Summary
Background
The incidence of breast cancer in sub-Saharan Africa is relatively low, but as survival from the disease in the region is poor, mortality rates are as high as in high-income countries. Stage at diagnosis is a major contributing factor to poor survival from breast cancer. We aimed to do a systematic review and meta-analysis on stage at diagnosis of breast cancer in sub-Saharan Africa to examine trends over time, and investigate sources of variations across the region.
Methods
We searched MEDLINE, Embase, Web of Knowledge, and Africa-Wide Information to identify studies on breast cancer stage at diagnosis in sub-Saharan African women published before Jan 1, 2014, and in any language. Random-effects meta-analyses were done to investigate between-study heterogeneity in percentage of late-stage breast cancer (stage III/IV), and meta-regression analyses to identify potential sources of variation. Percentages of women with late-stage breast cancer at diagnosis in sub-Saharan Africa were compared with similar estimates for black and white women in the USA from the Surveillance, Epidemiology, and End Results database.
Findings
83 studies were included, which consisted of 26788 women from 17 sub-Saharan African countries. There was wide between-study heterogeneity in the percentage of late-stage disease at diagnosis (median 74.7%, range 30.3–100%, I2=93.3%, p<0.0001). The percentage of patients with late-stage disease at diagnosis did not vary by region in black women, but was lower in non-black women from southern Africa than in black women in any region (absolute difference [AD] from black women in western Africa [reference group] −18.1%, 95% CI −28.2 to −8.0), and higher for populations from mixed (urban and rural) settings rather than urban settings (13.2%, 5.7 to 20.7, in analyses restricted to black women). The percentage of patients with late-stage disease at diagnosis in black Africans decreased over time (−10.5%, −19.3 to −1.6; for 2000 or later vs 1980 or before), but it was still higher around 2010 than it was in white and black women in the USA 40 years previously.
Interpretation
Strategies for early diagnosis of breast cancer should be regarded as a major priority by cancer control programmes in sub-Saharan Africa.
Funding
None.
Introduction
The incidence of breast cancer is highest in high-income countries (HICs), but has been rising in low-income and middle-income countries (LMICs).1,2 Survival rates for breast cancer are poorer in LMICs than in HICs and most deaths from breast cancer now occur in less developed parts of the world. In 2012, about 53% of all newly diagnosed cases of breast cancer, and about 58% of deaths, occurred in LMICs.3 Breast cancer incidence in LMICs is likely to increase further in forthcoming decades as a result of population ageing and increased adoption of the lifestyles of HICs.1,2
Breast cancer incidence in sub-Saharan Africa is among the lowest in the world. Estimated age-standardised rates in 2012 ranged from 27 cases per 100 000 women in middle Africa to 39 cases per 100 000 women in southern African regions. However, mortality due to cancer is as high as in high-incidence countries; estimated age-standardised rates in 2012 ranged from 15 deaths per 100 000 women in middle Africa to 20 deaths per 100 000 women in western Africa.3 These rates are higher than that of North America for the same year (age-standardised rate 14.8 cases per 100 000 women), which has a higher breast cancer incidence (age-standardised rate 91.6 cases per 100 000 women).3
Stage at diagnosis is a major determinant of survival from breast cancer; early-stage disease is associated with a better prognosis than late-stage disease,4 a pattern present in sub-Saharan Africa.5–8 Earlier stage at diagnosis, combined with therapeutic advances, was a major contributor to the sharp reductions in breast cancer mortality rates in the past two decades in most HICs.4 By contrast, most patients with breast cancer in sub-Saharan Africa present with late-stage disease, thought to be due to poor awareness, an absence of organised early detection programmes, and poor facilities for accurate and timely diagnosis and treatment.5,9–17 Variations in stage of breast cancer at diagnosis across sub-Saharan Africa and over time in some countries in sub-Saharan Africa have been previously reported in individual settings,5,7,9,13,18,19 but have not, to our knowledge, been examined systematically across sub-Saharan Africa.
In this study, we aimed to systematically review the published literature on stage at diagnosis of breast cancer in sub-Saharan Africa, examine trends over time, and investigate possible sources of between-study heterogeneity, which might help to identify appropriate approaches for stage-migration of this disease in the region.
Methods
Search strategy and selection criteria
For this systematic review and meta-analysis, we developed a study protocol (appendix p 1) based on the PRISMA guidelines (appendix p 4). We searched four databases (MEDLINE, Embase, Web of Knowledge, and Africa-Wide Information) to identify all studies published before Jan 1, 2014, which reported on stage at diagnosis of primary invasive breast cancer in women in sub-Saharan Africa. The UN classification20 was used to define sub-Saharan African countries and to group them according to region (ie, southern, eastern, western, and middle Africa). We did an initial keyword search and subsequent searches based on Medical Subject Headings (MeSH) with various combinations of search terms “breast cancer*”, “breast neoplasm*”, “breast carcinoma*”, “breast sarcoma*”, “breast tumor*”, “breast tumour*”, or “breast malignanc*”, AND “stage”, “presentation”, “grade”, “clinical features”, or “clinical findings”, AND “Africa” (appendix p 7). No restrictions were imposed on the ethnicity or race of women, whether diagnoses were done in public or private settings, age at diagnosis, or language of the publication.
We identified and reviewed articles in a two-step process. The first step consisted of a title and abstract review to identify records that were deemed potentially eligible for inclusion. This review was done by one of three authors (EJ-A, Id-S-S, or VM) to exclude publications that were duplicates; that were from north Africa (ie, Algeria, Egypt, Libya, Morocco, Sudan, Tunisia, and Western Sahara20); that did not focus on breast cancer (eg, studies of “all cancers”); that did not include women with breast cancer (eg, surveys on awareness); that did not provide information on stage (eg, pathology series, papers about screening); or that focused exclusively on breast cancer in men. Articles that restricted inclusion to a particular stage (eg, metastatic breast cancer) were also excluded. Reviews and conference proceedings were not included, but their references were cross-checked for completeness. Studies that included both female and male patients with breast cancer were included, even if they did not provide enough information to allow the exclusion of male patients, because men typically represented less than 2% of all study participants. A random sample of 50% of the total abstracts was independently reviewed by one of the other two authors, which showed no disagreements on which papers to select for full-text review.
Quality assessment and data extraction
In the second step, all full-text articles retrieved were reviewed to confirm eligibility and, if eligible, data were extracted. EJ-A assessed all articles for eligibility and extracted the data, using an adapted version of a pre-tested data entry electronic form.21 All articles were independently reviewed by one of the other two reviewers (Id-S-S or VM). Data were extracted from each eligible paper on the numbers of patients who presented in stages I, II, III, and IV at diagnosis, or at early (I/II) and late (III/IV) stages if only this combined information was provided; country; study design; study population and type of clinical setting (eg, primary, secondary, or tertiary clinical facility; population-based cancer registry; public, private, or mixed patients); year of diagnosis; race; average age at time of diagnosis (mean or median; if only age categories were reported the mean age was estimated from the mid-point and the reported numbers in each category); and methods and classification used to ascertain stage. Time at diagnosis in the original papers was either the time at clinical or pathological diagnosis.
If a study provided numbers for each specific American Joint Committee Cancer Tumour Node Metastases (TNM) category (eg, T2, N0, M0; appendix p 14), we used these to derive numbers in each one of the four stages. Whenever available, we extracted data on menopausal status, tumour characteristics (eg, histology, size, grade, receptor status), and time from first symptoms to diagnosis. Disagreements between extractors were discussed and a consensus reached. Most papers with missing information were from studies done several decades ago, hence no attempt was made to contact their authors because it was unlikely that the required information could still be retrieved. If there were several papers for the same study period, setting, and author, the paper with the most information on tumour stage was selected for inclusion.
The quality of the papers included in the review was assessed independently by two reviewers. An adapted version of the standardised quality assessment criteria developed by Eng and colleagues21 was used to assess the potential for selection and information bias as well as the availability of data on key variables (eg, age at diagnosis and year of diagnosis, tumour grade; details in the appendix; p 9). A quality score ranging from 0–28 (low to high quality) was given to each paper.
