Abstract
Objectives
Higher systolic blood pressure (SBP) visit-to-visit variability (SBPV) has been associated with increased risk of adverse events in patients with chronic kidney disease (CKD), but the association of SBPV in advanced non-dialysis dependent CKD (NDD-CKD) with mortality after the transition to end-stage renal disease (ESRD) remains unknown.
Methods
Among 17,729 US veterans transitioning to dialysis between 10/2007–9/2011, we assessed SBPV calculated from the standard deviation of at least three intra-individual outpatient SBP values during the last year prior to dialysis transition (“prelude period”). Outcomes included factors associated with higher prelude SBPV and post-transition all-cause, cardiovascular, and infection-related mortality, assessed using multivariable linear regression and Cox and competing risk regressions, respectively, adjusted for demographics, comorbidities, medications, cardiovascular medication adherence, SBP, body mass index, estimated glomerular filtration rate, and type of vascular access.
Results
Modifiable clinical factors associated with higher prelude SBPV included higher SBP, use of antihypertensive medications and erythropoiesis-stimulating agents, inadequate cardiovascular medication adherence, and catheter use. After multivariable adjustment, higher prelude SBPV was significantly associated with higher post-ESRD all-cause and infection-related mortality, but not cardiovascular mortality (hazard/subhazard ratios [95% CI] for the highest (vs. lowest) quartile of SBPV, 1.08 [1.01–1.16], 1.02 [0.89–1.15], and 1.41 [1.10–1.80] for all-cause, cardiovascular, and infection-related mortality, respectively).
Conclusions
High pre-ESRD SBPV is potentially modifiable and associated with higher all-cause and infection-related mortality following dialysis initiation. Further studies are needed to test whether modification of pre-ESRD SBPV can improve clinical outcomes in incident ESRD patients.
Keywords: systolic blood pressure, variability, mortality, cardiovascular disease, chronic kidney disease, end-stage renal disease
INTRODUCTION
A growing number of studies have reported that visit-to-visit variability in systolic blood pressure (SBPV) is independently associated with adverse clinical outcomes such as all-cause and cardiovascular mortality,[1–6] coronary heart disease,[7–9] heart failure,[10] and stroke,[11–13] mostly in patients with hypertension or in those with end-stage renal disease (ESRD) on hemodialysis. Over the course of chronic kidney disease (CKD) progression, patients with CKD develop a wide variety of cardiovascular complications such as endothelial dysfunction,[14] arterial stiffness,[15] and left ventricular diastolic dysfunction,[16] all of which are also associated with high SBPV;[17–19] and indeed, some recent studies have shown similar associations of SBPV with outcomes among patients with non-dialysis dependent CKD (NDD-CKD).[20–25] These studies, however, have primarily focused on patients with CKD stage 3 or 4; and thus it remains unknown if these associations apply to those with advanced NDD-CKD transitioning to ESRD, a unique patient population who experience the highest mortality immediately after the transition to dialysis and suffer from an exceptionally high health and economic burden.[26]
Given the peculiar risk profile and the pervasive nature of hypertension in this vulnerable population during the transition period,[27] the question whether high SBPV in advanced NDD-CKD holds prognostic significance on post-transition mortality is of paramount relevance. With this background in mind, we hypothesized that advanced NDD-CKD patients with higher SBPV are at greater risk of mortality following dialysis initiation. To test this hypothesis, we investigated the association of SBPV in the pre-ESRD transition period with post-ESRD all-cause, cardiovascular, and infection-related mortality, using a large nationally representative cohort of US veterans with advanced NDD-CKD transitioning to dialysis.
METHODS
Study Population
We analyzed longitudinal data from the Transition of Care in CKD (TC-CKD) study, a retrospective cohort study examining US veterans with advanced NDD-CKD transitioning to dialysis from October 1, 2007 through September 30, 2011.[28–30] A total of 52,172 US veterans were identified from the US Renal Data System (USRDS)[26] as a source population. The algorithm for the cohort definition is shown in Supplemental Figure 1. In the present study, we used all SBP values measured during outpatient clinical encounters in any Veterans Affairs (VA) facility; therefore, patients without any outpatient systolic blood pressure (SBP) measurements at a VA facility were excluded (n = 19,533). We also excluded those who had less than three outpatient SBP measurements recorded on different days within one year prior to dialysis initiation (i.e., one-year “prelude period”) (n = 14,645) and who were missing follow-up data (n = 265), resulting in a study population of 17,729 patients.
Exposure Variable
The primary exposure of interest was SBPV over the one-year prelude period. SBPV was defined as the standard deviation (SD) of the intra-individual outpatient SBP values in each patient measured during the same prelude period. We categorized the SBPV values into quartiles (<11.6, 11.6-<15.7, 15.7-<20.4, and ≥20.4 mmHg), using the lowest SBPV quartile as reference.
Covariates
Data from the USRDS Patient and Medical Evidence files were used to determine patients’ baseline demographic characteristics and type of vascular access at the time of dialysis initiation. Information about comorbidities was extracted from the VA Inpatient and Outpatient Medical SAS Datasets,[31] using the International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic and procedure codes and Current Procedural Terminology codes, as well as from VA/Centers for Medicare and Medicaid Services (CMS) data. The Charlson Comorbidity Index score was calculated using the Deyo modification for administrative datasets, without including kidney disease.[32] Cardiovascular disease was defined as the presence of diagnostic codes for angina, coronary artery disease, myocardial infarction, or cerebrovascular disease. Medication data were collected from both CMS Data (Medicare Part D) and VA pharmacy dispensation records.[33] Patients who received at least one dispensation of medications within the one-year prelude period were recorded as having been treated with these medications. Cardiovascular medication adherence was defined as the proportion of days covered by a drug during the one-year prelude period, capped at 100%.[30] Laboratory data were obtained from VA research databases as previously described,[34, 35] and their baseline values were defined as the average of each covariate during the one-year prelude period preceding dialysis initiation. Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration equation.[36]
Outcome Assessment
The co-primary outcomes of interest were all-cause, cardiovascular, and infection-related mortality after dialysis initiation. The start of the follow-up period was the date of dialysis initiation, and patients were followed up until death or other censoring events including kidney transplantation, loss of follow-up, or the last date of available follow-up (December 27, 2012 and October 6, 2011 for all-cause and cause-specific mortality, respectively).[28–30] All-cause mortality data, censoring events, and associated dates were obtained from VA and USRDS data sources.[26] Cause-specific mortality data were obtained from USRDS.
