Abstract
Background
As an option for minimally invasive thoracic surgery, robot-assisted thoracic surgery (RATS) has shown comparable perioperative outcomes to those achieved by traditional video-assisted thoracic surgery (VATS). It is unknown whether RATS might have any potential benefits in more complex thoracic surgical procedures, which usually require open surgery instead of VATS. The current study presents a preliminary result regarding the use of RATS in complex thoracic operations in an attempt to address this unresolved question.
Methods
Data from a prospectively collected and maintained surgical database were collected on patients who underwent RATS between February 2012 and August 2014. We defined complex RATS as those operations involving difficult dissections, complex sutures or excision of very large tumors (>8 cm) which would have required open surgery in our hospital before the introduction of RATS. The characteristics and peri-operative outcomes of patients receiving complex RATS were reviewed.
Results
Of the 120 patients undergoing RATS, 30 of them were classified as having undergone complex RATS, 21 to remove lung tumors and 9 to remove mediastinal tumors. The indications for complex RATS included 21 difficult dissections, 10 complex sutures, and 7 very large tumors (8 patients had two indications). There are three conversions to thoracotomy for pulmonary arterial bleeding. There was one mortality resulted from post-pneumonectomy pulmonary hypertension and sepsis. Patients with difficult dissection had longer operative time and hospital stay, and more bleeding and postoperative morbidity.
Conclusions
RATS for complex thoracic procedures is feasible, especially for complex suturing and excision of very large mediastinal tumors, but more attention is needed for patients needing difficult dissections. Advanced preparation for conversion is necessary during this difficult operation.
Keywords: Thoracic surgery, robot-assisted surgery
Introduction
In the early 1990s, the introduction of video-assisted thoracic surgery (VATS) opened the first page of minimal invasive surgery for thoracic diseases. VATS has since proved to be a feasible and safe alternative to open surgery with additional benefits that include shorter hospital stay, decreased acute postoperative pain, reduced inflammation, enhanced recovery, and better tolerance of adjuvant therapy (1-3). However, VATS still has limitations in performing complex procedures such as sleeve lobectomy or dealing with dense hilar structure.
Robot-assisted thoracic surgery (RATS) has recently come to the forefront as a new platform for minimally invasive thoracic surgery. Initial results of robotic lobectomy have shown the same advantages achieved by VATS compared with open thoracotomy are maintained (4-6). With more expensive and advanced technology, RATS is expected to provide additional benefits to VATS, but the evidence so far is not supportive enough for VATS surgeons to change their practice (7,8). We have tried to use RATS not only in routine operations but also in more complex thoracic procedures, which formerly required open surgery, since the robot is an ideal tool to perform delicate surgical maneuvers in vulnerable and difficult to reach anatomical areas. In this report we share our initial experience using RATS for complex thoracic procedures.
Methodd
Patients
This study was approved by the Research Ethics Committee of National Taiwan University Hospital/Taipei/Taiwan (201609048RINC). It presents the review of a prospectively collected and maintained surgical database of patients receiving RATS. From February 2012 to August 2014, 120 patients underwent RATS in National Taiwan University Hospital. Among them, we enrolled in the study 30 patients in need of complex procedures, who we hoped could avoid open surgery by undergoing RATS. We defined complex RATS as those operations requiring difficult dissections (Figure 1), complex sutures (Figure 2) or excisions of very large tumors (>8 cm) (Figure 3). These complex procedures had been routinely performed with traditional open surgery (thoracotomy or sternotomy) in our department before the introduction of RATS. Information regarding preoperative characteristics, operative details, and postoperative course were recorded prospectively.