Data analysis
The primary outcome was percentage (p34) of breast cancer diagnosed at late stages (stages III/IV), defined as p34=n34/n, where n34 is the number of women who presented at stages III or IV and n is the number of women with known stage information. The suite of metan and metaprop commands from Stata (version 13) were used to graphically display population-specific late-stage percentages and to estimate pooled percentages using random effect models. The metaprop command was specifically designed to model binary data, thereby allowing for proportions near boundaries (ie, in this instance near 100% late-stage cancer). Between-population heterogeneity was assessed using I2 statistic and the p value for heterogeneity (Cochrane’s Q statistic). To examine potential sources of heterogeneity, population-specific estimates were stratified by relevant clinicoepidemiological variables, and meta-regression analyses were done to identify independent correlates of percentage of late-stage disease. Study-level determinants of late-stage disease are expressed as absolute differences (AD) in the percentage of patients with late-stage disease (p34). Analyses were first done in all study populations (black and non-black African) and then in black African populations only. The latter analyses excluded non-black African populations, which were from South Africa, because of their known privileged access to health care. The potential for small study bias was assessed using funnel plots and the Egger test.22
To compare late-stage breast cancer in sub-Saharan Africa with corresponding figures for white women and black women in the USA, relevant data were extracted from the Surveillance, Epidemiology, and End Results (SEER) database, which includes information on all cases of invasive primary breast cancer in women from nine US population-based cancer registries23 for two time periods: 1973–2002 and 1998–2011. The SEER database provided numbers of in-situ, localised, regional, and distant (metastatic) breast cancer cases as well as numbers with unknown or missing stage. There were no age restrictions. The SEER summary staging classification was used to estimate the percentage of patients with regional or distant disease (proxy for stages III/IV) out of all patients with breast cancers of known stage.
Role of the funding source
There was no funding source for this study. EJ-A, VM, and Id-S-S had full access to all the data in the study and EJ-A and Id-S-S had final responsibility for the decision to submit for publication.
Results
Our search retrieved 675 articles, of which 170 were considered as potentially relevant (figure 1). The full text was retrieved for all of these articles except for six, which could not be traced through institutional libraries or direct contact with the authors (attempts to contact authors proved futile). The sample sizes of two of the untraceable studies24,25 were 47 and 120 according to Edmund and colleagues.26
Figure 1.
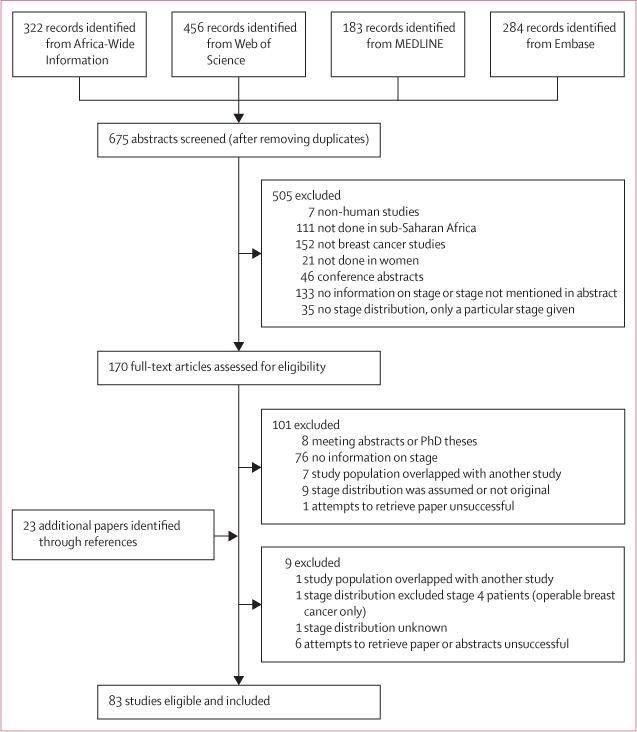
Study selection
The full-text review identified 83 eligible papers from 17 sub-Saharan African countries consisting of late-stage disease estimates for 91 distinct study populations; five studies provided separate estimates for different subsets of participants (ie, for pregnant or lactating and non-pregnant or non-lactating women27 or different racial groups12,28–30). For three studies,31–33 we obtained estimates that differed from those published because T3N1M0 tumours in the original articles were classified as stage II, but they should be stage III according to the 7th edition of the American Joint Committee on Cancer Breast Cancer Staging Manual.34 Four studies35–38 provided information on the tumour (T1–4) only and, for these, T3/T4 was regarded as a proxy for stages III/IV. The characteristics of the included studies are summarised in table 1; study-specific details and references are given in the appendix (p 14). They comprised 26 788 patients with breast cancer, with sample sizes ranging from 12 to 2346 (median 141; appendix p 14). Stage information was available for 24 213 (90.4%) patients. 36 studies (43%) were from Nigeria (8407 patients with cancer staging) and 16 studies (19%) were from South Africa (10 182 patients with cancer staging). 35 studies (42%) were consecutive case series and the remaining were convenience case series (ie, patients seen in pathology or radiotherapy departments only or studies in which not all eligible patients who reported at the surgery or oncology clinics were included; table 1). The average age at diagnosis was less than 45 years in 34% of studies, between 45–49 years in 43% of studies, and 50 years or older in 19% of studies. Age was not reported in only three studies (4%; table 1). The mean year of diagnosis ranged from 1960 to 2011, and was 2000 or later for 40% of the studies.
Table 1.
Study characteristics Declaration of interests
| Studies | Study populations | Patients with breast cancer | Patients with known breast cancer, n (%) | |
|---|---|---|---|---|
| Total | 83 | 91 | 26 788 | 24 213 (90.4%) |
|
| ||||
| Race | ||||
| Black† | 75 | 76 | 18 805 | 16 669 (88.6%) |
| Non-black‡ | 8 | 15 | 7983 | 7544 (94.5%) |
|
| ||||
| Region or country | ||||
| Western Africa | 48 | 49 | ||
| Nigeria | 36 | 37 | 8623 | 8407 (97.5%) |
| Benin | 2 | 2 | 204 | 204 (100%) |
| Ghana | 5 | 5 | 1969 | 1191 (60.5%) |
| Mali | 2 | 2 | 324 | 324 (100%) |
| Other§ | 3 | 3 | 797 | 719 (90.2%) |
| Eastern or middle Africa | 19 | 19 | ||
| Tanzania | 5 | 5 | 1310 | 1151 (87.7%) |
| Kenya | 2 | 2 | 287 | 157 (54.7%) |
| Ethiopia | 3 | 3 | 1267 | 841 (66.4%) |
| Madagascar | 2 | 2 | 289 | 233 (80.6%) |
| Uganda | 3 | 3 | 562 | 502 (89.3%) |
| Other¶ | 4 | 4 | 445 | 302 (67.9%) |
| Southern Africa | 16 | 23 | ||
| South Africa | 16 | 23 | 10 711 | 10 182 (95.1%) |
|
| ||||
| Study design | ||||
| Convenience case series | 48 | 55 | 10 780 | 9788 (90.8%) |
| Consecutive case series | 35 | 36 | 16 008 | 14 425 (90.1%) |
|
| ||||
| Study population | ||||
| Urban | 27 | 34 | 15 571 | 14 208 (91.2%) |
| Mixed (rural and urban) | 56 | 57 | 11 217 | 10 005 (89.2%) |
|
| ||||
| Type of health facility | ||||
| Tertiary, secondary, or primary‖ | 9 | 12 | 1639 | 1503 (91.7%) |
| Tertiary | 72 | 77 | 24 742 | 22 399 (90.5%) |
| Not reported in original study | 2 | 2 | 407 | 311 (76.4%) |
|
| ||||
| Age at diagnosis (years)** | ||||
| <45 years | 28 | 29 | 5475 | 4840 (88.4%) |
| >45 to <50 years | 36 | 37 | 7882 | 7218 (91.6%) |
| >50 years | 16 | 22 | 11 056 | 9841 (89.0%) |
| Not reported in original study | 3 | 3 | 2375 | 2314 (97.4%) |
|
| ||||
| Year of diagnosis†† | ||||
| Before 1980 | 11 | 16 | 3971 | 3782 (95.2%) |
| 1980–1999 | 32 | 34 | 11 125 | 10 737 (96.5%) |
| 2000 or after | 33 | 33 | 8648 | 6733 (77.8%) |
| Not reported in original study | 7 | 8 | 3044 | 2961 (97.3%) |
|
| ||||
| Staging methods | ||||
| Clinical and imaging | 25 | 26 | 10 416 | 9516 (91.4%) |
| Clinical only | 10 | 10 | 975 | 967 (99.2%) |
| Not reported in original study | 48 | 55 | 15 397 | 13 730 (89.2%) |
|
| ||||
| Staging classification | ||||
| TNM | 50 | 57 | 20 388 | 18 048 (88.5%) |
| Manchester | 11 | 11 | 1436 | 1426 (99.3%) |
| Not reported in original study | 22 | 23 | 4964 | 4739 (95.5%) |
|
| ||||
| Study quality scores‡‡ | ||||
| >23 (highest quality) | 12 | 12 | 4067 | 3569 (87.8%) |
| 22–20 | 26 | 27 | 6181 | 5721 (92.6%) |
| 19–17 | 31 | 38 | 14 541 | 13 327 (91.7%) |
| <17 (lowest quality) | 14 | 14 | 1999 | 1596 (79.8%) |
Data are n or n (%). TNM=Tumour, Lymph Node, and Metastasis staging system.