Statistical analysis
Baseline patient characteristics were summarized according to SBPV categories, and presented as number (percent) for categorical variables and the mean ± standard deviation (SD) for continuous variables with a normal distribution or median (25th, 75th percentiles) for those with a skewed distribution. Differences across categories were assessed using analysis of variance and chi-squared tests for continuous and categorical variables, respectively. We performed multivariable linear regression to identify factors independently associated with SBPV. Based on a priori knowledge and their availability in this study, the following explanatory variables were included: sociodemographics (age, sex, race, and marital status), comorbidities (diabetes mellitus, cardiovascular disease, congestive heart failure [CHF], peripheral vascular disease, lung disease, liver disease, and Charlson comorbidity index), SBP, body mass index [BMI], vascular access type, medications (angiotensin-converting enzyme inhibitors [ACEIs]/angiotensin receptor blockers [ARBs], β-blockers, calcium channel blockers [CCBs], diuretics, vasodilators, statins, and erythropoietin stimulating agents [ESAs]), cardiovascular medication adherence, and laboratory parameters (serum albumin, blood hemoglobin, and eGFR). Variance inflation factors were calculated to examine substantial multicollinearity among these parameters, and values >5.0 were considered to indicate collinearity. The association between SBPV and mortality was estimated using Cox proportional hazards models for all-cause death and Fine and Gray competing risks regression for cause-specific deaths by treating deaths from other causes as competing events.[37] Models were incrementally adjusted for the following potential confounders based on theoretical considerations: model 1 unadjusted; model 2 adjusted for age, sex, race/ethnicity, and marital status; model 3 additionally accounted for comorbidities (hypertension, diabetes mellitus, cardiovascular disease, CHF, peripheral vascular disease, lung disease, liver disease, and Charlson comorbidity index), and SBP, BMI, and eGFR levels averaged over the one-year prelude period; and model 4 additionally included medications, cardiovascular medication adherence, and type of vascular access (arteriovenous fistula, arteriovenous graft, or catheter).
We conducted several sensitivity analyses to evaluate the robustness of our main findings. SBPV was also defined as the coefficient of variation of the intra-individual outpatient SBP values (i.e., SD/mean SBP), and the associations of the relative SBPV index (in quartiles) with outcomes were evaluated. The associations between SBPV and mortality were examined in subgroups of patients stratified by age, race, BMI, SBP, eGFR, and presence/absence of select comorbidities. Potential interactions were formally tested by including relevant interaction terms. We also investigated whether accounting for serum albumin and blood hemoglobin levels further attenuates the SBPV-mortality associations in the group of 15,615 patients with available albumin and hemoglobin measurements as an additional model (model 5).
Compared to patients in the main cohort (n = 17,729), those who were excluded from the source cohort (n = 34,443) were older (71.6 versus 67.8 years) and were less likely to be male (92.5% versus 98.1%), African-American (20.5% versus 31.4%), and diabetic (49.4% versus 73.0%). Of the variables included in multivariable models, data points were missing for race (0.2%), BMI (<0.01%), eGFR (0.7%), vascular access type (7.5%), serum albumin (3.3%), and blood hemoglobin (2.3%). Information about cause of death was also missing in 4,337 of the 9,064 (52.2%) who died in our study population. Compared to patients with missing cause of death, those without missing cause of death were less likely to be African-American (21.6% versus 25.6%) and had a slightly higher prevalence of cardiovascular disease (57.1% versus 53.3%), CHF (66.7% versus 63.0%), and chronic pulmonary disease (56.3% versus 51.1%) (Supplemental Table 1). Of the 17,729 patients in our study population, 16,275 (91.8%) had complete data available for the main adjusted multivariable model (model 4). Due to the relatively low proportion of missingness, missing data was not imputed. The reported P values are two-sided and reported as significant at <0.05 for all analyses. All analyses were conducted using STATA/MP Version 14 (STATA Corporation, College Station, TX). The study was approved by the Institutional Review Boards of the Memphis and Long Beach VA Medical Centers, with exemption from informed consent.
RESULTS
Baseline Characteristics
Overall, the mean±SD age at baseline was 67.8±11.2 years; 98.1% were male; 31.4% were African-American; and 73.0% were diabetic. The median (25th, 75th percentiles) eGFR level was 15.7 (11.9, 22.2) mL/min/1.73m2. During the one-year prelude period, patients had a median (25th, 75th percentiles) of 10 (5, 16) outpatient SBP measurements. The mean±SD prelude SBPV was 16.4±7.0 mmHg. Baseline characteristics in the overall cohort and stratified by SBPV quartiles are presented in Table 1. Compared to patients with lower SBPV, those with higher SBPV were younger; were more likely to be African-American and unmarried; had higher systolic and diastolic BPs; and had a higher prevalence of hypertension, diabetes, cardiovascular disease, and peripheral vascular disease. They were also more likely to use antihypertensive medications; were less likely to adhere to cardiovascular medications; and had lower serum albumin, blood hemoglobin, and eGFR levels.
Table 1.
