26-31. 10.5761/atcs.oa.12.00203 [DOI] [PubMed] [Google Scholar]
- 10.Garcia JP, Iacono A, Kon ZN, et al. Ambulatory extracorporeal membrane oxygenation: a new approach for bridge-to-lung transplantation. J Thorac Cardiovasc Surg 2010;139:e137-9. 10.1016/j.jtcvs.2009.12.021 [DOI] [PubMed] [Google Scholar]
- 11.Hirose H, Yamane K, Marhefka G, et al. Right ventricular rupture and tamponade caused by malposition of the Avalon cannula for venovenous extracorporeal membrane oxygenation. J Cardiothorac Surg 2012;7:36. 10.1186/1749-8090-7-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biscotti M, Bacchetta M. The "sport model": extracorporeal membrane oxygenation using the subclavian artery. Ann Thorac Surg 2014;98:1487-9. 10.1016/j.athoracsur.2014.02.069 [DOI] [PubMed] [Google Scholar]
- 13.Chamogeorgakis T, Lima B, Shafii AE, et al. Outcomes of axillary artery side graft cannulation for extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 2013;145:1088-92. 10.1016/j.jtcvs.2012.08.070 [DOI] [PubMed] [Google Scholar]
- 14.Strueber M, Hoeper MM, Fischer S, et al. Bridge to thoracic organ transplantation in patients with pulmonary arterial hypertension using a pumpless lung assist device. Am J Transplant 2009;9:853-7. 10.1111/j.1600-6143.2009.02549.x [DOI] [PubMed] [Google Scholar]
- 15.Schmid C, Philipp A, Hilker M, et al. Bridge to lung transplantation through a pulmonary artery to left atrial oxygenator circuit. Ann Thorac Surg 2008;85:1202-5. 10.1016/j.athoracsur.2007.12.032 [DOI] [PubMed] [Google Scholar]
- 16.Kon ZN, Pasrija C, Shah A, et al. Venovenous Extracorporeal Membrane Oxygenation With Atrial Septostomy as a Bridge to Lung Transplantation. Ann Thorac Surg 2016;101:1166-9. 10.1016/j.athoracsur.2015.03.127 [DOI] [PubMed] [Google Scholar]
- 17.Rupprecht L, Lunz D, Philipp A, et al. Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel 2015;7:320-6. [PMC free article] [PubMed] [Google Scholar]
- 18.Ius F, Sommer W, Tudorache I, et al. Veno-veno-arterial extracorporeal membrane oxygenation for respiratory failure with severe haemodynamic impairment: technique and early outcomes. Interact Cardiovasc Thorac Surg 2015;20:761-7. 10.1093/icvts/ivv035 [DOI] [PubMed] [Google Scholar]
- 19.Reeb J, Olland A, Renaud S, et al. Vascular access for extracorporeal life support: tips and tricks. J Thorac Dis 2016;8:S353-63. 10.21037/jtd.2016.04.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Combes A, Bacchetta M, Brodie D, et al. Extracorporeal membrane oxygenation for respiratory failure in adults. Curr Opin Crit Care 2012;18:99-104. 10.1097/MCC.0b013e32834ef412 [DOI] [PubMed] [Google Scholar]
- 21.Garcia JP, Kon ZN, Evans C, et al. Ambulatory veno-venous extracorporeal membrane oxygenation: innovation and pitfalls. J Thorac Cardiovasc Surg 2011;142:755-61. 10.1016/j.jtcvs.2011.07.029 [DOI] [PubMed] [Google Scholar]
- 22.Gattinoni L, Carlesso E, Langer T. Clinical review: Extracorporeal membrane oxygenation. Crit Care 2011;15:243. 10.1186/cc10490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangi AA, Mason DP, Yun JJ, et al. Bridge to lung transplantation using short-term ambulatory extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 2010;140:713-5. 10.1016/j.jtcvs.2010.02.029 [DOI] [PubMed] [Google Scholar]
- 24.Ko Y, Cho YH, Park YH, et al. Feasibility and Safety of Early Physical Therapy and Active Mobilization for Patients on Extracorporeal Membrane Oxygenation. ASAIO J 2015;61:564-8. 10.1097/MAT.0000000000000239 [DOI] [PubMed] [Google Scholar]
- 25.Hayanga AJ, Aboagye J, Esper S, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation in the United States: an evolving strategy in the management of rapidly advancing pulmonary disease. J Thorac Cardiovasc Surg 2015;149:291-6. 10.1016/j.jtcvs.2014.08.072 [DOI] [PubMed] [Google Scholar]
- 26.Hayanga JA, Murphy E, Girgis RE, et al. Extracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation in Patients Over Age 70 Years: A Case Report. Transplant Proc 2017;49:218-20. 10.1016/j.transproceed.2016.11.025 [DOI] [PubMed] [Google Scholar]
- 27.Hayanga JW, Lira A, Aboagye JK, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation: what lessons might we learn from volume and expertise? Interact Cardiovasc Thorac Surg 2016;22:406-10. 10.1093/icvts/ivv379 [DOI] [PubMed] [Google Scholar]
- 28.Lang G, Taghavi S, Aigner C, et al. Primary lung transplantation after bridge with extracorporeal membrane oxygenation: a plea for a shift in our paradigms for indications. Transplantation 2012;93:729-36. 10.1097/TP.0b013e318246f8e1 [DOI] [PubMed] [Google Scholar]
- 29.Weig T, Irlbeck M, Frey L, et al. Parameters associated with short- and midterm survival in bridging to lung transplantation with extracorporeal membrane oxygenation. Clin Transplant 2013;27:E563-70. [DOI] [PubMed] [Google Scholar]
- 30.Crotti S, Iotti GA, Lissoni A, et al. Organ allocation waiting time during extracorporeal bridge to lung transplant affects outcomes. Chest 2013;144:1018-25. 10.1378/chest.12-1141 [DOI] [PubMed] [Google Scholar]
- 31.Shafii AE, Mason DP, Brown CR, et al. Growing experience with extracorporeal membrane oxygenation as a bridge to lung transplantation. ASAIO J 2012;58:526-9. 10.1097/MAT.0b013e31826417d8 [DOI] [PubMed] [Google Scholar]
- 32.Lafarge M, Mordant P, Thabut G, et al. Experience of extracorporeal membrane oxygenation as a bridge to lung transplantation in France. J Heart Lung Transplant 2013;32:905-13. 10.1016/j.healun.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 33.Toyoda Y, Bhama JK, Shigemura N, et al. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. J Thorac Cardiovasc Surg 2013;145:1065-70; discussion 70-1. 10.1016/j.jtcvs.2012.12.067 [DOI] [PubMed] [Google Scholar]
- 34.Inci I, Klinzing S, Schneiter D, et al. Outcome of Extracorporeal Membrane Oxygenation as a Bridge To Lung Transplantation: An Institutional Experience and Literature Review. Transplantation 2015;99:1667-71. 10.1097/TP.0000000000000653 [DOI] [PubMed] [Google Scholar]