Five studies provided separate estimates for different subsets of participants (ie, for pregnant or lactating and non-pregnant or non-lactating women27 or different ethnic groups12,28–30).
Includes seven southern African studies12,28–30,39–41 that reported estimates for black women only; one southern African study18 that presented only an overall (all ethnic groups combined) estimate, but reported that >80% of their study population was black; nine studies5,37,38,42–47 from western and eastern Africa that were done exclusively in black women, as well as the remaining 58 studies from these two regions that did not report on race, but were assumed to have been done in predominantly black women (ie, >80% black; see appendix p 14), which corresponded to 76 study population groups because one Nigerian study27 presented separate estimates for pregnant or lactating and non-pregnant or non-lactating women (appendix p 14).
Includes 15 southern African study population groups: four studies14,48–50 that did not report on race but were assumed to be predominantly non-black, four studies36,51–53 that present only overall estimates but reported an ethnically mixed population with ≤80% being black, and four multi-ethnic studies12,28–30 that together reported separate estimates for seven non-black population groups (appendix p 14).
Includes one study from Guinea (178 cases, 124 cases with known stage), one from Niger (146 cases, 146 cases with known stage), and one from Senegal (473 cases, 449 cases with known stage).
Includes one study from Rwanda (145 cases, seven cases with known stage), one from Zimbabwe (84 cases, 79 cases with known stage), one from Eritrea (82 cases, 82 cases with known stage), and one from Democratic Republic of the Congo (formerly known as Zaire; 134 cases, 134 cases with known stage).
All studies that recruited participants from secondary and primary health centres also included a tertiary centre.
Mean or median age at breast cancer diagnosis. If only age categories were reported, mean or median age was estimated from the mid-point and the reported number in each age category. The three studies in which age was not reported in the original category did not provide sufficient information to allow their allocation into one of the three age categories: Ajekigbe54 reported that 50.8% of the participants were aged <50 years; Amir and colleagues55 reported that 90% of the participants were aged <50 years; and Pegoraro and colleagues36 reported that 50% were between aged 45–64 years (appendix p 14).
Middle year of the time interval during which patients were recruited.
Categories represent quartiles of the overall score distribution (appendix p 9).
There was wide variation in the distribution of stage at diagnosis in sub-Saharan Africa. For example, in studies that provided stage IV-specific estimates, the percentage of women diagnosed with stage IV breast cancer ranged from 4%56 to 70%27 (figure 2). Consequently, between-population heterogeneity was wide (I2=93.3%; p<0.0001) in the percentage of late-stage cancers (III/IV) (median 74.7%; range 30.3–100), with 59 (65%) of study populations yielding an estimate of greater than 70% (figure 3).
Figure 2. Study-specific breast cancer stage at diagnosis.
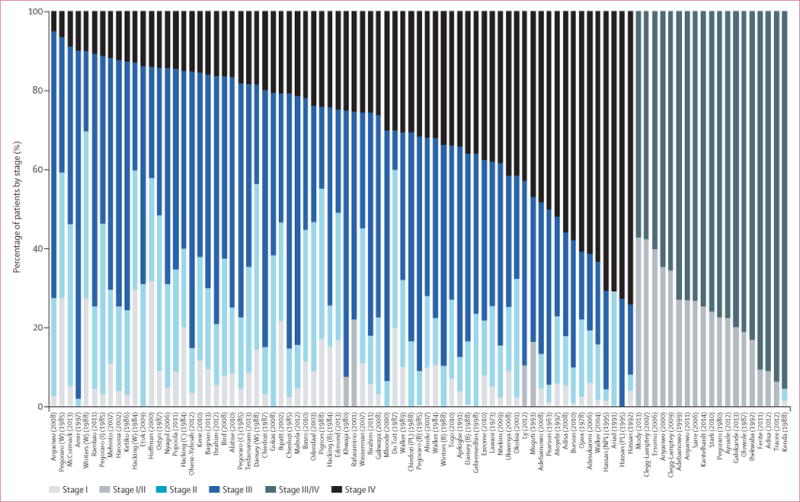
Study-specific distribution of stages I, II, III, and IV cancers. Percentage of T3/T4 cancers was used as a proxy for percentage of stage III/IV cancers in four studies.35–38 Percentage with metastases (M1) was given in three studies24,35,37 and was used as percentage of stage IV. Race as defined in table 1 and in the appendix (p 14). Study-specific references given in the appendix (p 14). B=black. C=coloured. I=Indian. NPL=non-pregnant or non-lactating women. PL=pregnant or lactating women. W=white.
Figure 3. Study-specific breast cancer stage at diagnosis.
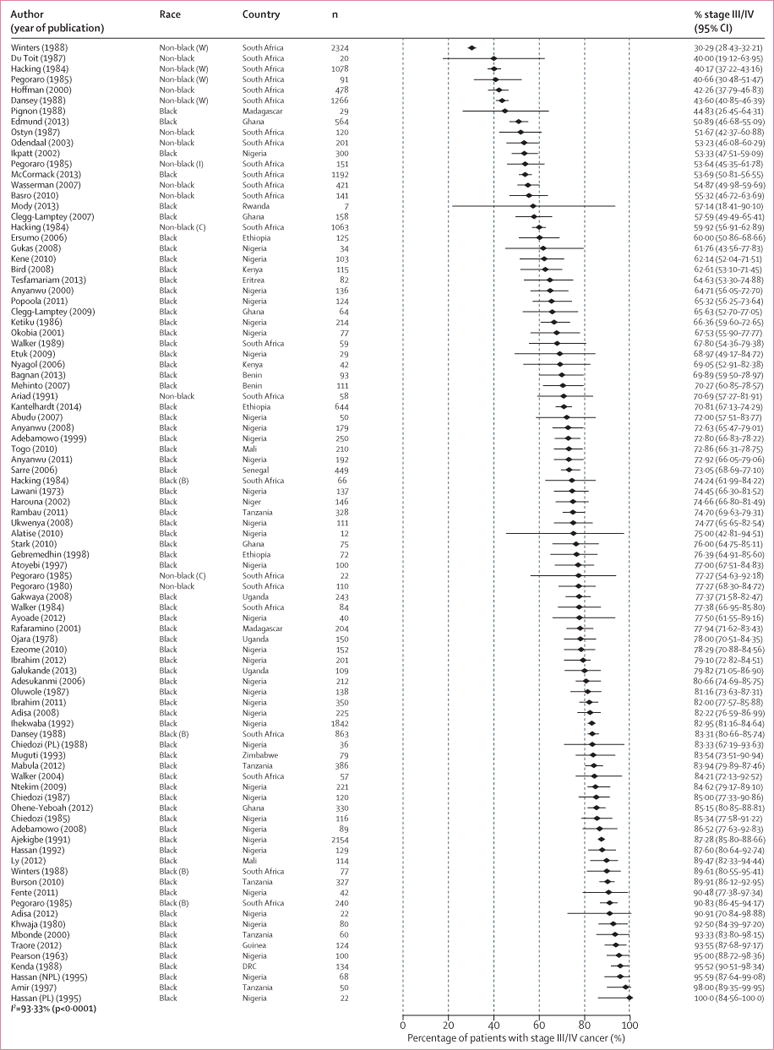
Study-specific percentage of late-stage disease (III/IV) ranked by increasing magnitude. Percentage of T3/T4 cancers was used as a proxy for percentage of stage III/IV cancers in four studies.35–38 Race as defined in table 1 and in the appendix (p 14). Study-specific references given in the appendix (p 14). B=black. C=coloured. DRC=Democratic Republic of the Congo. I=Indian. NPL=non-pregnant or non-lactating women. PL=pregnant or lactating women. W=white.
Nine studies from western and eastern Africa were done exclusively in black women.5,37,38,42–47 The remaining 58 studies did not report on race, but their populations were assumed to have the racial composition of their countries’ population and, hence, to consist predominantly (≥80%) of black women. Studies from South Africa included exclusively39–41 or predominantly (≥80%) black women;18 or predominantly (≥80%) non-black women (ie, white, Indian, or coloured women14,36,50–53); or provided separate estimates for black women and non-black women12,28–30 (appendix p 14).