Baseline patient characteristics according to quartiles of prelude SBPV
| All | Quartile of SBPV (mmHg)
|
P | ||||
|---|---|---|---|---|---|---|
| Q1 (lowest) | Q2 | Q3 | Q4 (highest) | |||
| <11.6 | 11.6 to <15.7 | 15.7 to <20.4 | ≥20.4 | |||
| (N = 17,729) | (n = 4,433) | (n = 4,432) | (n = 4,431) | (n = 4,433) | ||
| Age (years) | 67.8±11.2 | 69.5±11.6 | 68.1±11.3 | 67.3±10.9 | 66.2±11.0 | <0.001 |
| Sex (male) | 17,388 (98.1) | 4,361 (98.4) | 4,349 (98.1) | 4,350 (98.2) | 4,328 (97.6) | 0.071 |
| Race (African-American) | 5,559 (31.4) | 1,076 (24.3) | 1,372 (31.0) | 1,486 (33.5) | 1,625 (36.7) | <0.001 |
| Marital status (married) | 9,204 (51.9) | 2,540 (57.3) | 2,332 (52.6) | 2,223 (50.2) | 2,109 (47.6) | <0.001 |
| Systolic BP (mmHg) | 141.2±16.1 | 133.5±15.1 | 138.2±14.6 | 143.3±14.3 | 149.9±15.4 | <0.001 |
| Diastolic BP (mmHg) | 73.7±10.6 | 71.1±10.4 | 72.8±10.1 | 74.1±10.2 | 77.0±10.9 | <0.001 |
| Body mass index (kg/m2) | 29.9±6.6 | 30.0±6.5 | 30.4±6.6 | 30.1±6.6 | 29.2±6.4 | <0.001 |
| Hypertension | 17,327 (97.7) | 4,237 (95.6) | 4,329 (97.7) | 4,367 (98.6) | 4,394 (99.1) | <0.001 |
| Diabetes mellitus | 12,941 (73.0) | 2,921 (65.9) | 3,177 (71.7) | 3,382 (76.3) | 3,461 (78.1) | <0.001 |
| Cardiovascular disease* | 8,315 (46.9) | 2,012 (45.4) | 2,023 (45.6) | 2,150 (48.5) | 2,130 (48.0) | 0.003 |
| Congestive heart failure | 9,930 (56.0) | 2,455 (55.4) | 2,471 (55.8) | 2,519 (56.8) | 2,485 (56.1) | 0.55 |
| Peripheral vascular disease | 7,354 (41.5) | 1,773 (40.0) | 1,795 (40.5) | 1,914 (43.2) | 1,872 (42.2) | 0.007 |
| Chronic pulmonary disease | 8,136 (45.9) | 2,098 (47.3) | 2,098 (47.3) | 2,050 (46.3) | 1,890 (42.6) | <0.001 |
| Liver disease | 2,393 (13.5) | 595 (13.4) | 616 (13.9) | 587 (13.2) | 595 (13.4) | 0.83 |
| Malignancies | 4,401 (24.8) | 1,189 (26.8) | 1,212 (27.3) | 1,057 (23.9) | 943 (21.3) | <0.001 |
| AIDS/HIV | 244 (1.4) | 70 (1.6) | 60 (1.4) | 60 (1.4) | 54 (1.2) | 0.53 |
| Charlson Comorbidity Index | 5.0 (3.0, 7.0) | 5.0 (3.0, 7.0) | 5.0 (3.0, 7.0) | 5.0 (3.0, 7.0) | 5.0 (3.0, 7.0) | <0.001 |
| Vascular access type (catheter) | 12,345 (69.6) | 3,086 (69.6) | 3,027 (68.3) | 2,994 (67.6) | 3,238 (73.0) | <0.001 |
| Medications | ||||||
| ACEIs/ARBs | 11,238 (63.4) | 2,454 (55.4) | 2,793 (63.0) | 2,936 (66.3) | 3,055 (68.9) | <0.001 |
| β-blockers | 14,036 (79.2) | 3,161 (71.3) | 3,448 (77.8) | 3,679 (83.0) | 3,748 (84.5) | <0.001 |
| Calcium channel blockers | 13,047 (73.6) | 2,799 (63.1) | 3,158 (71.3) | 3,459 (78.1) | 3,631 (81.9) | <0.001 |
| Diuretics | 14,854 (83.8) | 3,428 (77.3) | 3,725 (84.0) | 3,862 (87.2) | 3,839 (86.6) | <0.001 |
| Vasodilators | 1,075 (6.1) | 126 (2.8) | 198 (4.5) | 307 (6.9) | 444 (10.0) | <0.001 |
| Statins | 12,772 (72.0) | 3,027 (68.3) | 3,189 (72.0) | 3,295 (74.4) | 3,261 (73.6) | <0.001 |
| Vitamin D analogs | 6,391 (36.0) | 1,453 (32.8) | 1,679 (37.9) | 1,713 (38.7) | 1,546 (34.9) | <0.001 |
| ESAs | 6,762 (38.1) | 1,231 (27.8) | 1,698 (38.3) | 1,946 (43.9) | 1,887 (42.6) | <0.001 |
| CV medication adherence (>80%) | 13,793 (77.8) | 3,640 (82.1) | 3,561 (80.3) | 3,407 (76.9) | 3,185 (71.8) | <0.001 |
| Laboratory parameters† | ||||||
| Serum albumin (g/dL) | 3.4±0.6 | 3.5±0.6 | 3.4±0.6 | 3.4±0.6 | 3.3±0.6 | <0.001 |
| Blood hemoglobin (g/dL) | 10.9±1.4 | 11.1±1.5 | 10.9±1.4 | 10.8±1.3 | 10.7±1.3 | <0.001 |
| eGFR (mL/min/1.73m2) | 15.7 (11.9, 22.2) | 16.3 (11.9, 24.7) | 15.7 (11.9, 22.5) | 15.4 (11.8, 21.2) | 15.6 (12.0, 21.0) | <0.001 |
Data are presented as number (percentage), mean ± standard deviation, or median (25th, 75th percentiles).
Cardiovascular disease include coronary artery disease, angina, myocardial infarction, or cerebrovascular disease.
All laboratory results averaged over the one-year prelude period.
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESA, erythropoietin stimulating agent; SBPV, systolic blood pressure variability
Factors associated with prelude SBPV
Table 2 shows the association of SBPV with baseline patient characteristics. After multivariable adjustment, higher SBP, presence of comorbidities such as diabetes, cardiovascular disease, and peripheral vascular disease, catheter use, and use of ACEIs/ARBs, β-blockers, vasodilators, and ESAs were associated with higher SBPV. In contrast, older age, married status, higher BMI, adequate cardiovascular medication adherence, and higher serum albumin were associated with lower SBPV.
Table 2.
