Abstract
South Africa has been engaged in pharmacovigilance (PV) activities to assess the impact of adverse drug reactions on public safety and health for 40 years. Activities have evolved from passive regulatory reporting to encompass active surveillance systems. The HIV and AIDS and TB epidemics stimulated pharmacoepidemiological research into the risks associated with medicines used in the standardised regimens of mass treatment programmes. Specific safety concerns, supported by robust local cohort data, have prompted major changes to national and international treatment policies.
This chapter describes the expanding body of local knowledge and the historical and emergent surveillance systems that address the burden of drug-related harms, noting the challenges to health system responsiveness. The South African context presents a unique opportunity to characterise the scale and nature of such harms in mass HIV and AIDS and TB treatment programmes. The use of complex regimens at scale poses new PV challenges. There is an urgent need to develop cohesive, sustainable systems to support evidence-based decisions on appropriate regimen choices, while minimising medicine-associated risks. The increasing use of computerised clinical, laboratory and dispensing records, with unique patient identifiers facilitating data linkage, will increase PV surveillance capacity.
A coherent national PV framework is an essential part of medicines policy, encompassing regulatory, programmatic and individual needs. Key pillars of this framework include: (i) consolidation and expansion of active and passive PV surveillance, optimising existing programmes; (ii) prioritising post-marketing monitoring within the new health products regulatory authority; and (iii) instilling a culture of active risk management in clinical practice through the creation of effective channels of communication and feedback into policy and practice.
Introduction
The use of medicines is unavoidable. We are exposed to medication even before birth, and exposure increases in frequency and variety until death. The system supporting the development, manufacture, regulation, marketing and use of these medicines is vast and influenced by complex social, environmental, financial and political factors at local and global levels.
A medication can be summarised in terms of its benefits, risks, and quality. In the modern era, preclinical and clinical trials are conducted under highly regulated conditions to identify the benefits of a candidate product as well as the major and common side-effects. Only once the benefits have been shown to outweigh the harms under clinical trial conditions, is the product licensed by a regulatory agency. Once marketed, medicines are rarely used in the specific, controlled conditions of the clinical trial. The frequency and severity of side-effects may be very different in the post-marketing phase when a medicine is used for longer periods of time in a heterogeneous patient population with a range of co-morbidities and concomitant medication, and for off-label indications. Detection of rare side-effects requires large sample sizes, so medicines must be monitored for performance throughout their lifespan.
Pharmacovigilance (PV) refers to the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem.1 The goal of PV is to optimise benefits and minimise risks, at the individual and population level. Responsibility should be shared by the pharmaceutical industry, drug regulators, health professionals, patients and the public.
Pharmacovigilance has evolved considerably over the last 20 years. Initially the primary focus was regulatory: identifying (diagnosing, reporting) signals1 of new or previously poorly described adverse drug reactions (ADRs) for registered medicines. There is increasing recognition of the importance of quantifying event rates and severity for known ADRs, as this may differ from pre-marketing incidence. Post-marketing research is needed that includes robust denominator data and methods that can identify risk factors and quantify incidence. Aligned with global evolution, PV in South Africa has expanded into a comprehensive science that links post-marketing activities with the pre-marketing process of drug development, and quantifies the risks and public health impact of medicines using more robust approaches such as cohort studies and registries. In South Africa, synergies between PV and the related activities of disease surveillance and health system strengthening have resulted in the recognition of PV as a critical public health discipline requiring integration into all aspects of health care. On the global stage, South Africa regularly contributes data, policies and expertise to the World Health Organization (WHO) International Drug Monitoring Programme and various vertical programme-driven initiatives co-ordinated by the WHO.
The focus of this review is on orthodox medicines used by humans, and methods to assess the direct impact of such medicines on human health; the review does not refer to environmental or indirect exposures, medical devices, complementary medicines or illicit drugs. We describe the evolution and scope of PV in South Africa and motivate for strengthening of this discipline as an essential and functional tool to improve patient care, clinical practice and public health.
An historical overview of pharmacovigilance in South Africa
A series of catastrophes, including the 1962 thalidomide disaster, were catalysts for the development of PV as a discipline (thalidomide was marketed as a sedative and anti-emetic in pregnancy and caused severe birth defects). It was internationally acknowledged that government intervention was required to regulate the manufacture and sale of medicines in order to ensure standards of safety, efficacy and quality. The Medicines and Related Substances Control Act 101 was promulgated in South Africa in 1965. In 1987, the National Adverse Drug Event Monitoring Centre (NADEMC), a unit of the Medicines Control Council (MCC), was established to facilitate ADR monitoring. The NADEMC managed the collection and review of voluntarily submitted ADR reports from health professionals, to detect signals of unknown or poorly understood ADRs, and South Africa became the first African member of the WHO International Drug Monitoring Programme in 1992.