Black women from South Africa presented much later than their non-black counterparts, but with marked between-population heterogeneity within each racial group (I2>97% for both groups; figure 4). Four South African studies examined racial differences (appendix p 11), which consistently showed a higher percentage of late-stage cancer in black Africans (range 74–91%) than white Africans (30–44%); the percentages of late-stage cancer in Indian and coloured women were intermediate, even when all the participants were diagnosed at the same health facility. However, these results were not adjusted for socioeconomic status because of a scarcity of information from the original publications.
Figure 4. Study-specific percentage of late-stage breast cancer at diagnosis, by region of sub-Saharan Africa.
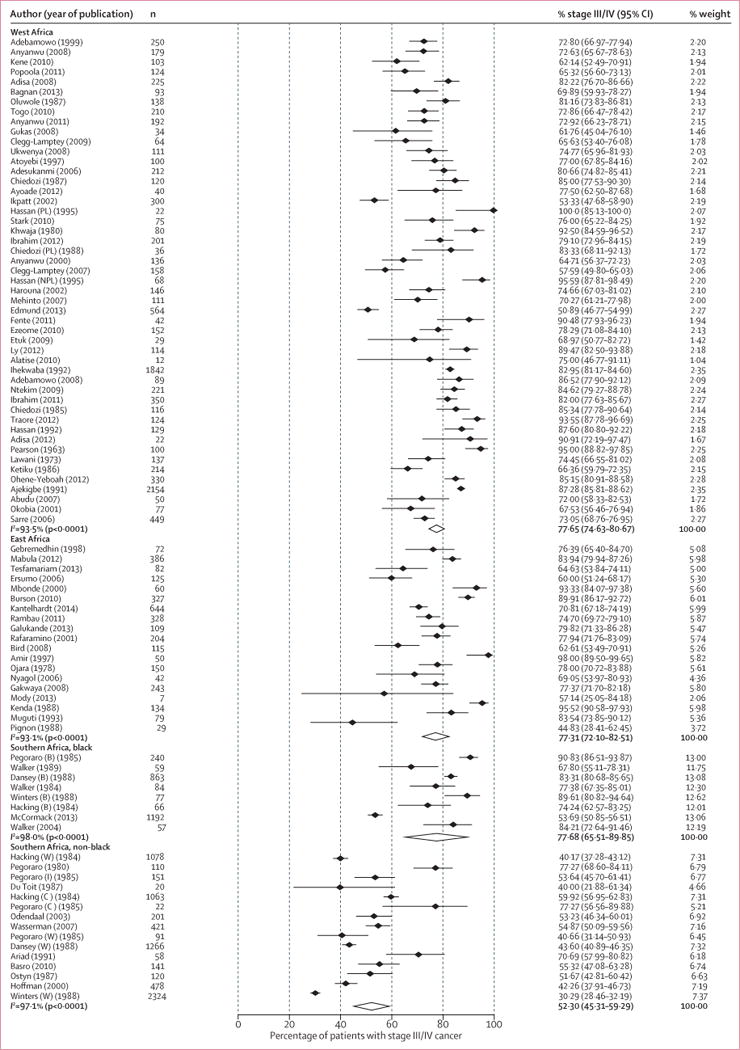
B=black African. C=coloured. I=Indian. NPL=non-pregnant or non-lactating women. PL=pregnant or lactating women. W=white. Study-specific references given in the appendix (p 14). *Weights are from random effects analyses.
Fully-adjusted meta-regression analysis (adjusting for region or race, study design, setting, facility type, age, and year of diagnosis) confirmed the difference between racial groups; the percentage of late-stage cancers was 18.1% lower (95% CI −28.2 to −8.0) for non-black women from South Africa than for black women in western Africa. By contrast, analysis restricted to black Africans revealed no difference in late-stage cancer diagnosis between the three sub-Saharan African regions (table 2).
Table 2.
Sources of between-population heterogeneity in the percentage of late-stage breast cancer (stages III/IV) from meta-regression analyses
| Patients with breast cancer, n | All study populations
|
Black populations only
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted analysis |
Region and race adjusted
analysis |
Fully-adjusted analysis*
|
Unadjusted analysis |
Fully-adjusted analysis†
|
|||||||
| AD (%) | 95% Cl | AD (%) | 95% Cl | AD (%) | 95% Cl | AD (%) | 95% Cl | AD (%) | 95% Cl | ||
| Region, race‡ | |||||||||||
|
| |||||||||||
| West Africa, black | 10845 | 0 (ref) | ‥ | ‥ | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ |
| East Africa, black | 3186 | −0.2 | −6.8 to 6.4 | ‥ | ‥ | −3.0 | −9.2 to 3.2 | −0.2 | −6.6 to 6.3 | −3.3 | −9.5 to 2.9 |
| Southern Africa, black | 2638 | 0.1 | −9.0 to 9.3 | ‥ | ‥ | 8.6 | −2.0 to 19.1 | 0.1 | −8.9 to 9.1 | 5.8 | −5.9 to 17.5 |
| Southern Africa, non-black | 7544 | −25.5 | −32.6 to −18.3 | ‥ | ‥ | −18.1 | −28.2 to −8.0 | ‥ | ‥ | ‥ | ‥ |
|
| |||||||||||
| Study design | |||||||||||
|
| |||||||||||
| Convenience case series | 14425 | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ |
| Consecutive case series | 9788 | −6.5 | −12.9 to −0.4 | −0.6 | −6.1 to 4.9 | −2.0 | −7.1 to 3.1 | −2.2 | −7.9 to 3.6 | −2.6 | −8.0 to 2.8 |
|
| |||||||||||
| Study population | |||||||||||
|
| |||||||||||
| Urban | 14208 | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ |
| Mixed (rural and urban) | 10 005 | 16.3 | 10.6 to 22.0 | 10.7 | 3.4 to 17.9 | 12.9 | 5.5 to 20·3 | 7.7 | 1.7 to 13.7 | 13.2 | 5.7 to 20.7 |
|
| |||||||||||
| Facility type | |||||||||||
|
| |||||||||||
| Tertiary | 22399 | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ |
| Tertiary, secondary, or primary | 1503 | −3.7 | −13.2 to 5.8 | −1.2 | −8.8 to 6.4 | −1.9 | −9.1 to 5.4 | −3.2 | −11.5 to 5.1 | −1.4 | −9.5 to 6.6 |
| Not reported in original study | 311 | −8.8 | −30.5 to 12.9 | 14.8 | −3.4 to 32.9 | 10.1 | −0.8 to 28.3 | ‥ | ‥ | ‥ | ‥ |
|
| |||||||||||
| Age at diagnosis (years)§ | |||||||||||
|
| |||||||||||
| <45 years | 4840 | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ |
| >45 to <50 years | 7218 | 0.8 | −6.0 to 7.8 | 0.3 | −5.5 to 6.0 | 3.9 | −2.0 to 9.9 | −0.3 | −6.2 to 5.6 | 3.9 | −2.3 to 10.1) |
| >50 years | 9841 | −13.2 | −21.2 to −5.3 | −6.2 | −14.4 to 2.0 | −1.7 | −9.9 to 6.4 | −3.9 | −12.0 to 4.1 | 1.8 | −8.9 to 12.4 |
| Not reported in original study | 2314 | 12.0 | −4.4 to 28.5 | 17.4 | 3.8 to 31.1 | 20.6 | 6.4 to 34.8 | 14.6 | −1.3 to 30.6 | 20.4 | 4.8 to 36.1 |
|
| |||||||||||
| Year of diagnosis¶ | |||||||||||
|
| |||||||||||
| Before 1980 | 3782 | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ |
| 1980–1999 | 10737 | 4.8 | −4.3 to 13.9 | −3.0 | −10.6 to 4.6 | −5.2 | −12.6 to 2.2 | −4.7 | −13.1 to 3.7 | −6.8 | −15.5 to 1.9 |
| 2000 or after | 6733 | 4.3 | −5.0 to 13.5 | −6.2 | −14.2 to 1.8 | −8.5 | −16.1 to −1.0 | −8.4 | −16.8 to −0.1 | −10.5 | −19.3 to −1.5 |
| Not reported in original study | 2961 | −6.5 | −19.9 to 6.9 | −7.3 | −18.2 to 3.6 | −8.5 | −19.0 to 2.1 | −16.5 | −29.7 to −3.3 | −12.5 | −25.9 to 1.8 |
|
| |||||||||||
| Staging methods | |||||||||||
|
| |||||||||||
| Clinical and imaging | 9516 | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ | 0 (ref) | ‥ |
| Clinical only | 967 | 5.0 | −6.4 to 16.4 | 2.3 | −7.0 to 11.6 | −1.4 | −10.4 to 7.6 | 3.0 | −5.9 to 11.9 | −1.3 | −10.3 to 7.8 |
| Not reported in original study | 13730 | 0.2 | −7.1 to 7.4 | 3.0 | 2.7 to 8.8 | 3.2 | −2.3 to 8.7 | 4.0 | −1.9 to 10.0 | 4.1 | −1.7 to 9.8 |
|
| |||||||||||
| Staging classification | |||||||||||
|
| |||||||||||
| TNM | 18048 | 0 (ref) | ‥ | 0 (ref) | ‥ | ‥ | ‥ | ‥ | ‥ | ‥ | ‥ |
| Manchester | 1426 | 2.4 | −7.6 to 12.5 | −3.7 | −12.1 to 4.6 | ‥ | ‥ | ‥ | ‥ | ‥ | ‥ |
| Not reported in original study | 4739 | 3.1 | −4.5 to 10.7 | −0.8 | −7.0 to 5.5 | ‥ | ‥ | ‥ | ‥ | ‥ | ‥ |
|
| |||||||||||
| Study quality scores‖ | ‥ | ‥ | ‥ | ‥ | ‥ | ‥ | |||||
|
| |||||||||||
| >23 (highest quality) | 3569 | 0 (ref) | ‥ | 0 (ref) | ‥ | ‥ | ‥ | ‥ | ‥ | ‥ | ‥ |
| 22–20 | 5721 | −2.1 | −12.7 to 8.5 | −0.1 | −8.5 to 8.3 | ‥ | ‥ | ‥ | ‥ | ‥ | ‥ |
| 19–17 | 13327 | −1.1 | −11.2 to 9.0 | 2.1 | −5.9 to 10.1 | ‥ | ‥ | ‥ | ‥ | ‥ | ‥ |
| <17 (lowest quality) | 1596 | 0.7 | −11.5 to 12.9 | 2.9 | −6.9 to 12.6 | ‥ | ‥ | ‥ | ‥ | ‥ | ‥ |
Data are n, absolute difference (%), or 95% Cl. Ref= reference category. AD=absolute difference. TNM=Tumour, Lymph Node, and Metastasis staging system.