Factors associated with prelude SBPV
| Characteristics | Coefficient* | (95% CI) | P value |
|---|---|---|---|
| Age (per 1 year) | −0.03 | (−0.04 to −0.02) | <0.001 |
| Sex (male vs. women) | 0.63 | (−0.06 to 1.31) | 0.072 |
| Race (African-American vs. white) | −0.01 | (−0.23 to 0.21) | 0.91 |
| Marital status (married vs. non-married) | −0.44 | (−0.64 to −0.25) | <0.001 |
| Systolic BP (per 1 mmHg) | 0.15 | (0.14 to 0.16) | <0.001 |
| Body mass index (per 1 kg/m2) | −0.10 | (−0.11 to −0.08) | <0.001 |
| Comorbidities (yes vs. no) | |||
| Diabetes mellitus | 0.64 | (0.38 to 0.90) | <0.001 |
| Cardiovascular disease† | 0.44 | (0.23 to 0.66) | <0.001 |
| Congestive heart failure | 0.08 | (−0.14 to 0.30) | 0.49 |
| Peripheral vascular disease | 0.39 | (0.18 to 0.60) | <0.001 |
| Chronic pulmonary disease | 0.07 | (−0.14 to 0.28) | 0.53 |
| Liver disease | −0.10 | (−0.39 to 0.20) | 0.52 |
| Charlson Comorbidity Index (per 1 unit) | −0.02 | (−0.08 to 0.03) | 0.37 |
| Vascular access type (catheter vs. others) | 0.34 | (0.13 to 0.56) | <0.001 |
| Medications (yes vs. no) | |||
| ACEIs/ARBs | 0.65 | (0.44 to 0.85) | <0.001 |
| β-blockers | 0.88 | (0.64 to 1.13) | <0.001 |
| Calcium channel blockers | 0.19 | (−0.05 to 0.42) | 0.12 |
| Diuretics | 0.27 | (−0.02 to 0.55) | 0.067 |
| Vasodilators | 0.96 | (0.56 to 1.36) | <0.001 |
| Statins | 0.15 | (−0.08 to 0.37) | 0.21 |
| ESAs | 0.74 | (0.53 to 0.95) | <0.001 |
| CV medication adherence (>80% vs. ≤80%) | −0.80 | (−1.02 to −0.57) | <0.001 |
| Laboratory parameters‡ | |||
| Serum albumin (per 1 g/dL) | −0.82 | (−1.00 to −0.65) | <0.001 |
| Blood hemoglobin (per 1 g/dL) | 0.008 | (−0.07 to 0.08) | 0.84 |
| eGFR (per 1 mL/min/1.73m2) | 0.002 | (−0.006 to 0.01) | 0.53 |
Coefficient for multivariable linear regression models. Value of coefficient represents change in SBPV (mmHg) per 1 unit change in each factor. Positive and negative numbers indicate higher and lower BPV per 1 unit change in factors, respectively. The variance inflation factors of these parameters were all less than 5.
Cardiovascular disease include coronary artery disease, angina, myocardial infarction, or cerebrovascular disease.
All laboratory results averaged over the one-year prelude period.
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; eGFR, estimated glomerular filtration rate; ESA, erythropoietin stimulating agent; SBPV, systolic blood pressure variability
Association of Pre-ESRD SBPV with Post-ESRD All-Cause Mortality
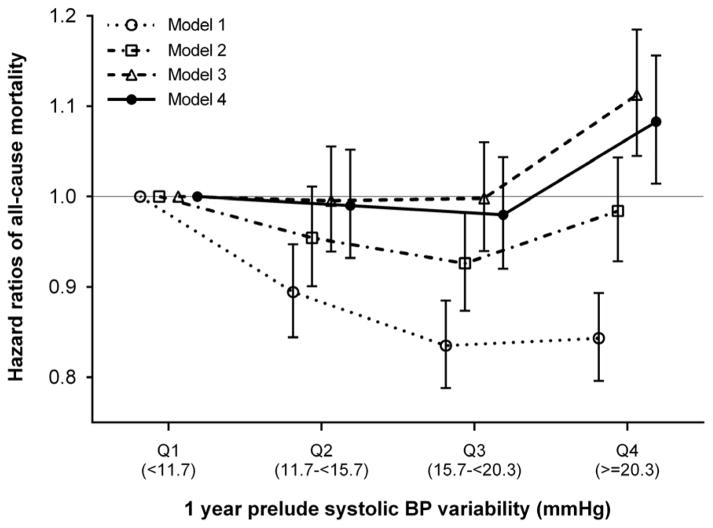
There were 9,064 all-cause deaths during a median follow-up of 2.0 years (25th, 75th percentiles, 1.1, 3.1 years; total time at risk, 37,969 patient-years) following dialysis initiation (crude incidence rate, 238.7 per 1000 patient-years; 95% confidence interval [CI], 233.9–243.7). Figure 1 shows the unadjusted- and multivariable-adjusted hazard ratios (HRs) associated with pre-ESRD SBPV quartiles. In the crude model, SBPV quartiles were inversely associated with all-cause mortality, with significantly lower death risks seen in higher SBPV quartiles. After multivariable adjustment, the association between SBPV and all-cause mortality was substantially attenuated and remained statistically significant only for the highest SBPV category (adjusted HRs [95% CI] for SBPV quartiles 2 through 4 [vs. quartile 1], 0.99 [0.93–1.05], 0.98 [0.92–1.04], and 1.08 [1.01–1.16], respectively, in model 4; Figure 1). The association remained essentially unchanged when SBPV was quantified as coefficient of variation of SBP (Supplemental Figure 2).
Figure 1. Association of prelude SBPV with all-cause mortality after dialysis initiation.
Models are as follows: model 1 is unadjusted; model 2 is adjusted for age, sex, race/ethnicity, and marital status; model 3 is adjusted for the variables in model 2 plus comorbidities (hypertension, cardiovascular disease, congestive heart failure, peripheral vascular disease, lung disease, diabetes mellitus, liver disease, and Charlson comorbidity index) and systolic BP, BMI, and eGFR averaged over the one-year prelude period; and model 4 is adjusted for the variables in model 3 plus medications (ACEIs/ARBs, β-blockers, calcium channel blockers, vasodilators, diuretics, statins, and ESAs), cardiovascular medication adherence, and type of vascular access (arteriovenous fistula, arteriovenous graft, or catheter).
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; ESA, erythropoietin stimulating agent; SBPV, systolic blood pressure variability
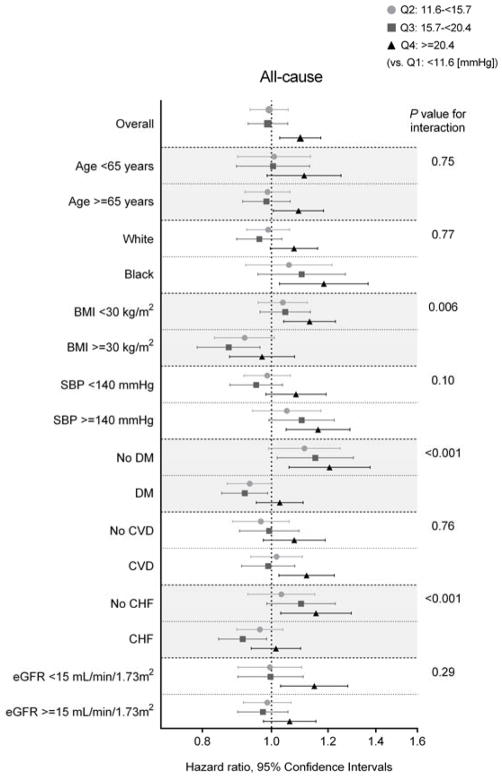
In subgroup analyses, higher SBPV was associated with higher all-cause mortality across most subgroups (Figure 2). Statistically significant interactions were present for BMI, diabetes, and CHF, with greater contributions of higher SBPV to all-cause mortality among patients with BMI <30 kg/m2, those without diabetes, and those without CHF. Results were similar after additional adjustment for serum albumin and blood hemoglobin levels, albeit without reaching statistical significance (Supplemental Table 2).