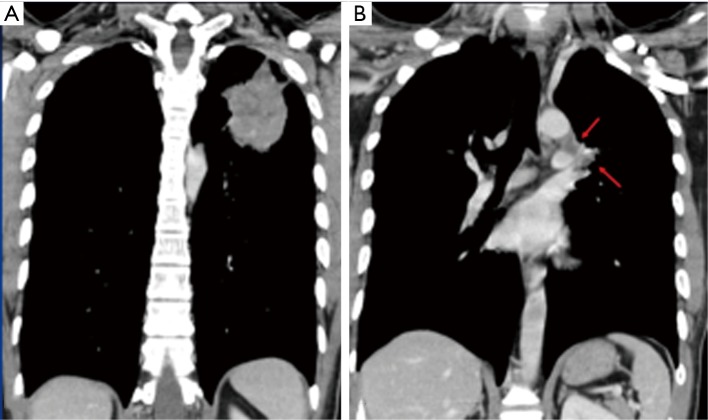
Figure 1.
Indication of difficult dissection: a 31-year-old male (patient No. 1) with pleomorphic carcinoma underwent neoadjuvant CCRT, followed by robotic LUL lobectomy and lymph node dissection. (A) The primary tumor; (B) the arrows indicate N2 lymph nodes encasing pulmonary artery. CCRT, concurrent chemoradiation therapy; LUL, left upper lobe.
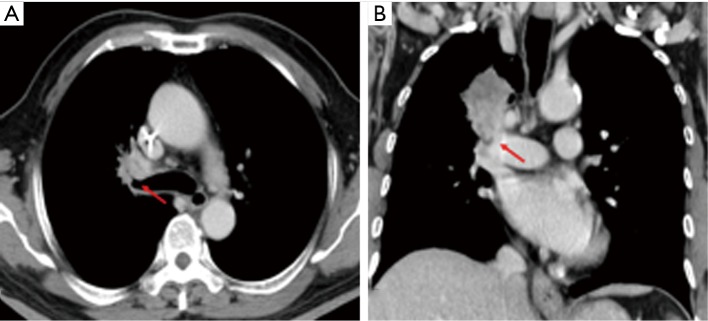
Figure 2.
Indication of difficult dissection and complex suture: a 69-year-old male (patient No. 22) with pleomorphic carcinoma underwent neoadjuvant CCRT, followed by robotic RUL sleeve lobectomy and lymph node dissection. (A) The primary tumor encases RUL bronchus. The arrow indicates the orifice of RUL bronchus; (B) the arrow indicates the primary tumor invading pulmonary artery. CCRT, concurrent chemoradiation therapy; RUL, right upper lobe.
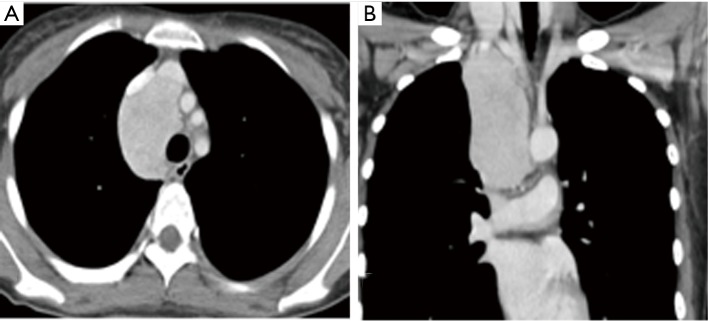
Figure 3.
Indication of very large tumor: a 24-year-old female (patient No. 25) with Castleman’s disease underwent robotic tumor excision through right pleural cavity. The CT images of axial (A) and coronal (B) views show a 9.4×4.5×4.3 cm tumor at middle mediastinum.
Surgery
We performed a variety of thoracoscopic operations using the 4-arm da Vinci Surgical Robotic System (Intuitive Surgical, Sunnyvale, CA, USA). All procedures were performed under general anesthesia. One-lung ventilation was achieved by use of a double-lumen endotracheal tube. A master–slave surgical cart was placed behind the patient’s head. The surgical instruments were controlled by the right and left arms while the endoscope (high-resolution 30- or 0-degree) was attached to the center arm. Trocars were positioned in a triangulation pattern being at least 8 cm apart to allow adequate range of motion of the external arms. Minor modifications were otherwise necessary depending on the procedures performed. Standard stapling devices were used.