The adverse events following immunization (AEFI) targeted spontaneous reporting (TSR) system of the expanded programme for immunization (EPI) was established in 1998 with strong links to the NADEMC. Targeted spontaneous reporting solicits reports of specific, pre-defined serious events for a group or groups of medicines and/or patient groups.
In 2003, ADR reporting guidelines for the pharmaceutical industry were issued by the MCC; the guidelines aimed to improve the quality and quantity of reports submitted and encourage a proactive approach to safety monitoring. During the same year, the PV expert committee of the MCC was constituted to advise the MCC on post-marketing safety issues. In 2003, the national antiretroviral (ARV) treatment programme was launched, with government-funded ARVs becoming accessible to thousands of patients. Integral to the roll-out was strengthening of the national spontaneous reporting system and implementation of “focused surveillance and novel pharmacovigilance methods for addressing key research questions”.2 Targeted spontaneous reporting systems for ADRs in patients on ARVs were established within provincial ARV programmes.3
National awareness grew around the importance of reporting ADRs, particularly reactions to ARVs, resulting in increased reporting rates. There was a parallel increase in studies examining the effects of ADRs on adherence and regimen substitutions4 as well as the impact of HIV on the risk of ADRs to vaccines and TB medicines.5,6 In 2011, the South African National Department of Health (NDoH) programmatic PV unit reported on its decentralised system, a TSR system for ARVs and TB medicines aimed at using ADR reports as a clinical tool to improve ARV and TB medicine use.7
In 2012, reports of maternal deaths caused by serious nevirapine (NVP)-induced ADRs raised concerns about the safety of ARVs in pregnancy and prompted the NDoH to change first-line ARVs in pregnant women from a NVP- to an efavirenz (EFV)-based regimen.8 Pregnancy safety concerns prompted the NDoH to pilot a national pregnancy exposure registry and birth defect surveillance system (PER/BDS) in eThekwini District, KwaZulu-Natal (KZN) in 2013, to monitor the safety of all medicines commonly used by pregnant women.9
Current pharmacovigilance systems and research in South Africa
The HIV and AIDS epidemic has generated tremendous advances in the science of PV in South Africa, and an appreciation of the impact of drug-related morbidity and mortality on adherence, treatment policies, health systems and public health.
Pharmacotherapy is a key intervention in public health programmes and the mainstay of clinical practice. In South Africa, PV initiatives are largely driven by three key stakeholders: regulators and the pharmaceutical industry (focused on products); public health programmes (focused on systems); and healthcare providers and clinicians (focused on patients). While each of these groups works to minimise drug-related harm and improve patient outcomes, they have different immediate objectives, capacities and tools at their disposal to investigate and respond to safety issues.
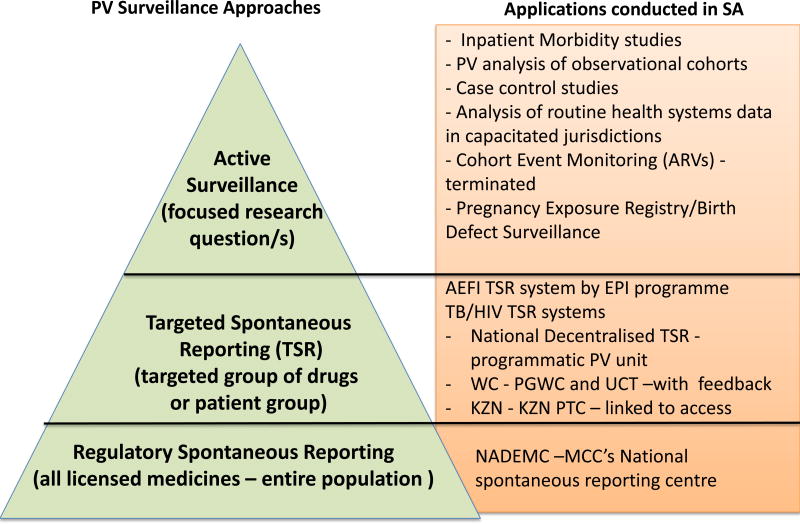
In certain cases or for certain medicines or patient populations, a more targeted and/or active surveillance system is required to augment the MCC’s spontaneous reporting system. Targeted spontaneous reporting systems that solicit more specific information are used to monitor the safety of vaccines and HIV and TB medicines because of their widespread use and the potential impact of real or perceived drug safety problems on the viability of disease management programmes in which they are employed. In cases where a more focused safety question needs to be answered, active surveillance and/or research activities are undertaken. For instance, inpatient morbidity and mortality studies have been conducted at South African hospitals to determine the burden of ADRs on medical ward admissions and deaths. Similarly, case control and cohort studies have been conducted to assess the safety of ARVs used as first-line treatment in the public sector.
