Ever since the results of the ELCAP study (1), which reported the malignancy rate of subsolid nodules to be about 5 times higher than that of solid nodules, it is common knowledge among radiologists and pulmonologists that these specific nodules require special attention and management.
Malignant subsolid nodules mostly correspond to the spectrum of lung adenocarcinomas, which consists of three subtypes as defined by IASLC/ETS/ERS (2) and WHO classifications of lung tumors (3). Adenocarcinoma in situ and minimally invasive adenocarcinoma, which are defined on pathology by no invasive component and an invasive component of less than 5 mm, respectively, have shown an excellent prognosis with a 5-year survival after surgical resection reported to be between 98% and 100% (2,4). Prognosis is less good however for invasive adenocarcinomas, defined on pathology by an invasive component of more than 5 mm, stressing the need for more invasive management strategies.
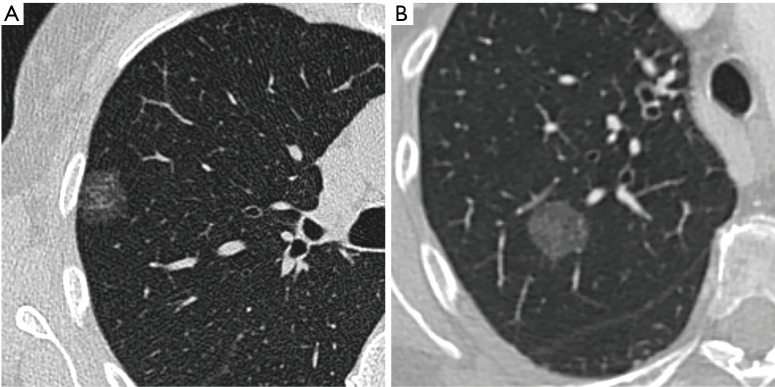
Pure ground-glass nodules (pGGNs), which are defined by opposition to part-solid nodules by the absence of a solid component on CT, have first been thought to correspond mostly to benign or very slowly evolving lesions and thus been discarded by some physicians. However, recent studies have proven that those nodules often correspond to invasive adenocarcinomas, representing for example 39% of the nodules in a Korean series of 46 pGGNs (5) and 40% in a Chinese series of 94 pGGNs (6). This makes pGGNs a very different entity in comparison to part-solid nodules, which show by contrast good radio-pathological correlations between solid component on CT and invasive foci on pathology for nodules corresponding to adenocarcinomas (7,8), putting aside a few false positive of solid component on CT such as alveolar collapses, fibrotic scars or mucinous components (9,10). This significant overlap in imaging features of in situ/minimally invasive and invasive adenocarcinomas manifesting as pGGNs on CT, as shown in Figure 1, can be explained by several reasons. On the one hand, the limited resolution of CT scan may hinder detection of small invasive foci (11). On the other hand, specific adenocarcinoma subtypes, such as papillary or micropapillary adenocarcinomas, can display large invasive foci punctuated with aerated areas, which may therefore appear on CT as a ground-glass areas instead of solid components.
Figure 1.
Imaging features overlap between different grades of lung adenocarcinomas presenting as pure ground-glass nodules. Chest CT scans displayed with lung window show respectively: (A) a pGGN of 20 mm in a 70-year-old female confirmed as an adenocarcinoma in situ; (B) a pGGN of 21 mm in a 66-year-old female patient confirmed on pathology as an invasive adenocarcinoma. pGGNs, pure ground-glass nodules.
According to the difference of prognosis between AIS/MIA and invasive adenocarcinomas, it is critical to differentiate those lesions non-invasively so as to allow optimal management for patients. The first step when discovering a pGGN is, according to the latest guidelines (12), to control it by CT 6 to 12 months later, enabling to exclude from 33% to 67% of benign inflammatory and infectious lesions manifesting as pGGNs (13,14). Once this step is done, the main issue we have today is that the tools currently available to differentiate extremely slow growing in situ or minimally invasive lesions from faster growing invasive adenocarcinomas in persistent pGGNs remain relatively limited. Aside from the historic nodule size criteria, which remains the most used and validated (12), other criteria such as lobulated margins, tumor/lung interface, lobulated contours, and nodule attenuation were suggested, but struggle to reach consensus due to varying results among different studies (6,15). In order to put light on this question, Heidinger et al. analyzed in a recently published study the relationship between pGGNs’ diameter, volume, attenuation, roundness and the size and number of invasive foci, as well as age and gender of patients (16). The main conclusion of the authors of this study was that among all the existing criteria on a single CT, nodule diameter wasn’t significantly less correlated with size and number of invasive foci on pathology than were attenuation, volume or roundness, and may therefore be sufficient alone for pGGNs’ risk evaluation. This result may be valuable since diameter is a simple measurement and, while advanced measurement techniques such as semi-automatic segmentation have significantly developed for subsolid nodules (7,17) and are now usable in clinical practice, many centers are still not properly equipped.