Results
Demographic data, diagnosis, complications, duration of drain use and hospital stay are shown in detail in Tables 1 and 2. The study population consisted of 30 elective patients, 19 males and 11 females with ages ranging from 24 to 85 years (median 60 years). Among the 19 primary lung cancer patients (3 stage-II and 16 stage-III), 7 patients received neoadjuvant therapy (4 chemotherapy and 3 concurrent chemoradiation therapy). The surgical treatments consisted of one pneumonectomy, 16 lobectomies (including 5 sleeve lobectomy and 2 bronchoplasty), and two wedge resections for Pancoast tumor. There were three conversions to thoracotomy for major pulmonary arterial bleeding. Numerous metastases (>20) from colon cancer and thyroid cancer were resected via small wedge resection and tumor enucleation, followed by delicate suture by robotic arm to preserve lung function as much as possible. There were 5 anterior mediastinal tumor resections, 4 thymomas and 1 carcinoid; 3 middle mediastinal tumor resections, 2 liposarcomas and 1 case of Castleman’s disease; and one posterior mediastinal tumor resection (esophageal leiomyoma). The indications for using RATS included 21 difficult dissections, 10 complex sutures, and 7 very large tumors. Eight of the patients had two indications. There was one death resulting from post-pneumonectomy pulmonary hypertension and sepsis. There were six postoperative morbidities, which were related to the underlying disease rather than the operation itself.
Table 1. Patients underwent RATS for complex procedure.
| Patient No./gender/age | Diagnosis | Reason to use RATS | Operative method | *Console time | Operative time | Drain tube duration (d) | Hospital stay (d) | Morbidity/mortality |
|---|---|---|---|---|---|---|---|---|
| 1/M/31 | Lung pleomorphic carcinoma s/p CCRT | Difficult dissection | LUL lobectomy | 327 | 387 | 4 | 6 | No |
| 2/M/75 | Lung adenocarcinoma, bronchiectasis | Difficult dissection | RLL lobectomy | 280 | 340 | 8 | 25 | Pneumonia |
| 3/M/44 | Lung SCC pericardium invasion s/p CCRT | Difficult dissection | LUL lobectomy, partial pericardiectomy | 480 | 600 | 6 | 8 | No |
| 4/M/74 | Lung adenocarcinoma, old TB | Difficult dissection | RUL lobectomy | 480 | 540 | 8 | 10 | No |
| 5/F/65 | Lung adenocarcinoma s/p chemotherapy | Difficult dissection | RUL lobectomy | 240 | 300 | 4 | 7 | No |
| 6/M/67 | Thymic carcinoma, 8.0×5.5×2.8 cm | Huge tumor | Thymothymectomy | 110 | 220 | 5 | 7 | No |
| 7/F/61 | Lung adenocarcinoma, locally advanced | Difficult dissection, complex suture | RUL sleeve lobectomy | 480 | 540 | 6 | 8 | No |
| 8/M/72 | Lung adenocarcinoma, locally advanced | Difficult dissection | LLL lobectomy | Conversion | 300 | 14 | 17 | Prolonged air leak |
| 9/M/58 | Liposarcoma, pericardium invasion, 9×8×6 cm | Difficult dissection, huge tumor | Tumor excision with pericardiectomy | 200 | 418 | 5 | 12 | No |
| 10/F/72 | Lung adenocarcinoma, locally advanced | Difficult dissection | Left pneumonectomy | 145 | 200 | 15 | 33 | Mortality |
| 11/F/60 | Thyroid papillary carcinoma with lung and mediastinum metastases | Difficult dissection, complex suture | RLL B6 segmentectomy, multiple wedge/tumor enucleation | 420 | 480 | 4 | 10 | No |