1. Regulatory pharmacovigilance
Passive surveillance
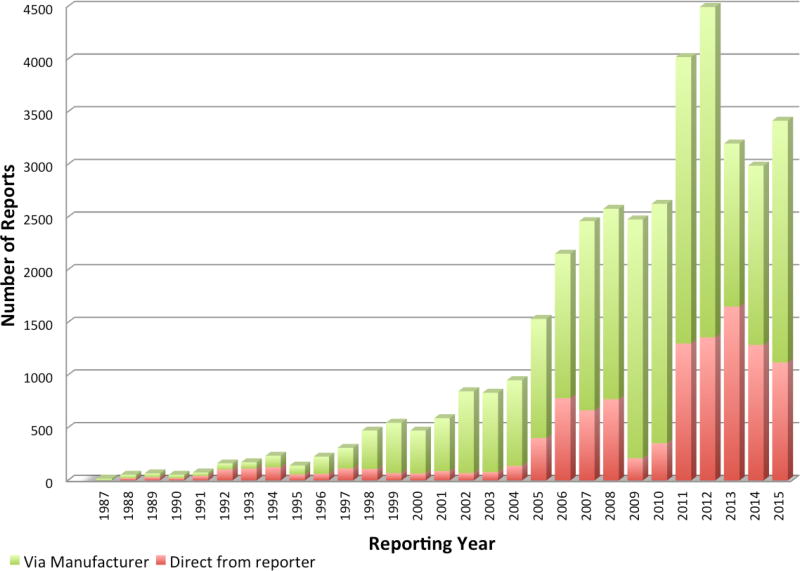
Spontaneous reporting of ADRs to the NADEMC by health professionals remains the cornerstone of local medicines safety data collection. While reporting rates are still extremely low, they have increased over time with approximately 62 reports/million capita received in 2015. Despite the surge in reporting rates after the introduction of public sector ARVs after 2003, many of the reports submitted directly to the programmatic PV systems were not incorporated into the NADEMC database.10 Unlike licensing, post-marketing PV generates no direct income for the regulator, and the discipline has suffered from resource constraints, limiting capacity for analysis, feedback and expansion.
Passive surveillance systems such as spontaneous reporting (including TSR) are useful in identifying new signals or ADR trends, but they are unable to quantify the risk of a particular harm. They cannot quantify ADR incidence or identify risk factors in the absence of a reliable background event rate in the unexposed population or other comparator groups. Therefore passive systems need to be augmented with active surveillance approaches.
Active surveillance
In well-resourced settings, pharmaceutical manufacturers are now required to submit risk management plans (RMPs), which includes a commitment to conduct post-marketing studies as part of their registration dossiers at the time of licence application. Similar requirements for RMPs are being introduced in South Africa with regard to new medicines and expanded or new indications for already-registered products (e.g. the use of tenofovir for pre-exposure prophylaxis). In addition to local spontaneous reports, the MCC routinely reviews warnings issued by other regulators, post-marketing safety studies published in the literature, media reports, and unpublished data from pharmaceutical manufacturers.
Despite its limitations, spontaneous ADR reporting does provide an opportunity for regulators to interact directly with healthcare providers. This opportunity should be exploited, both to encourage reporting and to improve clinical case management individually and collectively.
It is envisaged that in 2017 a new parastatal agency, the South African Health Products Regulatory Authority (SAHPRA) will replace the MCC. Regulations have been updated and a new infrastructure will be developed. This restructuring represents the opportunity to prioritise PV as a well-resourced, successful regulatory function of the new organisation. There is international recognition of the need to strengthen and prioritise post-marketing PV activities, while streamlining the licensing process through greater reliance on assessment reports written by well-resourced, mature regulatory authorities with greater capacity than South Africa for dossier review rather than conducting the entire dossier review afresh locally at time of licensing. This approach could reduce the registration time for novel and essential medicines; improve monitoring and evaluation of already-marketed products; and adapt decisions for local conditions.
In order to adopt a risk-based approach that focuses on patient safety at the individual and population levels, South African regulators need to expand their PV resources considerably; they must develop active surveillance capacity by co-opting the local research community, the pharmaceutical industry, medical aid programmes and hospitals to provide critical data for monitoring and risk assessment of registered products. However, careful consideration must be given to which international approaches are relevant and how these could be adapted to the South African context.
