Abstract
Synthetic cathinones in bath salts products are psychostimulant drugs of abuse, and 3,4-methylenedioxypyrovalerone (MDPV) is a common constituent of these products. Oral MDPV has been show to stimulate locomotor activity but reinforcing, locomotor and appetitive stimulus effects of oral MDPV are unknown. Choice procedures evaluated preference for 0.03, 0.10, 0.30, and 1.00 mg/mL MDPV solutions versus 0.10 mg/mL quinine solution or water. To verify that oral MDPV produced pharmacological effects, locomotor activity was monitored during and after consumption of water, quinine, or MDPV solutions. Conditioned place preference (CPP) tested the apparent appetitive effects of a preferred concentration of oral MDPV with locomotor stimulant effects (0.30 mg/mL), using water as a control, and compared with results from intraperitoneally-administered MDPV. Consumption of MDPV solutions (0.03 – 1.00 mg/mL) was low when the alternative fluid was water, but a history of MDPV consumption increased MDPV choice. When paired with a quinine control solution, MDPV solutions (0.03 – 0.30 mg/mL) were almost exclusively preferred, and treatment with the catecholamine synthesis inhibitor αMPT decreased MDPV choice. Consumption of MDPV concentrations (0.1 – 1.0 mg/mL) stimulated locomotor activity. Chronic (10 day) access to 0.30 mg/mL MDPV resulted in escalated consumption, but locomotor effects did not systematically change across the access period. Finally, consumption of 0.30 mg/mL MDPV elicited CPP with a magnitude similar to the preference observed following intraperitoneal administration of MDPV. Consistent with human abuse patterns, oral MDPV has reinforcing effects in the mouse which are most likely related to its psychostimulant-like pharmacological profile.
Keywords: MDPV, bath salts, oral self-administration, conditioned place preference, locomotor activity, reinforcement
1.0 Introduction
Analogs of the plant-derived psychostimulant cathinone (all based on the β-ketophenethylamine structure) have recently emerged as drugs of abuse. Commonly referred to as bath salts, these preparations have no legitimate use in bathing and are purchased as “legal highs” mimicking the effects of illicit psychostimulant drugs like cocaine and amphetamine (Coppola and Mondola, 2012; Prosser and Nelson, 2012). In 2014, the United Nations Office on Drugs and Crime reported that one quarter of all new psychoactive substances detected worldwide are synthetic cathinones (UNODC, 2014), which are self-administered by nasal, oral, rectal, or intravenous routes (Johnson and Johnson, 2014). In the United States, 3,4-methylenedioxypyrovalerone (MDPV) is the bath salts constituent most commonly detected by Emergency Department screens on blood and urine samples from those experiencing untoward effects after exposure to these products (US Drug Enforcement Administration, 2011; Kyle et al., 2011; Spiller et al., 2011; Borek and Holstege, 2012; Murray et al., 2012).
Consistent with the profile of other psychostimulants, in vitro studies have revealed that MDPV has cocaine-like inhibitory effects at dopamine and norepinephrine uptake transporters (Baumann et al., 2013; Simmler et al., 2013). In vivo studies of rodents trained to discriminate methamphetamine or cocaine have reported that MDPV substitutes for both psychostimulants (Gatch et al., 2013; Collins et al., 2016; Gannon et al., 2016). Similarly, we previously trained mice to discriminate MDPV from saline and reported that 3,4-methylenedioxymethamphetamine and methamphetamine generalize to MDPV (Fantegrossi et al., 2013). In rodents, locomotor stimulation has also been observed following administration of MDPV (Fuwa et al., 2007; Huang et al., 2012; Aarde et al., 2013; Fantegrossi et al., 2013).
A study in rats proposes that phase I metabolites of MDPV may inhibit expression of locomotor stimulant effects after subcutaneous administration (Anizan et al., 2016). Thus, it seems plausible that formation of these same phase I metabolites could also impact the reinforcing effects of MDPV, suggesting that route of administration may be an important factor to consider when evaluating the abuse liability of this compound. Intravenous self-administration of MDPV has been described in rats (e.g., Watterson et al., 2012; Aarde et al., 2013, 2015a, 2015b; Schindler et al., 2016; Gannon et al., 2017), however there is a paucity of information related to the abuse-related effects of orally administered MDPV. The aims of the current study were therefore (1) to determine whether concentrations of MDPV would elicit locomotor stimulant effects, (2) to determine whether MDPV is preferentially chosen over alternative fluids in a 2-bottle choice paradigm, (3) to use conditioned place preference (CPP) to determine whether voluntary consumption of a preferred MDPV concentration with locomotor stimulant effects would also induce appetitive stimulus effects, and (4) to determine whether any observed appetitive stimulus effects are similar to those observed following IP administration of MDPV. In all studies of oral MDPV, mice voluntarily consumed MDPV solutions in either forced-choice or 2-bottle choice paradigms, allowing us to evaluate pharmacological activity (locomotor stimulant effects and place preference) and choice behavior/reinforcing effects, respectively.