There are however a few concerns and limits for the use of the largest diameter of the nodule as a single criterion. Indeed, as Heidinger et al. stress in their article (16), the largest nodule diameter shows at most a weak statistical correlation with the number and size of invasive foci on pathology. For this reason, in routine practice, CT follow-up and evaluation of nodule growth is one of the best tools to evaluate pGGNs’ invasiveness. Similarly to what happens with solid nodules, 2D size alone may not be the best criteria to evaluate growth in pGGNs, which are known to have much slower volume doubling times than their solid counterparts. Even for subsolid nodules corresponding to adenocarcinomas, reported doubling times are of 813±375 days for pGGNs (18), i.e., much higher than those found in solid lung cancer nodules, with a median volume doubling time of 98 days (19). Furthermore, it is known that pGGNs may not only increase in size, but also in attenuation with or without appearance of a solid component, which are also risk factors of invasive lesions (20). Thus, while volume, attenuation and mass may not be more useful than a simple diameter for prognosis evaluation on a single CT, the situation may be quite different when it comes to follow-up.
The investigation of multiple invasive foci in the latter study (16) is an interesting endeavour. Indeed, current classifications are based on the largest invasive foci for the pathologist, and on the largest solid component on CT for the radiologist in the case of part-solid nodules. This raises the question to know how the number of invasive foci might affect lesion aggressiveness and patient prognosis compared to the size of the largest invasive foci. Further research is needed to properly answer this question.
Coming back to routine clinical practice, the Fleischner society recently released its 2017 guidelines for subsolid nodules (12), where axial diameter is also a key factor for pGGNs’ management. According to these, pGGNs of less than 6 mm shouldn’t warrant any particular follow-up, although an alternative of 2 and 4 years follow-up is proposed for pGGNs close to the 6 mm threshold and judged more suspicious. This is to reflect the results of a recent Japanese study (21) which showed that among 439 pGGNs of less than 5 mm, 10% eventually grew and 0.9% turned out to be adenocarcinomas, half of them invasive. For nodules larger than 6 mm, the guidelines propose follow-up CTs at 2 and 4 years after the first 6–12 months control CT and to refer patients for surgery in the case of significant growth and/or appearance of a solid component. The rational for those 2 years follow-up interval is that according to current data, a pGGN takes on average 3 to 4 years to grow and/or develop solid component (12).
In the future, emerging techniques may help us to further differentiate these lesions. Texture analysis may play a role in those advances and enable us to extract some additional features from those pGGNs. Although the effect of CT protocol parameters and different CT vendor/model on texture parameters remain an issue, several studies showed that parameters such as homogeneity and entropy might help differentiate invasive lesions in pGGNs (22,23). The constant evolution of semi-automatic segmentation and computer-aided techniques, may also be of use in the evaluation of those nodules, by reducing interobserver variability, enabling to more reliably differentiate pure and part-solid nodules and increasing the sensitivity of detection for small changes during follow-up.
As a conclusion, pGGNs shouldn’t be underestimated as they correspond to invasive adenocarcinomas in up to 40% of cases. According to current guidelines and recent studies, it is still recommended to use the largest diameter of the nodules for their evaluation, although the diagnostic performance of this criterion to identify invasive adenocarcinomas remains moderate at best. Further research is needed to identify more efficient diagnostic criteria for stratifying the risk in pGGNs.
Acknowledgements
None.