Regardless of the approach, local research unequivocally demonstrates the need to strengthen spontaneous reporting by training health professionals on detection and reporting of ADRs; the provision of reliable therapeutic advice in real time such as that provided nationally by the Medicines Information Centre (MIC) at the University of Cape Town (UCT); and individual and collective feedback and communication of ADR reports and other PV data.11,12 This will enhance public support for drug safety surveillance and optimise the benefits of ADR reports on patient care and public health.
2. Programmatic and clinical pharmacovigilance systems and research
Passive surveillance
Vaccinations administered to infants and children have shown proven efficacy in reducing the incidence of common childhood infections responsible for much paediatric morbidity and mortality in the past. Other high-risks groups also benefit from immunization requiring enhanced AEFI surveillance, e.g. influenza vaccines in pregnant women and the elderly. In these examples, events may be incorrectly attributed to the vaccine because of a temporal relationship between the administration of the vaccine and the clinical signs. Serious or potentially vaccine-related AEFIs are submitted routinely to the NADEMC, while programmatic errors are investigated and managed within the EPI programme. Here too, the system needs to be strengthened in terms of data analysis and feedback. In particular, a multidisciplinary, independent AEFI causality assessment committee should be established to review serious reports promptly and assess the relationship (causal, contributory or coincidental) between the event and the vaccine(s), enabling timeous and decisive response.
In 2005, a TSR reporting system for patients on ARVs was implemented in the Western Cape through a partnership between the provincial government and UCT’s MIC. The system has expanded to include ADRs to TB treatment, and more recently it has also encouraged reporting on all medicines. Quarterly newsletters summarising the data and including important case studies and ‘learning points’ are circulated. Potential prescribing errors are addressed through direct feedback to the reporting clinician.
In 2004, KZN’s PV committee implemented a mandatory reporting system requiring clinicians to submit an ADR report when toxicity prompted changes in ARV treatment regimens. The system elicited 3 923 reports in its first year (2007), providing useful information on the drugs commonly implicated in ADRs and necessitating treatment substitution.13 The programme instituted a culture of reporting where none had existed previously. However, the barriers to reporting faced by clinicians in resource-limited settings pose a challenge to the success of a mandatory reporting programme that is linked to treatment access.
In order to develop a responsive, clinically valuable PV system for ARV/TB medicines, the NDoH’s programmatic PV unit piloted a decentralised TSR system of multidisciplinary PV clusters at district level. This approach involves the submission of ADR reports to the national unit and routine review for causality and preventability by a multidisciplinary team, with a strong focus on feedback provided by both the local review team and the national unit.7,14 This system is being rolled out in all provinces. Challenges remain, and the sustainability and value of this model will need to be assessed. During an initial analysis, 48% of reports were found to be of poor quality and unevaluable.7 A subsequent analysis found that 41% had to be excluded due to poor quality or because the reports related to ARV inefficacy, not toxicity, or to medicines not included in the programme.14 Moreover, the confusion arising from the need to complete two forms – for both programmatic and regulatory reporting – has tended to undermine efforts to streamline ADR reporting. These findings reflect the need for better co-ordination between the regulatory and programmatic surveillance systems, and highlight again the importance of ongoing training, support and feedback. Efforts are currently underway to harmonise the PV systems, ensuring clear allocation of roles and responsibilities within each programme and appropriate sharing of data. Electronic reporting tools using mobile phones and computers, and integrated into other e-health applications, offer new development and streamlining opportunities.
Active surveillance (studies, cohorts and registries)
Weak healthcare systems, poorly resourced regulatory authorities, permeable geographical borders allowing a growing trade in substandard and counterfeit medicines, and complex and varied cultures of drug use and sharing, all contribute to drug-related morbidity and mortality in Africa. In addition, individual health can be affected by poor nutrition, HIV, TB and malaria, which alter the physiological response to medications and require sophisticated and often erratically accessible drug combinations. Policy makers need to respond to these challenges through the provision of sound local data to quantify the size and severity of ADR-related problems and to identify the extent to which they are preventable – and if so, how. Data from passive surveillance systems are not designed to achieve this.
Some progress has been made locally in quantifying the burden of serious ADRs. These data are derived from (i) morbidity studies in inpatient settings; (ii) PV-related data from disease-specific observational cohorts; (iii) analysis of routine service data using record linkage approaches; and (iv) development of patient registries.