| 12/M/75 | Lung adenocarcinoma, locally advanced | Difficult dissection | LUL lobectomy | Conversion | 540 | 17 | 20 | Arrhythmia, persistent effusion |
| 13/M/85 | Pancoast tumor, locally advanced | Difficult dissection | Tumor excision with RUL wedge resection | 112 | 184 | 7 | 11 | No |
| 14/M/58 | Liposarcoma upper mediastinum and thoracic inlet | Difficult dissection | Tumor excision | 300 | 600 | 7 | 9 | No |
| 15/M/67 | Lung cancer, right upper lobe extending to RML | Difficult dissection | RUL sleeve lobectomy | Conversion | 476 | 16 | 23 | Pneumonia |
| 16/F/47 | Colon cancer metastasis | Complex suture | Multiple wedge/tumor enucleation | 284 | 360 | 5 | 6 | No |
| 17/M/62 | Thymoma, type AB 8.3×7.6×3.4 cm | Huge tumor | Thymothymectomy | 180 | 274 | 3 | 5 | No |
| 18/F/63 | Lung adenocarcinoma, N2 | Difficult dissection, complex suture | RUL sleeve lobectomy with B6 segmentectomy | 660 | 758 | 7 | 9 | No |
| 19/M/74 | Lung adenocarcinoma, invasion of aorta adventitia | Difficult dissection | LUL lobectomy | 265 | 300 | 7 | 9 | No |
| 20/M/43 | Lung SCC | Complex suture | RUL lobectomy with bronchoplasty | 200 | 289 | 3 | 4 | No |
| 21/M/62 | Thymic cancer s/p chemotherapy | Difficult dissection | Tumor and LN excision | 230 | 274 | 3 | 5 | No |
| 22/M/69 | Pleomorphic carcinoma s/p neoadjuvant chemotherapy | Difficult dissection, complex suture | RUL sleeve lobectomy | 510 | 598 | 7 | 12 | No |
| 23/M/35 | Esophageal leiomyoma 5.8×4.4×3.9 cm | Complex suture | Tumor excision, repair esophageal laceration | 120 | 186 | 6 | 8 | No |
| 24/F/60 | Lung adenocarcinoma s/p chemotherapy N2 | Difficult dissection | LUL lobectomy | 400 | 540 | 12 | 34 | Pulmonary embolism |
| 25/F/24 | Castleman’s disease, 9.4×4.5×4.3 cm | Huge tumor | Tumor excision | 85 | 180 | 3 | 4 | No |
| 26/M/32 | Pancoast tumor s/p CCRT 8.5×5.5×4.0 cm | Difficult dissection, huge tumor | Tumor excision with RUL wedge resection | 660 | 768 | 20 | 24 | Hemothorax |
| 27/F/42 | Lung adenocarcinoma | Complex suture | RUL lobectomy with bronchoplasty | 178 | 227 | 3 | 5 | No |
| 28/F/36 | Invasive thymoma, type B1 11.5×8.5×2.6 cm | Huge tumor | Thymothymectomy | 156 | 204 | 7 | 9 | No |
| 29/F/55 | Lung carcinoid | Difficult dissection, complex suture | LUL sleeve lobectomy | 510 | 600 | 4 | 7 | No |
| 30/M/45 | Mediastinal atypical carcinoid | Huge tumor, complex suture | Tumor excision with thymectomy | 224 | 284 | 4 | 5 | No |
*, Console time is the overall duration of time the surgeon spent using the robotic system during the operation. RATS, robot-assisted thoracoscopic surgery; CCRT, Concurrent chemoradiation therapy; LUL, left upper lobe; RUL, right upper lobe; SCC, squamous cell carcinoma; TB, tuberculosis; RML, right middle lobe; RLL, right lower lobe; LLL, left lower lobe.
Table 2. Disease category.
| Tumor type | N |
|---|---|
| Lung tumor (N=21) | |
| Stage for primary lung cancer | |
| II | 3 |
| III | 16 |
| Histology | |
| Non-small cell primary | |
| Adenocarcinoma | 14 |
| SCC | 2 |
| Pleomorphic carcinoma | 2 |
| Carcinoid | 1 |
| Metastasis | |
| Colon | 1 |
| Thyroid | 1 |
| Mediastinal tumor (N=9) | |
| Masaoka stage for thymic tumor | |
| II | 1 |
| III | 3 |
| Histology | |
| Thymoma | 4 |
| Liposarcoma | 2 |
| Castleman’s disease | 1 |
| Carcinoid | 1 |
| Leiomyoma | 1 |
SCC, squamous cell carcinoma.