Adjusted for all other variables, except for staging classification and study quality because of concerns of overadjustment,
Adjusted for all other variables, except region or race, staging classification, and study quality.
The study population was classified as black if 80% of participants or more were black (appendix p 14, figure 3).
Mean or median age at breast cancer diagnosis. If only age categories were reported mean or median age was estimated from the mid-point and the reported number in each age category.
Taken as the middle year of the period during which patient recruitment took place.
Categories defined using quartiles of the overall score distribution. Analyses were not further adjusted for the other variables in the table because most of them were integrated into the study quality scores (appendix p 9).
After adjustment for region or race, no differences in late-stage disease were observed between consecutive or convenience case series, or by type of health facility (table 2). Studies done in mixed urban or rural populations had a higher percentage of women with late-stage disease than those done in urban populations, and this finding remained significant in the fully adjusted model (AD 12.9%, 95% CI 5.5 to 20.3) and in the analysis restricted to black Africans (AD 13.2%, 5.7 to 20.7; table 2).
A smaller percentage of women aged 50 years or older had late-stage disease than those younger than 45 years (AD −13.2%, 95% CI −21.2 to −5.3), but most studies of older women consisted predominantly of non-black South Africans. Consequently, the age difference attenuated markedly on adjustment for region and race, and disappeared in analyses restricted to black Africans (table 2). A slight improvement in stage at diagnosis was observed over time (appendix p 12). In the fully-adjusted meta-regression model, the percentage of women with late-stage disease was lower in black Africans diagnosed since 2000 compared with women diagnosed before 1980 (AD −10.5%, 95% CI −19.3 to −1.6; table 2). In analyses restricted to black Africans, the percentage of women with late-stage cancer was lower in studies that did not report year of diagnosis than studies published before 1980, but this finding was not statistically significant (table 2). Because the years of publication of these studies ranged from 2002 to 2011, it is likely that patients recruited into these studies would have been diagnosed in recent years.
The TNM or the Manchester staging classification (appendix p 14) were used in most studies, but this information was missing in 21 studies (table 1). No clear differences in the percentage of late-stage disease were observed between studies that reported the staging classification used and studies that did not, or between studies done in facilities where there was access to imaging methods (eg, radiographs)—either routinely or in clinically suspicious cases—and studies done in settings without imaging facilities (table 2).
Few studies reported on tumour characteristics or duration of symptoms (appendix p 21). In studies of black African populations that reported on these characteristics, late-stage disease at diagnosis was positively associated with mean tumour size (Pearson correlation coefficient r=0.63, p=0.004, based on data from 19 studies), but not with self-reported mean duration of symptoms (r=−0.14, p=0.42, 35 studies) or with percentages of tumours classified as invasive ductal carcinomas (r=0.09, p=0.50, 53 studies), oestrogen-receptor positive (r=−0.03, p=0.91, 15 studies), or grade 3 (r=0.21, p=0.26, 32 studies; appendix p 21).
The median study quality score was 19.5 (IQR 17.5–21.5), with no evidence of regional or racial differences. No variation in the percentage of women diagnosed with late-stage breast cancer was observed by study quality (table 2). The funnel plot (appendix p 13) and the value of the Egger’s test for small study bias (p=0.01) were difficult to interpret because of the marked between-population heterogeneity.
The proportion of women with late-stage breast cancer at diagnosis declined markedly in the USA between 1973 and 2011: from 50% to 27% in white women, and from 60% to 32% in black women23 (figure 5). By contrast, most study-specific estimates of late-stage disease in black sub-Saharan African women remained well above 60% from the 1970s to 2011, albeit with some indication of a slight downward trend in some settings (figure 5). Notably, the proportion of late-stage disease in black women in sub-Saharan Africa in the most recent study years (around 2010) was still higher than in black women from the USA 40 years previously. The proportion of women with late-stage disease in southern Africa remained unchanged for non-black Africans, but seemed to decline somewhat in black Africans. Remarkably, only two studies were done after 2000 in the southern African region. Both studies were done in South Africa: one in non-black Africans14 and one in black Africans.18 By contrast, the number of studies from eastern and western Africa published after 2000 was higher than in previous decades, although most had relatively small sample sizes.
Figure 5. Trends in stage of breast cancer at diagnosis in sub-Saharan Africa in 1960–2011, and in the USA in 1973–2002 and 1988–2011.
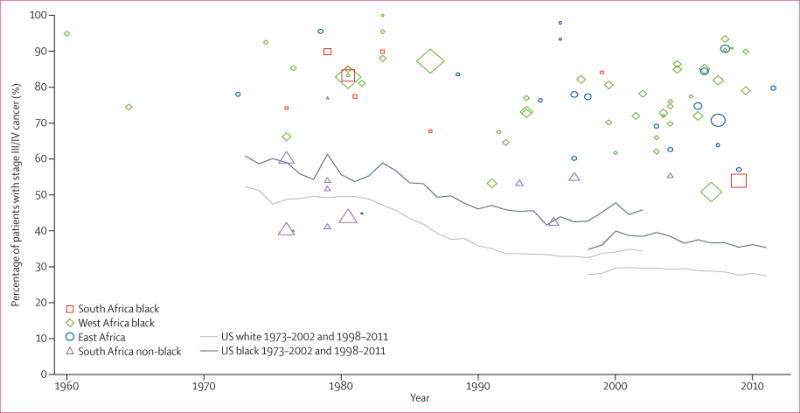
The US estimates represent percentage of patients with breast cancer with regional or distant disease (as a proxy for stages III/IV) out of all patients with known stage in the Surveillance Epidemiology End Results (SEER) database (see Methods); the SEER summary staging classification was used for both time periods: 1973–2002 (based on 365 695 white women and 31 781 black women with breast cancer in the USA) and 1998–2011 (based on 780 137 white women and 96 526 black women with breast cancer in the USA). The discontinuity between the two time series was due to a change in staging classification. The sub-Saharan Africa estimates correspond to percentage of patients with stage III/IV breast cancer at diagnosis; the size of the point estimate symbols are proportional to the size of the study.