Inpatient morbidity studies
The profile of ADRs and their impact on public health varies across settings,15 and the nature and frequency of ADRs in South Africa differs significantly from other countries, being influenced by the population structure, burden of disease and the risk profiles of commonly used drugs.16,17
A cross-sectional survey conducted in 2013 reviewed adult medical admissions at four geographically diverse South African hospitals. One hundred and sixty-four of 1 951 medical admissions (8.4%) were the direct result of an ADR: female sex, polypharmacy, comorbidities and ARVs were independent risk factors.16 An earlier study found that 6.3% of 665 medical ward admissions were ADR-related.17 Both studies noted a bimodal age distribution of serious ADRs: those due to drugs used in management of non-communicable diseases in older patients (similar to the pattern seen in high-income countries15), and those due to medicines used in the management of HIV and TB, driven by the burden of these diseases in South Africa. Forty-five per cent of ADRs were classified as preventable: due to inappropriate and excessive prescribing, inadequate therapeutic monitoring, poor adherence and poor knowledge of drug interactions.16 In the 2013 survey, ADRs contributed to death in 2.9% of medical admissions, and 16% of deaths were ADR-related, much higher rates of mortality than reported in high-income countries.18 Tenofovir, rifampicin and co-trimoxazole were most commonly implicated. In contrast, warfarin, non-steroidal anti-inflammatory drugs, heparin, selective serotonin re-uptake inhibitor antidepressants and corticosteroids were most commonly implicated in high-income countries.19 Similar data for other patient groups (paediatric, surgical, psychiatric and cancer patients, and pregnant women) are lacking in South Africa.
These data illustrate the burden of serious ADRs due to ARVs and anti-TB treatment, which are frequently prescribed concomitantly. Healthcare workers lack confidence in their ability to diagnose and manage ADRs11 and 18% of nurses’ queries to the National HIV and TB Health Care Worker Hotline were clinical questions about ADRs.12, 20
Disease-specific cohorts
Observational studies of HIV (case-series,21 case-control22 and cohort studies4,23) have highlighted safety and effectiveness concerns for commonly used ARVs. Notably, data on the safety of stavudine contributed to local and international changes in treatment policy. Subsequent reports assessed the positive impact of these policy changes on patient safety.24, 25 In paediatrics and dermatology, observational research addressed the use of nevirapine and ritonavir (when used as a single active protease inhibitor).26,27 These examples demonstrate the value of well-designed and conducted cohort studies in PV research.
Health service data-mining
In higher-income countries, data mining of large linked databases has contributed significantly to PV. The UK Clinical Practice Research Database (CPRD), a government-led initiative, houses the clinical data of millions of British citizens and has served as a resource for several pivotal PV studies.28 In South Africa, a recent analysis of 56 298 patients in a large private sector disease-management programme database identified reverse transcriptase ARVs (efavirenz, zidovudine and stavudine) as risk factors for incident diabetes.29 Efforts are already underway in South Africa to develop capacity for record linkage using routine health system data.4 This process requires support from database custodians, appropriate investment in the further development of electronic records and registers, and a governance structure that ensures the research conducted is robust, ethical and in the interests of public health.
Registries
In response to concerns about the safety of efavirenz (EFV) in pregnancy, and notwithstanding reassuring global pooled analyses,30 the NDoH programmatic PV unit piloted a pregnancy exposure registry/birth defect surveillance (PER/BDS) programme in 2013. The registry was established as a rolling cohort aimed at understanding the effect that ARVs and other medicines commonly used in pregnancy have on maternal and neonatal outcomes. In the first year, 10 417 pregnancies were assessed, with first trimester exposure to ARVs being the priority. The pilot programme demonstrated the value of the PER/BDS surveillance system as an approach to assess potential associations between exposures to certain medicines over the course of pregnancy and adverse birth outcomes such as stillbirth, preterm delivery, low birth weight, neonatal death and congenital anomalies identified at birth, while providing further reassuring evidence of the safety of the first-line ARV regimen in pregnancy. The Western Cape is currently developing a pregnancy registry that is integrated into and strengthening existing routine clinical data-collection systems.
Robust active surveillance systems in representative populations are often able to supply many of the answers that programmatic PV is designed to deliver but often poorly able to achieve. A sentinel approach to the use of active PV could be an efficient way of limiting programmatic PV interventions to those that have direct value to clinical practice with minimal additional burden on practitioners.
Reflections: Gaps, challenges and opportunities
Over the last 20 years, owing largely to local and international funding of expanded HIV and TB treatment programmes, South Africa has developed a better understanding of the burden of ADRs on both the healthcare system and healthcare consumers. Approximately one in 12 medical ward admissions are due to ADRs, and ADRs account for 16% of deaths among adult medical admissions. Almost half of these deaths are preventable, which indicates the need for research and monitoring to inform and transform clinical practice.