Figure 2. Adjusted hazard ratios (95% CIs) of all-cause mortality after dialysis initiation associated with prelude SBPV quartiles in selected subgroups.
Model is adjusted for age, sex, race/ethnicity, marital status, comorbidities (hypertension, cardiovascular disease, congestive heart failure, peripheral vascular disease, lung disease, diabetes mellitus, liver disease, and Charlson comorbidity index), systolic BP, BMI, and eGFR averaged over the one-year prelude period, medications (ACEIs/ARBs, β-blockers, calcium channel blockers, vasodilators, diuretics, statins, and ESAs), cardiovascular medication adherence, and type of vascular access (arteriovenous fistula, arteriovenous graft, or catheter).
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CHF, congestive heart failure; CVD, cardiovascular disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; ESA, erythropoietin stimulating agent; SBPV, systolic blood pressure variability
Association of Pre-ESRD SBPV with Post-ESRD Cardiovascular and Infection-Related Mortality
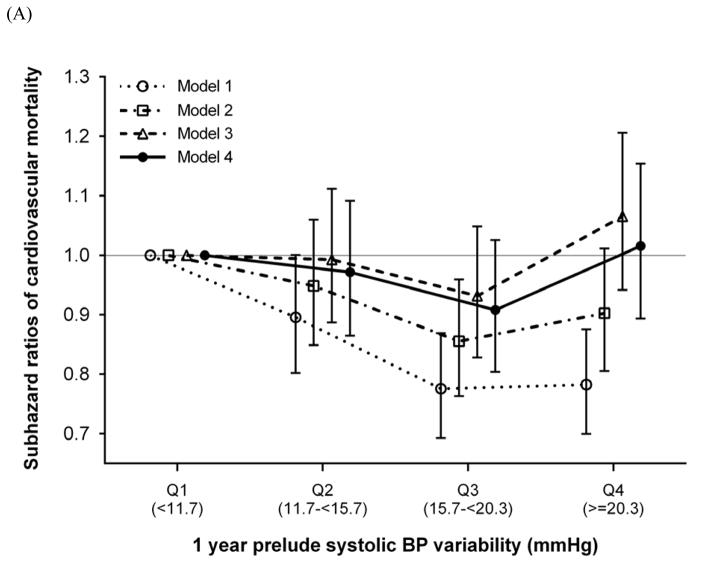
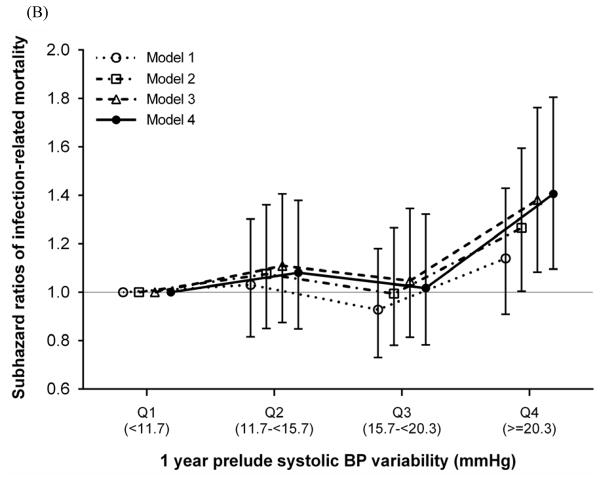
During a median follow-up of 1.3 years (25th, 75th percentiles, 0.5, 2.3 years) following dialysis initiation, 2,363 and 574 deaths occurred from cardiovascular and infection-related causes, respectively, and 1,790 deaths occurred from other causes. In our crude model, SBPV quartiles were inversely associated with cardiovascular mortality, with significantly lower death risks seen in higher SBPV quartiles. This association was considerably attenuated and no longer significant after multivariable adjustment (adjusted SHRs [95% CI] for SBPV quartiles 2 through 4 [vs. quartile 1], 0.97 [0.86–1.09], 0.91 [0.80–1.03], and 1.02 [0.89–1.15], respectively, in model 4; Figure 3). In contrast, higher SBPV quartiles were associated with higher infection-related mortality in all models (adjusted SHRs [95% CI] for SBPV quartiles 2 through 4 [vs. quartile 1], 1.08 [0.85–1.38], 1.02 [0.78–1.32], and 1.41 [1.10–1.80], respectively, in model 4; Figure 3). The associations were largely similar for SBPV defined as coefficient of variation of SBP (Supplemental Figure 3).
Figure 3. Association of prelude SBPV with (A) cardiovascular and (B) infection-related mortality after dialysis initiation.
Models are as follows: model 1 is unadjusted; model 2 is adjusted for age, sex, race/ethnicity, and marital status; model 3 is adjusted for the variables in model 2 plus comorbidities (hypertension, cardiovascular disease, congestive heart failure, peripheral vascular disease, lung disease, diabetes mellitus, liver disease, and Charlson comorbidity index) and systolic BP, BMI, and eGFR averaged over the one-year prelude period; and model 4 is adjusted for the variables in model 3 plus medications (ACEIs/ARBs, β-blockers, calcium channel blockers, vasodilators, diuretics, statins, and ESAs), cardiovascular medication adherence, and type of vascular access (arteriovenous fistula, arteriovenous graft, or catheter).
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; ESA, erythropoietin stimulating agent; SBPV, systolic blood pressure variability
In subgroup analyses, the pattern of association between SBPV and cardiovascular mortality was qualitatively similar to that with all-cause mortality, while the association with infection-related mortality was generally consistent across selected subgroups (Supplemental Figure 4). The associations were robust to additional adjustment for serum albumin and blood hemoglobin levels (Supplemental Table 3).
DISCUSSION
In this large national cohort of 17,729 US veterans with advanced NDD-CKD transitioning to dialysis, we found that higher SBPV was associated with higher all-cause and infection-related mortality, but not cardiovascular mortality, following dialysis initiation. During the one-year prelude period, we also found that higher SBP, history of diabetes, cardiovascular disease, and peripheral vascular disease, use of antihypertensive medications and ESAs, inadequate cardiovascular medication adherence, and catheter use were all associated with higher SBPV.