Provenance: This is an invited Editorial commissioned by Section Editor Dr. Gang Shen, MMSC (The Second Affiliated Hospital Zhejiang University School of Medicine, Hangzhou, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178:1053-7. 10.2214/ajr.178.5.1781053 [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. 10.1513/pats.201107-042ST [DOI] [PubMed] [Google Scholar]
- 3.Lantuejoul S, Rouquette I, Brambilla E, et al. New WHO classification of lung adenocarcinoma and preneoplasia. Ann Pathol 2016;36:5-14. 10.1016/j.annpat.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 4.Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 2013;81:371-6. 10.1016/j.lungcan.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 5.Lim HJ, Ahn S, Lee KS, et al. Persistent pure ground-glass opacity lung nodules ≥ 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest 2013;144:1291-9. 10.1378/chest.12-2987 [DOI] [PubMed] [Google Scholar]
- 6.Jin X, Zhao SH, Gao J, et al. CT characteristics and pathological implications of early stage (T1N0M0) lung adenocarcinoma with pure ground-glass opacity. Eur Radiol 2015;25:2532-40. 10.1007/s00330-015-3637-z [DOI] [PubMed] [Google Scholar]
- 7.Cohen JG, Goo JM, Yoo RE, et al. Software performance in segmenting ground-glass and solid components of subsolid nodules in pulmonary adenocarcinomas. Eur Radiol 2016;26:4465-74. 10.1007/s00330-016-4317-3 [DOI] [PubMed] [Google Scholar]
- 8.Cohen JG, Reymond E, Lederlin M, et al. Differentiating pre- and minimally invasive from invasive adenocarcinoma using CT-features in persistent pulmonary part-solid nodules in Caucasian patients. Eur J Radiol 2015;84:738-44. 10.1016/j.ejrad.2014.12.031 [DOI] [PubMed] [Google Scholar]
- 9.Yang ZG, Sone S, Takashima S, et al. High-resolution CT analysis of small peripheral lung adenocarcinomas revealed on screening helical CT. AJR Am J Roentgenol 2001;176:1399-407. 10.2214/ajr.176.6.1761399 [DOI] [PubMed] [Google Scholar]
- 10.Borczuk AC. Assessment of invasion in lung adenocarcinoma classification, including adenocarcinoma in situ and minimally invasive adenocarcinoma. Mod Pathol 2012;25 Suppl 1:S1-10. 10.1038/modpathol.2011.151 [DOI] [PubMed] [Google Scholar]
- 11.Lee HY, Choi YL, Lee KS, et al. Pure ground-glass opacity neoplastic lung nodules: histopathology, imaging, and management. AJR Am J Roentgenol 2014;202:W224-33. 10.2214/AJR.13.11819 [DOI] [PubMed] [Google Scholar]
- 12.MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. 10.1148/radiol.2017161659 [DOI] [PubMed] [Google Scholar]
- 13.Felix L, Serra-Tosio G, Lantuejoul S, et al. CT characteristics of resolving ground-glass opacities in a lung cancer screening programme. Eur J Radiol 2011;77:410-6. 10.1016/j.ejrad.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 14.Yu JY, Lee B, Ju S, et al. Proportion and characteristics of transient nodules in a retrospective analysis of pulmonary nodules. Thorac Cancer 2012;3:224-8. 10.1111/j.1759-7714.2011.00101.x [DOI] [PubMed] [Google Scholar]
- 15.Lee SM, Park CM, Goo JM, et al. Invasive pulmonary adenocarcinomas versus preinvasive lesions appearing as ground-glass nodules: differentiation by using CT features. Radiology 2013;268:265-73. 10.1148/radiol.13120949 [DOI] [PubMed] [Google Scholar]
- 16.Heidinger BH, Anderson KR, Nemec U, et al. Lung Adenocarcinoma Manifesting as Pure Ground-Glass Nodules: Correlating CT Size, Volume, Density, and Roundness with Histopathologic Invasion and Size. J Thorac Oncol 2017;12:1288-98. 10.1016/j.jtho.2017.05.017 [DOI] [PubMed] [Google Scholar]
- 17.Cohen JG, Kim H, Park SB, et al. Comparison of the effects of model-based iterative reconstruction and filtered back projection algorithms on software measurements in pulmonary subsolid nodules. Eur Radiol 2017;27:3266-74. 10.1007/s00330-016-4716-5 [DOI] [PubMed] [Google Scholar]
- 18.Oda S, Awai K, Murao K, et al. Volume-doubling time of pulmonary nodules with ground-glass opacity at multidetector CT: Assessment with computer-aided three-dimensional volumetry. Acad Radiol 2011;18:63-9. 10.1016/j.acra.2010.08.022 [DOI] [PubMed] [Google Scholar]
- 19.Henschke CI, Yankelevitz DF, Yip R, et al. Lung cancers diagnosed at annual CT screening: volume doubling times. Radiology 2012;263:578-83. 10.1148/radiol.12102489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen JG, Reymond E, Jankowski A, et al. Lung adenocarcinomas: correlation of computed tomography and pathology findings. Diagn Interv Imaging 2016;97:955-63. 10.1016/j.diii.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 21.Kakinuma R, Muramatsu Y, Kusumoto M, et al. Solitary Pure Ground-Glass Nodules 5 mm or Smaller: Frequency of Growth. Radiology 2015;276:873-82. 10.1148/radiol.2015141071 [DOI] [PubMed] [Google Scholar]
- 22.Son JY, Lee HY, Kim JH, et al. Quantitative CT analysis of pulmonary ground-glass opacity nodules for distinguishing invasive adenocarcinoma from non-invasive or minimally invasive adenocarcinoma: the added value of using iodine mapping. Eur Radiol 2016;26:43-54. 10.1007/s00330-015-3816-y [DOI] [PubMed] [Google Scholar]
- 23.Hwang IP, Park CM, Park SJ, et al. Persistent Pure Ground-Glass Nodules Larger Than 5 mm: Differentiation of Invasive Pulmonary Adenocarcinomas From Preinvasive Lesions or Minimally Invasive Adenocarcinomas Using Texture Analysis. Invest Radiol 2015;50:798-804. 10.1097/RLI.0000000000000186 [DOI] [PubMed] [Google Scholar]