In the follow-up of lung cancer patients (median 30 months, range 18–42 months), there were 4 recurrences with two distant recurrences and two intrathoracic recurrences (one contralateral lymph node and one malignant pleural effusion). There was no cancer-related mortality. The two patients with distant metastasis continued their systemic therapy after their operation, and no intrathoracic recurrence was detected. One (liposarcoma) of the 9 patients with mediastinal tumor had local recurrence 6 months after resection, and the other 8 patients remained disease free (median 19 months, range 12–31 months).
In the analysis of indications for complex RATS, patients with difficult dissection had longer operative time and hospital stay, and more bleeding and postoperative morbidity (Table 3).
Table 3. Perioperative outcomes of patients with or without indication of difficult dissection.
| Perioperative outcomes | Not difficult dissection (n=9) | Difficult dissection (n=21) | P value |
|---|---|---|---|
| Console time (min) | 170.8±61.7 | 372.2±163.7 | 0.002 |
| Operative time (min) | 247.1±59.0 | 464.0±167.9 | 0.001 |
| Major vessel bleeding | 0/9 | 11/21 | 0.006 |
| Blood loss (mL) | 84.4±80.4 | 681.2±242.7 | 0.024 |
| Conversion | 0/9 | 3/21 | 0.232 |
| Drain tube duration (d) | 4.3±1.5 | 8.6±5.0 | 0.018 |
| Hospital stay (d) | 5.8±1.8 | 14.2±8.8 | 0.008 |
| Morbidity | 0/9 | 7/21 | 0.048 |
| Mortality | 0/9 | 1/21 | 0.506 |
Discussion
While robotic systems have been widely used in urological and gynecological operations, their application is thoracic surgery is relatively uncommon despite reports in the literature demonstrating their safety and feasibility in performing lobectomies, thymectomies, and esophagectomies (9-12). We started using RATS with the same indication protocol as for VATS, which has been performed more than 10,000 times in our institute. We did not find that RATS showed significant advantages over VATS, while some reports have indicated that RATS requires a longer operative time and costs more money than VATS, but only provides a similar perioperative outcome (7,8,13,14). In other words, the data discourages a proficient VATS surgeon from investing the time and resources necessary to develop RATS if it is only used to perform similar procedures to VATS. Therefore, we tried to extend the indication for RATS by taken into consideration its advantages. In the present study, we have demonstrated the feasibility of RATS to do difficult dissections, complex sutures and excisions of very large tumors, which often require open surgery if RATS is not used. RATS required smaller incisions compared to open surgery and resulted in lower morbidity and mortality, improved mental health, and shorter hospital stay (6). However, the long term outcomes need to be evaluated in future studies.
For difficult dissections in thoracic surgery, such as dense hilar structures caused by malignant or inflammatory lymphadenopathy, open surgery is frequently indicated to manage possible injury of major vessels. Instead of open surgery, we used RATS to dissect lymph nodes anatomically off adherent vessels, making good use of its dexterous arms and tremor filtering with optional motion downscaling, which translates large hand movements into precise surgical maneuvers. The radical dissection of mediastinal and hilar lymph nodes increases the number of excised lymph nodes and contributes to accurate cancer staging (15-17), especially when surgery follows chemotherapy or radiation therapy. Despite this advantage, we still experienced a number of vascular injuries during these challenging procedures, most of which could be managed by compression or direct suturing repair, since the robotic arms also allow for steady compression and delicate suturing. There were three conversions out of 21 difficult dissection cases due to loss of camera vision by blood, from which we discovered that an experienced tableside assistant and ready-for-conversion preparation are both mandatory safety precautions for such difficult operations.