Optimising the safety of medicines to minimise patient harm is a shared responsibility requiring the co-ordinated and complementary efforts of key stakeholders. In August 2012, a multi-stakeholder meeting made recommendations aimed at strengthening national PV; however, no national policy exists.31 The current transition from the MCC to SAHPRA is an opportunity to strengthen and prioritise PV activities nationally and to expand this essential safety net to better monitor the risk of harm, including harms associated with complementary medicines and medical devices. Importantly, this requires a dedicated budget.
In keeping with global trends, PV activities in South Africa are transitioning from reliance on passive surveillance reporting to a more dynamic science involving active surveillance with cohort studies, record-linkage projects and the establishment of patient registries. In South Africa, these active surveillance systems have been largely confined to investigating the effects of HIV and TB medicines, with resultant positive public health interventions. Attention needs to be paid to medicines for non-communicable diseases such as diabetes, hypertension, inflammatory conditions and stroke, as these are the other major contributors of drug-related hospitalisations, particularly among the elderly.32 Technological advances and progress towards an electronic health information system will do much to support the expansion of PV research in both the public and private sectors, largely through data mining of large linked databases and the interrogation of existing patient cohorts. Where present, unique patient identifiers should be exploited to link medicine use and ADRs, including in pregnant women and children. These activities need to be governed by sound ethical and scientific principles, building on what has been learnt in South Africa and elsewhere.
Perhaps the most critical shortcoming of the national PV programme is lack of communication. Healthcare providers who responsibly submit ADR reports to the national reporting centres are rarely given any useful feedback that would give relevance and meaning to the reporting process. Concurrently, strategies for public education and feedback are required, both to enhance individual patient self-reporting of ADRs, and community and media understanding of what is meant by the risk-benefit of medicines. Much can be gained by working with international regulatory agencies, including in Africa, where new centres of excellence in PV are being established.33
Thus far the regulatory and programmatic PV programmes have largely operated in parallel, missing the opportunity to share and benefit from each other’s data and expertise. This has had a detrimental effect on the PV programme and caused confusion around reporting requirements.16 The benefits of provincial pilot programmes have either not been sustained, or have not been expanded to the national programme. While ADR reporting from clinical trials is largely well managed, reports from observational research studies such as demographic platforms or cohort studies also need to be strengthened through advocacy and closer collaboration between researchers and the PV programmes, and the proactive development of a more consultative regulatory framework.
Ideally, existing South African PV systems needs to be assessed critically in terms of their ability to inform treatment policies and patient care and improve outcomes. In January 2016, the European Medicines Agency released its strategy on measuring the impact of PV activities. This document recognises the importance of developing standardised methods for modelling the health impact of PV decisions and activities based on epidemiological parameters such as “population attributable risk, prevalence of exposure, behavioural change data, regimen or drug switching of therapies, etc.). Key data sources for impact studies will include electronic health records, drug prescription, dispensing and utilisation data, and patient registries.”34 South Africa is in the process of building these data systems to facilitate such impact assessments on a large scale.
Conclusion and recommendations
This review highlights the importance of a robust national PV system in order to reduce the significant burden of drug-induced disease, to inform treatment policies with real-world evidence, to improve outcomes of common diseases such as HIV, TB, hypertension and diabetes through optimal therapeutic management, and to ensure the safety of large-scale pharmacotherapeutic interventions such as vaccines, especially when newly introduced.
South Africa needs to develop a cohesive system that builds on the considerable progress already achieved. A considered PV policy framework is recommended that:
promotes consolidation and expansion of active and passive PV surveillance, optimising existing research and surveillance programmes;
prioritises post-marketing monitoring within the regulatory authority; and
instils a culture of active risk management in clinical practice through the creation of effective channels of communication and feedback into policy and practice.
This requires strong political commitment and leadership by senior policymakers, supported by real investment in infrastructure and training. Initiatives must be underpinned by a culture of drug safety awareness in which healthcare providers, patients, manufacturers, and policy makers feel confident in their knowledge of the risks and benefits of the products they promote, prescribe or use. All medicines have side-effects, which vary according to who uses them and how they are used. Understanding this not only alerts patients to potential risks, but importantly reassures patients about the relative safety and therapeutic benefits of medicines and vaccines. Having an effective national PV programme will reinforce patient and community confidence in the health system, while building the science base that supports rational and safe prescription of medicines.
Figure 1.
Number of ADRs reported per annum to the NADEMC (1987–2015)
Figure 2.