Although the concept that SBPV has a prognostic value for cardiovascular events is not new,[38–40] there has been limited evidence for its effect on outcomes among NDD-CKD population. In recent years, some observational studies have demonstrated its independent associations with all-cause mortality and cardiovascular and renal events in patients with NDD-CKD;[20–25] however, these studies consisted mostly of patients with CKD stage 3 or 4 and included a relatively small number of patients with advanced NDD-CKD. More importantly, all but one of these studies have examined the association of SBPV with outcomes that occurred before ESRD transition, presumably due to the lack of large databases linking pre-ESRD transition data to post-ESRD registries. In one retrospective cohort study of 374 elderly patients with NDD-CKD and hypertension, Iorio et al.[20] investigated the carry-over effect of SBPV on mortality after dialysis initiation by extending the observation of 34 out of 374 patients (9.0%) who initiated dialysis, but failed to show an association due to not detecting any fatal events over a mean follow-up of six months after dialysis initiation. In the present study, we therefore extended the previous observations to a large and unique cohort of patients with advanced NDD-CKD transitioning to dialysis, and for the first time demonstrated the prognostic impact of pre-ESRD SBPV with post-ESRD outcomes, including not only all-cause mortality but also cardiovascular and infection-related mortality.
Several potential explanations have been proposed for the mechanisms underlying higher SBPV, such as endothelial dysfunction,[17] increased arterial stiffness,[41, 42] disturbed baroreflex regulation of BP,[43] use of certain types of antihypertensive medications,[44] low medication adherence,[45] and social and lifestyle factors.[46] Increased SBPV can in turn cause greater stress on blood vessels and induce endothelial dysfunction and subclinical inflammation, serving as a potential direct mediator of early target-organ damage.[47, 48] In line with these plausible mechanisms linking SBPV to adverse outcomes, we identified both non-modifiable and modifiable factors associated with higher SBPV, including history of diabetes, cardiovascular disease, and peripheral vascular disease as non-modifiable factors; and higher SBP, use of antihypertensive medications and ESAs, low cardiovascular medication adherence and dialysis catheter use as vascular access as modifiable risk factors. When we accounted for all of these factors in the mortality risk estimates, the association of pre-ESRD SBPV with post-ESRD mortality, particularly with all-cause and cardiovascular mortality, was substantially modified; which may in turn support their potential involvement as underlying pathophysiological mechanisms in the SBPV-mortality relationship. Contrary to expectation, however, we found a weak U-shaped but not statistically significant association between pre-ESRD SBPV and post-ESRD cardiovascular mortality. This seemingly counterintuitive observation might be partly explained by survivorship bias in this unique study population, such that patients who had severely suffered from the above-mentioned cardiovascular complications and had higher SBPV may have died before reaching ESRD. Notably, there was an inverse association of pre-ESRD SBPV with post-ESRD mortality in the unadjusted model, which was substantially confounded by patient characteristics such as younger age and higher SBP (recently reported to be associated with lower post-ESRD mortality in the same study population.[49]) Nonetheless, it is important to note that a similar weak U-shaped association has also been reported between SBPV and fatal coronary heart disease or nonfatal myocardial infarction in a high-risk hypertensive population.[50] In this context, it is of particular interest that higher pre-ESRD SBPV was consistently associated with higher post-ESRD infection-related mortality. Considering the increased risk of infection in later stages of CKD, it is plausible that higher SBPV in patients with advanced NDD-CKD reflects non-fatal infectious events accompanied by hypotensive episodes during the prelude period, which could be harbingers of future deaths from infectious causes.
SBPV is a complex construct which is not readily available in daily clinical practice, but given the considerable uncertainty about the optimal approach to BP management in advanced NDD-CKD patients, there are several potential clinical and prognostic implications from our study. First, physicians need to be aware of the post-ESRD death risk, particularly infection-related death risk, associated with high pre-ESRD SBPV. Given the independent associations of pre-ESRD SBPV with some potentially modifiable clinical factors such as SBP, use of antihypertensive medications and ESAs, cardiovascular medication adherence, and catheter use, SBPV could become a treatment target through interventions aimed at these characteristics. The effect of such interventions on patient outcomes will have to be tested in future studies.
Our study has several limitations that should be acknowledged. First, this study was observational, and hence, the results do not allow us to infer causality but merely associations. Second, our cohort consisted of predominantly male US veterans, and only 1.9% of the main cohort were women; thus, the results may not apply to women or patients from other geographical areas. Also of note, the majority of patients (73%) in our study population were diabetic. Third, the effect of longitudinal changes in SBPV and other potential confounders such as cardiovascular medications over the post-ESRD follow-up period was not accounted for; therefore, it is possible that such time-dependent factors might affect the observed associations. However, the obtained results with the use of fixed pre-ESRD baseline covariates would still be of value, providing potential prognostic implications for post-ESRD outcomes in patients with advanced NDD-CKD. Fourth, we examined only SBPV but not diastolic BP variability separately; however, some previous studies have demonstrated poor associations of visit-to-visit diastolic BP variability with clinical outcomes both in NDD-CKD patients[22] and in the general population.[2] Finally, as with all observational studies, we cannot eliminate the possibility of unmeasured confounders such as proteinuria.
In conclusion, in this large national cohort of US veterans with advanced NDD-CKD transitioning to dialysis, a greater pre-ESRD SBPV, a potentially modifiable risk factor, was independently associated with higher all-cause and infection-related mortality after dialysis initiation. Our findings suggest the prognostic importance of pre-ESRD SBPV on post-ESRD outcomes and the need for careful consideration to high SBPV in CKD patients during the transition period. Further studies are needed to test whether modification of pre-ESRD SBPV can improve clinical outcomes among incident ESRD patients.
Supplementary Material
Acknowledgments
Source of Funding:
This study is supported by grant 5U01DK102163 from the National Institute of Health (NIH) to KKZ and CPK, and by resources from the US Department of Veterans Affairs. The data reported here have been supplied in part by the United States Renal Data System (USRDS). Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
CPK and KKZ are employees of the Department of Veterans affairs. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the Department of Veterans Affairs or the US government. The results of this paper have not been published previously in whole or part.
Abbreviations
- CHF
congestive heart failure
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- NDD-CKD
non-dialysis dependent chronic kidney disease
- SBPV
systolic blood pressure visit-to-visit variability
Footnotes
Disclosures:
None of the authors have relevant conflicts of interest.