There was one case of mortality in a patient who had a difficult hilum dissection. The cause of mortality was sepsis combined with post-pneumonectomy pulmonary hypertension, which was not directly related to the robotic surgery. Nevertheless, cases of difficult dissection did result in significantly prolonged operative time and hospital stay as well as more postoperative morbidities when compared to cases that did not involve difficult dissection. From our initial experience, we would not regard “difficult dissection” as an ideal indication for RATS, although we would not rule out its potential advantage even in cases of difficult dissection.
In contrast, we would encourage the use of RATS to perform complex sutures, such as sleeve lobectomies. Some experienced experts are capable of using traditional VATS to do this complex procedure, but it is still not commonly attempted after a decade long development of VATS. The dexterous robotic arms with 7 degrees of freedom provide hand-like articulation and allow for more controlled and precise handling and suturing, which makes bronchial anastomosis an ideal application. Moreover, the console system provides surgeons an ergonomically comfortable position with minimum fatigue during this relatively long operation. Prior to our study, there has been only one bronchoplastic lobectomy and one sleeve lobectomy performed by RATS reported in the literature (18,19). We performed two bronchoplastic lobectomies and five sleeve lobectomies with one conversion to open thoracotomy. The key for success of this procedure is getting accustomed to the robotic suturing technique because of the absence of tactile feedback, which may result in breaking the suture during knot tying.
While open surgery is still the standard approach for resection of malignant mediastinal tumors, the minimally invasive approach has gained popularity due its desirable outcomes of less trauma, morbidity, and hospital stay, in addition to its better cosmetic results. However, VATS is not suitable for diseases arising in the deep mediastinal area, such as the thoracic inlet, which is a delicate and difficult-to-reach anatomic region with vulnerable structures (especially large vessels and nerves). The characteristics of robotic systems, however, facilitate delicate dissection in these narrow regions and results in better short- and long-term clinical outcomes compared to open surgery (20-23). However, these reports have been limited to early-stage and relatively small tumors. We went further by using RATS for larger tumors in the mediastinum and found it unexpectedly easy to manipulate very large tumors by using the powerful robotic arms to lift the tumor and create a dissection plane. When repair of a pericardial or esophageal defect following tumor excision is needed, RATS provided additional benefits in the suturing. Although his application of RATS may cause a great debate, we made sure the integrity of the tumor was maintained to avoid any dissemination in the pleural cavity. Another limitation of both VATS and RATS has been the difficulty encountered in extruding very large tumors, which can be overcome by the technique of tumor axis rotation. The utility wound needed to squeeze the tumor out is then just slightly larger than its short axis and is usually much shorter than in traditional sternotomy or thoracotomy.
In conclusion, RATS can be a feasible and safe alternative to open surgery for complex procedures, and maintain the additional benefits of minimal invasive surgery.
Acknowledgements
Funding: This study was supported by the Ministry of Science and Technology (MOST 104-2314-B-002-182-MY3), National Taiwan University Hospital (NTUH.105-S3005), Taiwan Health Foundation, and Taiwan Society for the Chest Care of Taiwan.