Pharmacovigilance approaches conducted in South Africa
Footnotes
A Signal is defined as reported information on a possible causal relationship between an adverse event and a drug, the relationship being unknown or incompletely documented previously. Usually more than a single report is required to generate a signal, depending on the seriousness of the event and the quality of the information.
References
- 1.World Health Organization. Geneva: WHO; 2002. [cited 1 February 2017]. The importance of pharmacovigilance: Safety monitoring of medicinal products. [Internet]. URL: http://apps.who.int/medicinedocs/pdf/s4893e/s4893e.pdf. [Google Scholar]
- 2.South African National Department of Health. Pretoria: South African National Department of Health; 2003. [cited 1 February 2017]. Operational plan for comprehensive HIV and AIDS care, management and treatment for South Africa. [Internet]. URL: https://www.hsph.harvard.edu/population/aids/southafrica.aids.03.pdf. [Google Scholar]
- 3.World Health Organization. Geneva: WHO; 2013. [cited 1 February 2017]. HIV/AIDS programme. WHO technical brief: Surveillance of antiretroviral drug toxicity within antiretroviral treatment programmes. [Internet]. URL: apps.who.int/iris/bitstream/10665/91578/WHO_HIV_2013.124_eng.pdf?ua=1. [Google Scholar]
- 4.Boulle A, Orrell C, Kaplan R, et al. Substitutions due to antiretroviral toxicity or contraindication in the first three years of antiretroviral therapy in a large South African Cohort. Clin Infect Dis. 2007;45(2):254–60. doi: 10.1177/135965350701200508. [DOI] [PubMed] [Google Scholar]
- 5.Hesseling AC, Schaaf HS, Hanekom WA, et al. Danish Bacille Calmette-Guerin vaccine-induced disease in human immunodeficiency virus-infected children. Clin Infec Dis. 2003;37:1226–33. doi: 10.1086/378298. [DOI] [PubMed] [Google Scholar]
- 6.Schutz C, Ismail Z, Proxenos CJ, et al. Burden of antituberculosis and antiretroviral drug-induced liver injury at a secondary hospital in South Africa. S Afr Med J. 2012;102(6):506–11. doi: 10.7196/samj.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dheda M, Distefano K, Sunduzway K, et al. Decentralized HIV/AIDS pharmacovigilance in South Africa: Mpumalanga success and moving forward. J AIDS and HIV Research. 2013;5(9):370–9. [Google Scholar]
- 8.Moran NF. Maternal deaths due to adverse drug reactions to nevirapine-containing HAART: New recommendations for ARV therapy in pregnancy in South Africa. O&G Forum. 2012;22:29–32. [Google Scholar]
- 9.South African National Department of Health. The UBOMI Report: Understanding birth outcomes from mothers and infants: Report on the pilot phase of the national pregnancy exposure registry and birth defect surveillance (PER/BDS) system: A review of the initial 12 months of data and experiences. Pretoria: South African National Department of Health; 2015. [Google Scholar]
- 10.Maigetter K, Pollock AM, Kadam A, et al. Pharmacovigilance in India, Uganda and South Africa with reference to WHO’s minimum requirements. Int J Health Policy Manag. 2015;4(5):295–305. doi: 10.15171/ijhpm.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruud KW, Srinivas SC, Toverud E. Healthcare providers experiences with adverse drug reactions and adherence challenges in antiretroviral therapy of HIV patients in the Eastern Cape Province, South Africa. Eur J Clin Pharmacol. 2012;68:1321–8. doi: 10.1007/s00228-012-1254-1. [DOI] [PubMed] [Google Scholar]
- 12.Swart AM, Chisholm BS, Cohen K, et al. Analysis of queries from nurses to the South African National HIV and TB health care worker hotline. S Afr J HIV Med. 2013;14(4):179–82. [Google Scholar]
- 13.Manickum VK, Suleman F. Evaluating adverse drug reactions among HAART patients in a resource-constrained province of South Africa. Afr J AIDS Research. 2012;11(2):75–81. doi: 10.2989/16085906.2012.698050. [DOI] [PubMed] [Google Scholar]
- 14.Birbal S, Dheda M, Ojewole E, et al. Adverse drug reactions associated with antiretroviral therapy in South Africa. Afr J AIDS Research. 2016;15(3):243–8. doi: 10.2989/16085906.2016.1191519. [DOI] [PubMed] [Google Scholar]
- 15.Wiffen P, Gill M, Edwards J, Moore A. Adverse drug reactions in hospital patients. A systematic review of the prospective and retrospective studies. Bandolier Extra. 2002 Jun;:1–6. [Google Scholar]