References
- 1.Brunelli SM, Thadhani RI, Lynch KE, Ankers ED, Joffe MM, Boston R, et al. Association between long-term blood pressure variability and mortality among incident hemodialysis patients. Am J Kidney Dis. 2008;52(4):716–726. doi: 10.1053/j.ajkd.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 2.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57(2):160–166. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 3.Rossignol P, Cridlig J, Lehert P, Kessler M, Zannad F. Visit-to-visit blood pressure variability is a strong predictor of cardiovascular events in hemodialysis: insights from FOSIDIAL. Hypertension. 2012;60(2):339–346. doi: 10.1161/HYPERTENSIONAHA.111.190397. [DOI] [PubMed] [Google Scholar]
- 4.Selvarajah V, Pasea L, Ojha S, Wilkinson IB, Tomlinson LA. Pre-Dialysis Systolic Blood Pressure-Variability Is Independently Associated with All-Cause Mortality in Incident Haemodialysis Patients. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0086514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shafi T, Sozio SM, Bandeen-Roche KJ, Ephraim PL, Luly JR, St Peter WL, et al. Predialysis systolic BP variability and outcomes in hemodialysis patients. J Am Soc Nephrol. 2014;25(4):799–809. doi: 10.1681/ASN.2013060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C, Shlipak MG, Stawski RS, Peralta CA, Psaty BM, Harris TB, et al. Visit-to-Visit Blood Pressure Variability and Mortality and Cardiovascular Outcomes Among Older Adults: The Health, Aging, and Body Composition Study. Am J Hypertens. 2016 Sep 6; doi: 10.1093/ajh/hpw106. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierdomenico SD, Di Nicola M, Esposito AL, Di Mascio R, Ballone E, Lapenna D, Cuccurullo F. Prognostic value of different indices of blood pressure variability in hypertensive patients. Am J Hypertens. 2009;22(8):842–847. doi: 10.1038/ajh.2009.103. [DOI] [PubMed] [Google Scholar]
- 8.Poortvliet RK, Ford I, Lloyd SM, Sattar N, Mooijaart SP, de Craen AJ, Westendorp RG, et al. Blood pressure variability and cardiovascular risk in the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER) PLoS One. 2012;7(12):e52438. doi: 10.1371/journal.pone.0052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability, carotid atherosclerosis, and cardiovascular events in the European Lacidipine Study on Atherosclerosis. Circulation. 2012;126(5):569–578. doi: 10.1161/CIRCULATIONAHA.112.107565. [DOI] [PubMed] [Google Scholar]
- 10.Hata J, Arima H, Rothwell PM, Woodward M, Zoungas S, Anderson C, et al. Effects of visit-to-visit variability in systolic blood pressure on macrovascular and microvascular complications in patients with type 2 diabetes mellitus: the ADVANCE trial. Circulation. 2013;128(12):1325–1334. doi: 10.1161/CIRCULATIONAHA.113.002717. [DOI] [PubMed] [Google Scholar]
- 11.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 12.Shimbo D, Newman JD, Aragaki AK, LaMonte MJ, Bavry AA, Allison M, et al. Association between annual visit-to-visit blood pressure variability and stroke in postmenopausal women: data from the Women’s Health Initiative. Hypertension. 2012;60(3):625–630. doi: 10.1161/HYPERTENSIONAHA.112.193094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, Kovesdy CP. Association of Systolic Blood Pressure Variability With Mortality, Coronary Heart Disease, Stroke, and Renal Disease. J Am Coll Cardiol. 2016;68(13):1375–1386. doi: 10.1016/j.jacc.2016.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yilmaz MI, Stenvinkel P, Sonmez A, Saglam M, Yaman H, Kilic S, et al. Vascular health, systemic inflammation and progressive reduction in kidney function; clinical determinants and impact on cardiovascular outcomes. Nephrol Dial Transplant. 2011;26(11):3537–3543. doi: 10.1093/ndt/gfr081. [DOI] [PubMed] [Google Scholar]
- 15.Chen SC, Chang JM, Liu WC, Wang CS, Su HM, Chen HC. Arterial stiffness in patients with chronic kidney disease. Am J Med Sci. 2012;343(2):109–113. doi: 10.1097/MAJ.0b013e318223e814. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi SY, Rohani M, Lindholm B, Brodin LA, Lind B, Barany P, Alvestrand A, Seeberger A. Left ventricular function in patients with chronic kidney disease evaluated by colour tissue Doppler velocity imaging. Nephrol Dial Transplant. 2006;21(1):125–132. doi: 10.1093/ndt/gfi075. [DOI] [PubMed] [Google Scholar]
- 17.Diaz KM, Veerabhadrappa P, Kashem MA, Feairheller DL, Sturgeon KM, Williamson ST, et al. Relationship of visit-to-visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertens Res. 2012;35(1):55–61. doi: 10.1038/hr.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotsis V, Stabouli S, Karafillis I, Papakatsika S, Rizos Z, Miyakis S, et al. Arterial stiffness and 24 h ambulatory blood pressure monitoring in young healthy volunteers: the early vascular ageing Aristotle University Thessaloniki Study (EVA-ARIS Study) Atherosclerosis. 2011;219(1):194–199. doi: 10.1016/j.atherosclerosis.2011.07.111. [DOI] [PubMed] [Google Scholar]
- 19.Masugata H, Senda S, Murao K, Inukai M, Hosomi N, Iwado Y, et al. Visit-to-visit variability in blood pressure over a 1-year period is a marker of left ventricular diastolic dysfunction in treated hypertensive patients. Hypertens Res. 2011;34(7):846–850. doi: 10.1038/hr.2011.54. [DOI] [PubMed] [Google Scholar]
- 20.Di Iorio B, Pota A, Sirico ML, Torraca S, Di Micco L, Rubino R, et al. Blood pressure variability and outcomes in chronic kidney disease. Nephrol Dial Transplant. 2012;27(12):4404–4410. doi: 10.1093/ndt/gfs328. [DOI] [PubMed] [Google Scholar]
- 21.McMullan CJ, Bakris GL, Phillips RA, Forman JP. Association of BP variability with mortality among African Americans with CKD. Clin J Am Soc Nephrol. 2013;8(5):731–738. doi: 10.2215/CJN.10131012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallamaci F, Minutolo R, Leonardis D, D’Arrigo G, Tripepi G, Rapisarda F, et al. Long-term visit-to-visit office blood pressure variability increases the risk of adverse cardiovascular outcomes in patients with chronic kidney disease. Kidney Int. 2013;84(2):381–389. doi: 10.1038/ki.2013.132. [DOI] [PubMed] [Google Scholar]