Discussion
To our knowledge, this is the first systematic review of stage at diagnosis of breast cancer in sub-Saharan Africa. We compiled data from 83 studies consisting of 24 213 patients with staged cancers. The findings highlight two main issues. First, our findings show the paucity of data on one of the most important clinical prognostic markers of breast cancer in this region. Specifically, no published data from middle Africa were identified, and data from southern Africa were restricted to one country (South Africa), with only two studies done after 2000 (one in black Africans and another in non-black Africans). Furthermore, no study presented data from population-based cancer registries. Second, the findings show that most patients in sub-Saharan Africa (77% across all black study populations) were diagnosed at stages III/IV. Although this overall situation might seem grave, the presence of public-sector sub-Saharan Africa settings with improved stage profile needs to be highlighted because those settings reveal that progress in stage migration of breast cancer can be made within the public sector setting in which mammography is often unavailable. However, the reasons for the marked heterogeneity between populations, which is present even in analyses restricted to black Africans, are not entirely clear—no distinct patterns define the better settings. Late-stage breast cancer was, as expected, more frequent in black Africans than in non-black Africans; however, no clear differences in the percentage of late-stage cancers at diagnosis by region or type of health facility were observed in black African women, except that the percentage of late-stage cancers at diagnosis was lower in urban settings. There was evidence of stage migration of breast cancer over time in black Africans diagnosed after 2000, consistent with the downward trend within studies in late-stage disease at diagnosis described by one of the studies in this review. McCormack and colleagues18 reported a decrease in the frequency of stage III/IV cancers in South Africa from 66% in 2006–07 to 46% in 2010–12.
We did not find a strong association between age at diagnosis and late-stage cancer at diagnosis in black African women. Most patients were aged 35–49 years at diagnosis (approximately 10–15 years younger than patients in developed countries).57 This finding likely reflects the younger age structure of the sub-Saharan African population, consequent to higher fertility and shorter life expectancy, and the lower prevalence of risk factors in older generations than in young generations, rather than any inherent biological differences in disease aggressiveness between black and white patients. Consistent with this interpretation is the fact that, at a study level, late-stage cancer at diagnosis was not correlated with tumour grade, which could indicate that late-stage cancer at diagnosis is not entirely a consequence of black African women having more biologically aggressive forms of disease—indeed a 2014 review21 suggests that oestrogen-receptor-positive disease constitutes two-thirds of tumours in black women from sub-Saharan Africa. Late-stage disease was, however, positively correlated with mean tumour size (as expected given that tumour size is used to derive stage), consistent with delays in access to health care.
Increased breast cancer awareness and improvements in health care over time have been paralleled by decreases in tumour size and downstaging of breast cancer in other LMICs.58,59 However, studies have reported low levels of breast cancer awareness in the general population and health-care professionals in sub-Saharan Africa.60,61 The poor awareness contributes to the high frequency of late-stage cancer at diagnosis seen in sub-Saharan Africa.62,63 Other barriers to access, such as distance to health-care facility, also play a role in this region.64
Most studies used the TNM or the Manchester staging classifications, but only a quarter reported on the staging methods used. Of these, most studies relied on both clinical and imaging methods, but a few studies used clinical methods only. Although the clinical methods only approach leads to under-staging,65 most women in settings where imaging procedures are unavailable or unaffordable are likely to have presented at advanced stages when clinical methods might suffice.66 This is consistent with our finding of no differences in late-stage disease depending on whether staging methods were reported and, if reported, by the type used.
There was no correlation, at a study level, between percentage of late-stage disease and average self-reported duration of symptoms (ie, time between onset of symptoms and diagnosis). The extent to which this ecological-level association reflects a similar absence of an association at an individual level is unclear. Women might not recognise symptoms because of poor breast cancer awareness,67,68 or they might not accurately remember the dates on which they first noticed symptoms. Nevertheless, the average duration of symptoms was between 8 months and 12 months in most studies (appendix p 21), indicating that for the most part advanced stage at diagnosis might be a result of delayed diagnosis. Hence, a large window exists in which delays to diagnosis can be shortened.
The frequency of late-stage disease at diagnosis in black women in sub-Saharan Africa was higher than in white and black women from the USA in 1970–2010, including during the pre-mammography screening era (screening in the USA began in 197669). This shows that, through more rapid diagnosis of palpable clinical disease, considerable improvements can be made before expensive systems for the detection of preclinical disease are warranted. In sub-Saharan Africa, where mammography is often unavailable or unaffordable, stage migration through breast cancer awareness and improved access to diagnostic facilities, not mammographic screening, is urgently required.
Major strengths of this review include the detailed and inclusive search strategy, which included non-English publications; the large sample size of more than 24 000 women with breast cancer in the region; and the use of standard methods for study identification, and data extraction and synthesis. There were also limitations. The representativeness of the review might have been compromised by several factors. First, we included studies from only 17 of 49 sub-Saharan Africa countries, albeit together they represent 71% of the total population in the region, with most studies based on convenience samples of patients. Second, by definition, the large numbers of patients with breast cancer in the region who never reach a health-care facility could not be included. Dickens and colleagues64 showed that distance to a tertiary care facility was a major determinant of access to diagnosis even within a relatively small geographical area (ie, Soweto in Johannesburg, South Africa). Because the patients included in this review are, by definition, patients who were able to reach a health-care facility, predominantly tertiary centres, they might not be a representative sample of all patients with breast cancer in sub-Saharan Africa. Third, some participants might have been included in more than one study; to minimise this, whenever papers from the same institution and recruitment period were identified, we only included the paper that had the more comprehensive information on stage at diagnosis. Fourth, six potentially eligible papers could not be retrieved; the sample sizes for two of these papers are known to be small, and therefore their exclusion is not likely to have substantially affected our findings. Finally, the absence of information on staging methods and procedures in many studies and the absence of standardisation in staging procedures between studies, and possibly even within studies, might have obscured some of the findings. Staging is affected by neoadjuvant chemotherapy, but this treatment is not available in most sub-Saharan Africa settings.9 Neoadjuvant chemotherapy was mentioned in only two papers included in this review,14,70 and whether staging was ascertained before or after chemotherapy was not clear.
This review showed that the percentage of late-stage breast cancer at diagnosis in black populations from sub-Saharan Africa around 2010 was higher than in black and white populations in the USA 40 years previously. Cancer control strategies in the region should target early detection and diagnosis of symptomatic disease as one essential component of the strategy to improve survival from breast cancer. In most settings, symptom duration of 8–12 months shows that there is a considerable delay between symptom onset and diagnosis and thus a considerable time window exists in which to realistically achieve early detection and diagnosis. Population-level interventions for the stage migration of breast cancer have been shown to be successful in Tanzania71 and other LMICs, such as Malaysia.72 Several sub-Saharan Africa studies have shown improved survival rates in women diagnosed at earlier stages,6,19 which shows that early diagnosis coupled with timely and appropriate treatment can prevent deaths from this disease in this region.
Supplementary Material
Research in context.
Evidence before this study
We preliminarily searched MEDLINE with the terms Breast “Cancer” OR “Breast Carcinoma” AND “Stage” AND “Diagnosis” or “presentation” AND “Africa” OR “Sub-Saharan Africa”. No language restrictions were used. Previous studies have reported a wide variation in stage at diagnosis of breast cancer across sub-Saharan Africa, but none has examined trends in stage at diagnosis over time or investigated potential sources of variations across the region.
Added value of this study
We provide the most comprehensive synthesis to date of the available evidence on stage at diagnosis of breast cancer in sub-Saharan Africa. This review showed that most patients in sub-Saharan Africa were diagnosed at a late stage (stages III/IV). There was, however, a wide range of estimates across the region; the reasons for which were unclear. The percentage of women with late-stage disease at diagnosis was, as expected, higher in black women than non-black women; however, no clear differences exist in black women by region or type of health facility, except that the percentage was lower in urban settings than in rural or urban areas. This review also highlights the paucity of published data on breast cancer stage from certain parts of the region (eg, from middle Africa).
Implications of all the available evidence
Although some improvements in stage at diagnosis of breast cancer in sub-Saharan Africa have occurred over the past few decades, very advanced disease is still prevalent at diagnosis in many settings. Nevertheless, within the region, public-sector settings exist with a much improved stage profile, indicating that stage migration is achievable in such settings—ie, in the absence of organised screening. To prevent avoidable deaths from this potentially good-prognosis cancer, breast cancer control measures require a strong emphasis on early diagnosis and treatment. Earlier diagnosis is dependent on the time window in which the patient has symptomatic disease; thus efforts to promote early presentation and faster referrals, diagnosis, and treatment need strengthening.
Acknowledgments
We thank the Training Program in Nigeria for Non-Communicable Diseases Research (TRAPING-NCD; Fogarty International Center of the National Institutes of Health D43TW009106-CA) for providing funding support for EJ-A’s PhD research work at the London School of Hygiene & Tropical Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center of the National Institutes of Health.