- 16.Mouton JP, Njuguna C, Kramer N, et al. Adverse drug reactions causing admission to medical wards: A cross-sectional survey at 4 hospitals in South Africa. Medicine. 2016;95(19):1–9. doi: 10.1097/MD.0000000000003437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta U, Durrheim DN, Blockman M, Kredo T, Gounden R, Barnes KI. Adverse drug reactions in adult medical inpatients in a South African hospital serving a community with a high HIV/AIDS prevalence: prospective observational study. Br J Clin Pharmacol. 2008;65(3):396–406. doi: 10.1111/j.1365-2125.2007.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouton JP, Mehta U, Parrish AG, et al. Mortality from adverse drug reactions in adult medical inpatients at four hospitals in South Africa: a cross-sectional survey. Br J Clin Pharmacol. 2014;80(4):818–26. doi: 10.1111/bcp.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirmohammed M, Meakin JS, Green C, et al. Adverse drug reactions as a cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–9. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Njuguna C, Stewart A, Mouton JP, et al. Adverse drug reactions reported to a national HIV & tuberculosis health care worker hotline in South Africa: Description and prospective follow-up of reports. Drug Saf. 2016;39(2):159–69. doi: 10.1007/s40264-015-0359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stead D, Osler M, Boulle A, et al. Severe hyperlactataemia complicating stavudine first-line antiretroviral therapy in South Africa. Antivir Ther. 2008;13(7):937–43. [PubMed] [Google Scholar]
- 22.Osler M, Stead D, Rebe K, et al. Risk factors for and clinical characteristics of severe hyperlactataemia in patients receiving antiretroviral therapy: a case-control study. HIV Med. 2010;11(2):121–9. doi: 10.1111/j.1468-1293.2009.00754.x. [DOI] [PubMed] [Google Scholar]
- 23.Geddes R, Knight S, Moosa MY, et al. A high incidence of nucleoside reverse transcriptase inhibitor (NRTI)-induced lactic acidosis in HIV-infected patients in a South African context. S Afr Med J. 2006;96(8):722–4. [PubMed] [Google Scholar]
- 24.Njuguna C, Orrell C, Kaplan R, et al. Rates of switching antiretroviral drugs in a primary care service in South Africa before and after introduction of tenofovir. PloS One. 2013;8(5):e63596. doi: 10.1371/journal.pone.0063596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brennan AT, Davies MA, Bor J, et al. Has the phasing out of stavudine in accordance with changes in WHO guidelines led to a decrease in single-drug substitutions in first-line antiretroviral therapy for HIV in sub-Saharan Africa? AIDS. 2017;31(1):147–57. doi: 10.1097/QAD.0000000000001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies MA, Moutrie H, Eley B, et al. Virologic failure and second-line therapy in children in South Africa – the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr. 2011;56(3):270–8. doi: 10.1097/QAI.0b013e3182060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart A, Lehloenya R, Boulle A, et al. Severe antiretroviral-associated reactions in South African patients: a case series and case control analysis. Pharmacoepidemiol Drug Saf. 2016;25(11):1313–9. doi: 10.1002/pds.4067. [DOI] [PubMed] [Google Scholar]
- 28.Herret E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical practice research datalink (CPRD) Int J Epidemiol. 2015;44(3):827–36. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karamchand S, Leisegang R, Schomaker M, et al. Risk Factors for Incident Diabetes in a Cohort Taking First-Line Nonnucleoside Reverse Transcriptase Inhibitor-Based Antiretroviral Therapy. Medicine. 2016;95(9):e2844. doi: 10.1097/MD.0000000000002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ford N, Mofenson L, Shubber Z, et al. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and analysis. AIDS. 2014;28(S2):S123–31. doi: 10.1097/QAD.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 31.Mehta U, Dheda M, Steel G, et al. Strengthening pharmacovigilance in South Africa. S Afr Med J. 2014;104(2):104–6. doi: 10.7196/samj.7517. [DOI] [PubMed] [Google Scholar]
- 32.Tipping B, Kalula S, Badri M. The burden and risk factors for adverse drug events in older patients – a prospective cross-sectional study. S Afr Med J. 2006;96(12):1255–9. [PubMed] [Google Scholar]
- 33.Uppsala Monitoring Centre. Uppsala: 2017. [cited 1 February 2017]. Global Pharmacovigilance. [Internet]. URL: https://www.who-umc.org/global-pharmacovigilance/specialist centres/ [Google Scholar]
- 34.European Medicines Agency Pharmacovigilance Risk Assessment Committee (EMA PRAC) PRAC strategy on measuring the impact of Pharmacovigilance activities. [cited 3 March 2017];2016 [Internet]. URL: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2016/01/WC500199756.pdf.