- 23.Yokota K, Fukuda M, Matsui Y, Hoshide S, Shimada K, Kario K. Impact of visit-to-visit variability of blood pressure on deterioration of renal function in patients with non-diabetic chronic kidney disease. Hypertens Res. 2013;36(2):151–157. doi: 10.1038/hr.2012.145. [DOI] [PubMed] [Google Scholar]
- 24.McMullan CJ, Lambers Heerspink HJ, Parving HH, Dwyer JP, Forman JP, de Zeeuw D. Visit-to-visit variability in blood pressure and kidney and cardiovascular outcomes in patients with type 2 diabetes and nephropathy: a post hoc analysis from the RENAAL study and the Irbesartan Diabetic Nephropathy Trial. Am J Kidney Dis. 2014;64(5):714–722. doi: 10.1053/j.ajkd.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Chang TI, Tabada GH, Yang J, Tan TC, Go AS. Visit-to-visit variability of blood pressure and death, end-stage renal disease, and cardiovascular events in patients with chronic kidney disease. J Hypertens. 2016;34(2):244–252. doi: 10.1097/HJH.0000000000000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.United States Renal Data System. 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2014. [Google Scholar]
- 27.Robinson BM, Zhang J, Morgenstern H, Bradbury BD, Ng LJ, McCullough KP, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85(1):158–165. doi: 10.1038/ki.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sumida K, Molnar MZ, Potukuchi PK, Thomas F, Lu JL, Jing J, et al. Association of Slopes of Estimated Glomerular Filtration Rate With Post-End-Stage Renal Disease Mortality in Patients With Advanced Chronic Kidney Disease Transitioning to Dialysis. Mayo Clin Proc. 2016;91(2):196–207. doi: 10.1016/j.mayocp.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumida K, Molnar MZ, Potukuchi PK, Thomas F, Lu JL, Ravel VA, et al. Association between vascular access creation and deceleration of estimated glomerular filtration rate decline in late-stage chronic kidney disease patients transitioning to end-stage renal disease. Nephrol Dial Transplant. 2016 May 30; doi: 10.1093/ndt/gfw220. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molnar MZ, Gosmanova EO, Sumida K, Potukuchi PK, Lu JL, Jing J, et al. Predialysis Cardiovascular Disease Medication Adherence and Mortality After Transition to Dialysis. Am J Kidney Dis. 2016;68:609–618. doi: 10.1053/j.ajkd.2016.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US Department of Veterans Affairs. VIReC Research User Guide; VHA Medical SAS Inpatient Datasets FY2006–2007. Hines, IL: VA Information Resource Center; 2007. [Google Scholar]
- 32.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 33.VA Information Resource Center (VIReC) VHA Pharmacy Prescription Data. 2. Hines, IL: US Department of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; 2008. [Google Scholar]
- 34.Kovesdy CP, Norris KC, Boulware LE, Lu JL, Ma JZ, Streja E, et al. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015;132(16):1538–1548. doi: 10.1161/CIRCULATIONAHA.114.015124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovesdy CP, Alrifai A, Gosmanova EO, Lu JL, Canada RB, Wall BM, et al. Age and Outcomes Associated with BP in Patients with Incident CKD. Clin J Am Soc Nephrol. 2016;11(5):821–831. doi: 10.2215/CJN.08660815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 38.Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5(1):93–98. doi: 10.1097/00004872-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Frattola A, Parati G, Cuspidi C, Albini F, Mancia G. Prognostic value of 24-hour blood pressure variability. J Hypertens. 1993;11(10):1133–1137. doi: 10.1097/00004872-199310000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, et al. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension. 2000;36(5):901–906. doi: 10.1161/01.hyp.36.5.901. [DOI] [PubMed] [Google Scholar]
- 41.Shimbo D, Shea S, McClelland RL, Viera AJ, Mann D, Newman J, et al. Associations of aortic distensibility and arterial elasticity with long-term visit-to-visit blood pressure variability: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Hypertens. 2013;26(7):896–902. doi: 10.1093/ajh/hpt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okada H, Fukui M, Tanaka M, Inada S, Mineoka Y, Nakanishi N, et al. Visit-to-visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2012;220(1):155–159. doi: 10.1016/j.atherosclerosis.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 43.Hata Y, Muratani H, Kimura Y, Fukiyama K, Kawano Y, Ashida T, et al. Office blood pressure variability as a predictor of acute myocardial infarction in elderly patients receiving antihypertensive therapy. J Hum Hypertens. 2002;16(2):141–146. doi: 10.1038/sj.jhh.1001301. [DOI] [PubMed] [Google Scholar]
- 44.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375(9718):906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 45.Muntner P, Levitan EB, Joyce C, Holt E, Mann D, Oparil S, Krousel-Wood M. Association between antihypertensive medication adherence and visit-to-visit variability of blood pressure. J Clin Hypertens (Greenwich) 2013;15(2):112–117. doi: 10.1111/jch.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eguchi K, Hoshide S, Schwartz JE, Shimada K, Kario K. Visit-to-visit and ambulatory blood pressure variability as predictors of incident cardiovascular events in patients with hypertension. Am J Hypertens. 2012;25(9):962–968. doi: 10.1038/ajh.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eto M, Toba K, Akishita M, Kozaki K, Watanabe T, Kim S, et al. Reduced endothelial vasomotor function and enhanced neointimal formation after vascular injury in a rat model of blood pressure lability. Hypertens Res. 2003;26(12):991–998. doi: 10.1291/hypres.26.991. [DOI] [PubMed] [Google Scholar]
- 48.Rothwell PM. Does blood pressure variability modulate cardiovascular risk? Curr Hypertens Rep. 2011;13(3):177–186. doi: 10.1007/s11906-011-0201-3. [DOI] [PubMed] [Google Scholar]
- 49.Sumida K, Molnar MZ, Potukuchi PK, Thomas F, Lu JL, Ravel VA, et al. Blood Pressure Before Maintenance Dialysis and Subsequent Mortality. Am J Kidney Dis. 2017 doi: 10.1053/j.ajkd.2016.12.020. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, et al. Visit-to-Visit Variability of Blood Pressure and Coronary Heart Disease, Stroke, Heart Failure, and Mortality: A Cohort Study. Ann Intern Med. 2015;163(5):329–338. doi: 10.7326/M14-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.