Ethical Statement: This study was approved by the Research Ethics Committee of National Taiwan University Hospital/Taipei/Taiwan (201609048RINC).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. 10.1016/j.jtcvs.2009.03.030 [DOI] [PubMed] [Google Scholar]
- 2.Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. 10.1016/j.jtcvs.2009.04.026 [DOI] [PubMed] [Google Scholar]
- 3.Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 50. 10.1016/j.athoracsur.2006.12.029 [DOI] [PubMed] [Google Scholar]
- 4.Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. 10.1016/j.jtcvs.2011.10.055 [DOI] [PubMed] [Google Scholar]
- 5.Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604; discussion 604-5. 10.1016/j.athoracsur.2012.01.067 [DOI] [PubMed] [Google Scholar]
- 6.Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. 10.1016/j.jtcvs.2011.07.022 [DOI] [PubMed] [Google Scholar]
- 7.Lee BE, Korst RJ, Kletsman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. 10.1016/j.jtcvs.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 8.Augustin F, Bodner J, Maier H, et al. Robotic-assisted minimally invasive vs. thoracoscopic lung lobectomy: comparison of perioperative results in a learning curve setting. Langenbecks Arch Surg 2013;398:895-901. 10.1007/s00423-013-1090-5 [DOI] [PubMed] [Google Scholar]
- 9.Fernando HC, Erdem CC, Daly B, et al. Robotic assisted thoracic surgery for resection of an esophageal cyst. Dis Esophagus 2006;19:509-11. 10.1111/j.1442-2050.2006.00621.x [DOI] [PubMed] [Google Scholar]
- 10.Rea F, Schiavon M, Di Chiara F, et al. Single-institution experience on robot-assisted thoracoscopic operations for mediastinal diseases. Innovations (Phila) 2011;6:316-22. 10.1097/IMI.0b013e318235b783 [DOI] [PubMed] [Google Scholar]
- 11.Augustin F, Bodner J, Wykypiel H, et al. Perioperative results of robotic lung lobectomy: summary of literature. Surg Endosc 2012;26:1190-1. 10.1007/s00464-011-1975-8 [DOI] [PubMed] [Google Scholar]
- 12.Pardolesi A, Park B, Petrella F, et al. Robotic Anatomic Segmentectomy of the Lung: Technical Aspects and Initial Results. Ann Thorac Surg 2012;94:929-34. 10.1016/j.athoracsur.2012.04.086 [DOI] [PubMed] [Google Scholar]
- 13.Park BJ, Flores RM. Cost comparison of robotic, video-assisted thoracic surgery and thoracotomy approaches to pulmonary lobectomy. Thorac Surg Clin 2008;18:297-300, vii. 10.1016/j.thorsurg.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 14.Swanson SJ, Miller DL, McKenna RJ, Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. 10.1016/j.jtcvs.2013.09.046 [DOI] [PubMed] [Google Scholar]
- 15.Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. 10.1016/j.athoracsur.2009.04.039 [DOI] [PubMed] [Google Scholar]
- 16.Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 6-7. [DOI] [PubMed]
- 17.Nasir BS, Bryant AS, Minnich DJ, et al. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203-8; discussion 8-9. 10.1016/j.athoracsur.2014.02.051 [DOI] [PubMed] [Google Scholar]
- 18.Schmid T, Augustin F, Kainz G, et al. Hybrid video-assisted thoracic surgery-robotic minimally invasive right upper lobe sleeve lobectomy. Ann Thorac Surg 2011;91:1961-5. 10.1016/j.athoracsur.2010.08.079 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura H, Taniguchi Y, Miwa K, et al. A successful case of robotic bronchoplastic lobectomy for lung cancer. Ann Thorac Cardiovasc Surg 2013;19:478-80. 10.5761/atcs.cr.12.02020 [DOI] [PubMed] [Google Scholar]
- 20.Ye B, Li W, Ge XX, et al. Surgical treatment of early-stage thymomas: robot-assisted thoracoscopic surgery versus transsternal thymectomy. Surg Endosc 2014;28:122-6. 10.1007/s00464-013-3137-7 [DOI] [PubMed] [Google Scholar]
- 21.Weksler B, Tavares J, Newhook TE, et al. Robot-assisted thymectomy is superior to transsternal thymectomy. Surg Endosc 2012;26:261-6. 10.1007/s00464-011-1879-7 [DOI] [PubMed] [Google Scholar]
- 22.Seong YW, Kang CH, Choi JW, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg 2014;45:e68-73; discussion e73. [DOI] [PubMed]
- 23.Goldstein SD, Yang SC. Assessment of robotic thymectomy using the Myasthenia Gravis Foundation of America Guidelines. Ann Thorac Surg 2010;89:1080-5; discussion 5-6. 10.1016/j.athoracsur.2010.01.038 [DOI] [PubMed] [Google Scholar]