Footnotes
Contributors
EJ-A extracted data, analysed data, drafted the manuscript, and made subsequent revisions to the manuscript. VM had the idea for the study, extracted data, analysed data, and provided critical revisions to the report. CA provided critical revisions to the manuscript. Id-S-S had the idea for the study, extracted data, supervised data extraction and analysis, and revised the manuscript.
Declaration of interests
We declare no competing interests.
See Online for appendix
For more on Africa-Wide Information see https://www.ebscohost.com/academic/africa-wide-information
For more on the change in staging classification see http://seer.cancer.gov/seerstat/variables/seer/yr1973_2009/lrd_stage/index.html
Contributor Information
Elima Jedy-Agba, Department of Non-communicable Disease Epidemiology, London School of Hygiene & Tropical Medicine, London, UK; Institute of Human Virology, Abuja, Nigeria.
Valerie McCormack, Section of Environment and Radiation, International Agency for Research on Cancer, Lyon, France.
Prof Clement Adebamowo, Department of Epidemiology and Public Health, University of Maryland Marlene and Stewart Greenebaum Comprehensivew Cancer Center, Baltimore, MD, USA; Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD, USA.
Prof Isabel dos-Santos-Silva, Department of Non-communicable Disease Epidemiology, London School of Hygiene & Tropical Medicine, London, UK.
References
- 1.Akarolo-Anthony SN, Ogundiran TO, Adebamowo CA. Emerging breast cancer epidemic: Evidence from Africa. Breast Cancer Res. 2010;12(suppl 4):S8. doi: 10.1186/bcr2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle P, Autier P, Adebamowo C, et al. World Breast Cancer Report. Lyon: International Prevention Research Institute; 2012. [Google Scholar]
- 3.International Agency for Research on Cancer. GLOBOCAN. 2012 http://globocan.iarc.fr/Default.aspx (accessed Aug 13, 2015)
- 4.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kene TS, Odigie VI, Yusufu LM, Yusuf BO, Shehu SM, Kase JT. Pattern of presentation and survival of breast cancer in a teaching hospital in north Western Nigeria. Oman Med J. 2010;25:104–07. doi: 10.5001/omj.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantelhardt EJ, Zerche P, Mathewos A, et al. Breast cancer survival in Ethiopia: a cohort study of 1070 women. Int J Cancer. 2014;135:702–09. doi: 10.1002/ijc.28691. [DOI] [PubMed] [Google Scholar]
- 7.Gakwaya A, Kigula-Mugambe JB, Kavuma A, et al. Cancer of the breast: 5-year survival in a tertiary hospital in Uganda. Br J Cancer. 2008;99:63–67. doi: 10.1038/sj.bjc.6604435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantelhardt EJ, Zerche P, Trocchi P, et al. Breast cancer in sub-Saharan Africa: 1,000 patients with primary breast cancer in Addis Ababa followed for up to 5 years. J Clin Oncol. 2012;30(suppl):580. [Google Scholar]
- 9.Mabula JB, McHembe MD, Chalya PL, et al. Stage at diagnosis, clinicopathological and treatment patterns of breast cancer at Bugando Medical Centre in north-western Tanzania. Tanzan J Health Res. 2012;14:269–79. [PubMed] [Google Scholar]
- 10.Okobia MN, Osime U. Clinicopathological study of carcinoma of the breast in Benin City. Afr J Reprod Health. 2001;5:56–62. [PubMed] [Google Scholar]
- 11.Pearson JB. Carcinoma of the breast in Nigeria. A review of 100 patients. Br J Cancer. 1963;17:559–65. doi: 10.1038/bjc.1963.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winters Z, Mannell A, Esser JD. Breast cancer in black South Africans. S Afr J Surg. 1988;26:69–70. [PubMed] [Google Scholar]
- 13.Gebremedhin A, Shamebo M. Clinical profile of Ethiopian patients with breast cancer. East Afr Med J. 1998;75:640–43. [PubMed] [Google Scholar]
- 14.Basro S, Apffelstaedt JP. Breast cancer in young women in a limited-resource environment. World J Surg. 2010;34:1427–33. doi: 10.1007/s00268-009-0299-5. [DOI] [PubMed] [Google Scholar]
- 15.Ntekim A, Nufu FT, Campbell OB. Breast cancer in young women in Ibadan, Nigeria. Afr Health Sci. 2009;9:242–46. [PMC free article] [PubMed] [Google Scholar]
- 16.Anyanwu SN. Survival following treatment of primary breast cancer in eastern Nigeria. East Afr Med J. 2000;77:539–43. [PubMed] [Google Scholar]
- 17.Ezeome ER. Delays in presentation and treatment of breast cancer in Enugu, Nigeria. Niger J Clin Pract. 2010;13:311–16. [PubMed] [Google Scholar]
- 18.McCormack VA, Joffe M, van den Berg E, et al. Breast cancer receptor status and stage at diagnosis in over 1200 consecutive public hospital patients in Soweto, South Africa: a case series. Breast Cancer Res. 2013;15:R84. doi: 10.1186/bcr3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galukande M, Wabinga H, Mirembe F. Breast cancer survival experiences at a tertiary hospital in sub-Saharan Africa: a cohort study. World J Surg Oncol. 2015;13:220. doi: 10.1186/s12957-015-0632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United Nations Statistics Division. United Nations methods and classification: composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings. http://unstats.un.org/unsd/methods/m49/m49regin.htm (accessed April 11, 2016)
- 21.Eng A, McCormack V, dos-Santos-Silva I. Receptor-defined subtypes of breast cancer in indigenous populations in Africa: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001720. doi: 10.1371/journal.pmed.1001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Cancer Institute. Surveillance Epidemiology and End Results Program (SEER) SEER data, 1973–2013. http://seer.cancer.gov/data/ (accessed Aug 16, 2015)
- 24.Asumanu EVR, Naaeder SB. Pattern of breast disease in Ghana. Ghana Med J. 2000;34:206–10. [Google Scholar]
- 25.Quartey-Papafio JB, Anim JT. Cancer of the breast in Accra. Ghana Med J. 1980;19:159–62. [Google Scholar]
- 26.Edmund DM, Naaeder SB, Tettey Y, Gyasi RK. Breast cancer in Ghanaian women: what has changed? Am J Clin Pathol. 2013;140:97–102. doi: 10.1309/AJCPW7TZLS3BFFIU. [DOI] [PubMed] [Google Scholar]
- 27.Hassan I, Muhammed I, Attah MM, Mabogunje O. Breast cancer during pregnancy and lactation in Zaria, Nigeria. East Afr Med J. 1995;72:280–82. [PubMed] [Google Scholar]
- 28.Dansey RD, Hessel PA, Browde S, et al. Lack of a significant independent effect of race on survival in breast cancer. Cancer. 1988;61:1908–12. doi: 10.1002/1097-0142(19880501)61:9<1908::aid-cncr2820610931>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 29.Hacking EA, Dent DM, Gudgeon CA. Malignant tumours of the breast. Frequency distribution by age, race and stage at Groote Schuur Hospital, Cape Town, 1971–1981. S Afr Med J. 1984;65:323–24. [PubMed] [Google Scholar]
- 30.Pegoraro RJ, Nirmul D, Bryer JV. Clinical patterns of presentation of breast cancer in women of different racial groups in South Africa. S Afr Med J. 1985;68:808–10. [PubMed] [Google Scholar]
- 31.Alatise OI, Schrauzer GN. Lead exposure: a contributing cause of the current breast cancer epidemic in Nigerian women. Biol Trace Elem Res. 2010;136:127–39. doi: 10.1007/s12011-010-8608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehinto DKMS, Houngbe F, Padonou N. Traitement du cancer du sein chez la femme. Med Afr Noire. 2007;54:277–85. [Google Scholar]
- 33.Harouna YD, Boukary I, Kanou HM, et al. Le cancer du sein de la femme au Niger: epidemiologie et clinique a propos de 146 cas. Med Afr Noire. 2002;49:39–43. [Google Scholar]
- 34.American Joint Committee on Cancer. Breast cancer staging. https://cancerstaging.org/references-tools/quickreferences/Documents/BreastMedium.pdf (accessed March 31, 2016)
- 35.Ly M, Antoine M, Dembele AK, et al. High incidence of triple-negative tumors in sub-saharan Africa: a prospective study of breast cancer characteristics and risk factors in Malian women seen in a Bamako university hospital. Oncol. 2012;83:257–63. doi: 10.1159/000341541. [DOI] [PubMed] [Google Scholar]
- 36.Pegoraro RJ, Soutter WP, Joubert SM, Nirmul D, Bryer JV. Nuclear and cytoplasmic oestrogen receptors in human mammary carcinoma. S Afr Med J. 1980;58:807–13. [PubMed] [Google Scholar]
- 37.Rafaramino F, Rakotobe P, Pignon T. Management of breast cancer in Madagascar. Cancer Radiother. 2001;5:445–51. doi: 10.1016/s1278-3218(01)00115-9. [DOI] [PubMed] [Google Scholar]
- 38.Muguti GI. Experience with breast cancer in Zimbabwe. J R Coll Surg Edinb. 1993;38:75–78. [PubMed] [Google Scholar]
- 39.Walker AR, Walker BF, Tshabalala EN, Isaacson C, Segal I. Low survival of South African urban black women with breast cancer. Br J Cancer. 1984;49:241–44. doi: 10.1038/bjc.1984.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker ARP, Adam FI, Walker BF. Breast cancer in black African women: a changing situation. J R Soc Promot Health. 2004;124:81–85. doi: 10.1177/146642400412400212. [DOI] [PubMed] [Google Scholar]
- 41.Walker AR, Walker BF, Funani S, Walker AJ. Characteristics of black women with breast cancer in Soweto, South Africa. Cancer J. 1989;2:316–19. [Google Scholar]
- 42.Adebamowo CA, Adekunle OO. Case-controlled study of the epidemiological risk factors for breast cancer in Nigeria. Br J Surg. 1999;86:665–68. doi: 10.1046/j.1365-2168.1999.01117.x. [DOI] [PubMed] [Google Scholar]
- 43.Chiedozi LC. Breast-cancer in Nigeria. Cancer. 1985;55:653–57. doi: 10.1002/1097-0142(19850201)55:3<653::aid-cncr2820550330>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 44.Ihekwaba FN. Breast cancer in Nigerian women. Br J Surg. 1992;79:771–75. doi: 10.1002/bjs.1800790819. [DOI] [PubMed] [Google Scholar]
- 45.Ersumo T. Breast cancer in an Ethiopian population, Addis Ababa. East Cent Afr J Surg. 2006;11:81–86. [Google Scholar]
- 46.Ojara EA. Carcinoma of the male breast in Mulago Hospital, Kampala. East Afr Med J. 1978;55:489–91. [PubMed] [Google Scholar]
- 47.Pignon T, Ratsiaharovalala JJ, Randrianandriana S, et al. Breast diseases in women of 35 years of age, or younger, in Madagascar—clinical and prognostic analysis. J Eur Radiother. 1988;9:121–29. [Google Scholar]
- 48.Odendaal JdV, Apffelstaedt JP. Limited surgery and tamoxifen in the treatment of elderly breast cancer patients. World J Surg. 2003;27:125–29. doi: 10.1007/s00268-002-6530-2. [DOI] [PubMed] [Google Scholar]
- 49.Wasserman LJ, Apffelstaedt JP, Odendaal JV. Conservative management of breast cancer in the elderly in a developing country. World J Surg Oncol. 2007;5:108. doi: 10.1186/1477-7819-5-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ariad S, Seymour L, Bezwoda WR. Platelet-derived growth factor [PDGF] in plasma of breast cancer patients: correlation with stage and rate of progression. Breast Cancer Res Treat. 1991;20:11–17. doi: 10.1007/BF01833352. [DOI] [PubMed] [Google Scholar]
- 51.Hoffman M, de Pinho H, Cooper D, et al. Breast cancer incidence and determinants of cancer stage in the Western Cape. S Afr Med J. 2000;90:1212–16. [PubMed] [Google Scholar]
- 52.Ostyn C, Spector I, Bremner CG. Malignant breast tumours at Coronation Hospital, Johannesburg. A 10-year review. S Afr Med J. 1987;72:528–29. [PubMed] [Google Scholar]
- 53.du Toit RS, van Rensburg PS, Goedhals L. Paget’s disease of the breast. S Afr Med J. 1988;73:95–97. [PubMed] [Google Scholar]
- 54.Ajekigbe AT. Fear of mastectomy: the most common factor responsible for late presentation of carcinoma of the breast in Nigeria. Clin Oncol (R Coll Radiol) 1991;3:78–80. doi: 10.1016/s0936-6555(05)81167-7. [DOI] [PubMed] [Google Scholar]
- 55.Amir H, Azizi MR, Makwaya CK, Jessani S. TNM classification and breast cancer in an African population: a descriptive study. Cent Afr J Med. 1997;43:357–59. [PubMed] [Google Scholar]
- 56.Anyanwu SN. Temporal trends in breast cancer presentation in the third world. J Exp Clin Cancer Res. 2008;27:17. doi: 10.1186/1756-9966-27-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farooqi B, Smith B, Chowdhary M, Pavoni S, Modi A, Schnell F. Racial disparities in breast cancer diagnosis in Central Georgia in the United States. J Community Support Oncol. 2015;13:436–41. doi: 10.12788/jcso.0179. [DOI] [PubMed] [Google Scholar]
- 58.Harirchi I, Karbakhsh M, Montazeri A, et al. Decreasing trend of tumor size and downstaging in breast cancer in Iran: results of a 15-year study. Eur J Cancer Prev. 2010;19:126–30. doi: 10.1097/CEJ.0b013e328333d0b3. [DOI] [PubMed] [Google Scholar]
- 59.Harirchi I, Kolahdoozan S, Karbakhsh M, et al. Twenty years of breast cancer in Iran: downstaging without a formal screening program. Ann Oncol. 2011;22:93–97. doi: 10.1093/annonc/mdq303. [DOI] [PubMed] [Google Scholar]
- 60.Akhigbe AO, Omuemu VO. Knowledge, attitudes and practice of breast cancer screening among female health workers in a Nigerian urban city. BMC Cancer. 2009;9:203. doi: 10.1186/1471-2407-9-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moodley J, Cairncross L, Naiker T, Momberg M. Understanding pathways to breast cancer diagnosis among women in the Western Cape Province, South Africa: a qualitative study. BMJ Open. 2016;6:e009905. doi: 10.1136/bmjopen-2015-009905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tetteh DA, Faulkner SL. Sociocultural factors and breast cancer in sub-Saharan Africa: implications for diagnosis and management. Womens Health (Lond) 2016;12:147–56. doi: 10.2217/whe.15.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pruitt L, Mumuni T, Raikhel E, et al. Social barriers to diagnosis and treatment of breast cancer in patients presenting at a teaching hospital in Ibadan, Nigeria. Glob Public Health. 2015;10:331–44. doi: 10.1080/17441692.2014.974649. [DOI] [PubMed] [Google Scholar]
- 64.Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: a South African public hospital case series of over 1000 women. Int J Cancer. 2014;135:2173–82. doi: 10.1002/ijc.28861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohene-Yeboah M, Adjei E. Breast cancer in Kumasi, Ghana. Ghana Med J. 2012;46:8–13. [PMC free article] [PubMed] [Google Scholar]
- 66.Okobia M, Bunker C, Zmuda J, et al. Case–control study of risk factors for breast cancer in Nigerian women. Int J Cancer. 2006;119:2179–85. doi: 10.1002/ijc.22102. [DOI] [PubMed] [Google Scholar]
- 67.Zelle SG, Nyarko KM, Bosu WK, et al. Costs, effects and cost-effectiveness of breast cancer control in Ghana. Trop Med Int Health. 2012;17:1031–43. doi: 10.1111/j.1365-3156.2012.03021.x. [DOI] [PubMed] [Google Scholar]
- 68.Adisa AO, Arowolo OA, Akinkuolie AA, et al. Metastatic breast cancer in a Nigerian tertiary hospital. Afr Health Sci. 2011;11:279–84. [PMC free article] [PubMed] [Google Scholar]
- 69.American Cancer Society. History of cancer screening and early detection. http://www.cancer.org/cancer/cancerbasics/thehistoryofcancer/the-history-of-cancer-cancer-screening-and-early-detection (accessed Oct 20, 2016)
- 70.Clegg-Lamptey J, Dakubo J, Attobra YN. Why do breast cancer patients report late or abscond during treatment in Ghana? A pilot study. Ghana Med J. 2009;43:127–31. [PMC free article] [PubMed] [Google Scholar]
- 71.Ngoma T, Mandeli J, Holland JF. Downstaging cancer in rural Africa. Int J Cancer. 2015;136:2875–79. doi: 10.1002/ijc.29348. [DOI] [PubMed] [Google Scholar]
- 72.Devi BC, Tang TS, Corbex M. Reducing by half the percentage of late-stage presentation for breast and cervix cancer over 4 years: a pilot study of clinical downstaging in Sarawak, Malaysia. Ann Oncol. 2007;18:1172–76. doi: 10.1093/annonc/mdm105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