2.0 Methods
2.1 Subjects
All studies were carried out in accordance with the Guide for Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. The Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences approved all of the experimental protocols. Adult male NIH Swiss mice (Harlan Sprague Dawley) weighing 20–25 g on delivery were housed three animals per cage (15.24 × 25.40 × 12.70 cm3) in a temperature-controlled room in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility. Room conditions were maintained at 22±2 °C and 45–50% humidity, with lights set to a 12-h light/dark cycle. Animals were fed Lab Diet rodent chow (Laboratory Rodent Diet no. 5001, PMI Feeds, St Louis, MO) ad libitum. All test conditions used groups of either five or six mice, and all mice were drug naive (with the exception of surgical anesthetics for locomotor studies) before testing, unless otherwise noted. Mice were separated into experimental groups and singly-housed for the duration of these experiments.
2.2 Oral Self-Administration: Two-Bottle Choice Procedures
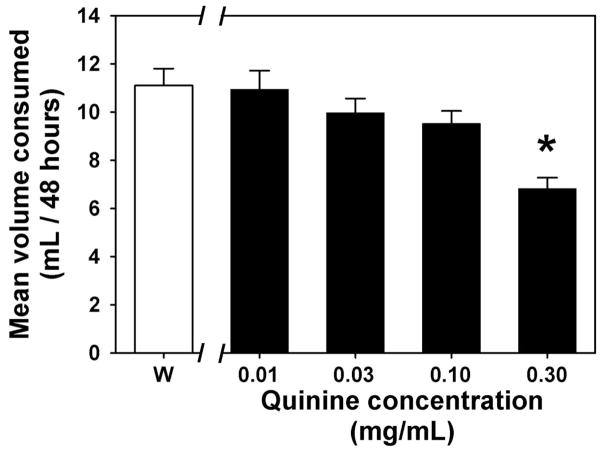
A continuous two-bottle choice paradigm was used to compare MDPV preference against a quinine solution control or against a water control. Before preference testing began, mice were presented with two bottles containing only water and baseline fluid intake was noted. Baseline fluid consumption patterns were noted for 4 days, after which water in both bottles was replaced with various quinine concentrations (0.01–0.30 mg/mL, presented in ascending order) for 4 days per concentration to determine an appropriate taste control, operationally defined as the highest quinine concentration at which fluid intake was similar to baseline levels. Bottles were briefly removed each day for measurements of the volume of fluid consumed and to allow for cleaning and refilling. The position of the bottles within the cage (left versus right side) was reversed each day. Since the 0.10 mg/mL quinine solution was the highest concentration that did not reduce total fluid consumption (see Figure 1), this concentration was chosen as the taste control for all MDPV choice studies.
Figure 1.
Fluid consumption when water (white bar, W) or various concentrations of quinine solution (black bars) were the only fluid options available for 48 hours. Asterisk indicates a significant difference from water consumption. Abscissa: Fluid available, with quinine concentration expressed as mg/mL on a log scale. Ordinate: Mean fluid consumption recorded over a 48 hour observation period.
For MDPV choice procedures, one bottle was filled with control solution (either water or 0.10 mg/mL quinine) while the other bottle was filled with a solution of MDPV (0.03–1.00 mg/mL), and mice were allowed to drink freely (24-hour access) for 4 days. After the fourth day, the MDPV solution was replaced with a different concentration of MDPV for four days. This paradigm continued until all mice were exposed to all the concentrations of MDPV, in randomized order. As before, bottles were briefly removed each day to measure the total volumes consumed, to clean and refill bottles, and switch the position of the bottles within the cage daily. MDPV preference was determined by dividing the volume of MDPV solution consumed by the total fluid volume (MDPV + control) consumed.
In studies evaluating the role of taste and drug history on preference, mice had either 0, 3, or 10 consecutive days of continuous (24 h access) exposure to a bottle containing a 0.30 mg/mL solution of MDPV as the only fluid available to drink, prior to commencement of choice procedures. During choice procedures, water was the control fluid. In all other respects, these studies were conducted identically to the MDPV vs. quinine experiments described above.
For choice studies gauging the relative contribution of taste versus pharmacological effects on apparent MDPV preference, choice procedures were conducted in two distinct groups of mice injected (IP) once per day for five consecutive days with either saline or 100 mg/kg of the catecholamine synthesis inhibitor alpha-methyl-para-tyrosine (αMPT). Following the third injection, mice could freely choose between quinine or 1.00 mg/mL MDPV for 4 consecutive days as described above. A pilot study using this αMPT dose for 3 consecutive days did not show any significant effects on locomotor activity (see Supplemental Figure 11)
2.3 Radiotelemetry of Locomotor Activity
All animals were implanted with radiotelemetry probes that monitor locomotor activity, as previously described (Fantegrossi et al., 2013). At least 7 days following surgery, fluid access was restricted to 3 h/day for all mice, and volumetric bottles containing either water, various concentrations of MDPV (0.03, 0.10, 0.30, or 1.00 mg/mL), or 0.10 mg/mL quinine were provided. Fluid order was randomized. Each cage was placed on a receiver situated inside a standard light- and sound-attenuating chamber. After 3 h fluid access, bottles were removed, consumption volumes were documented, and locomotor activity continued to be monitored for the next 3 h, allowing for a total of 6 h of continuous data collection. To determine whether consumption and locomotor effects of MDPV changed over time, two additional groups of animals were prepared with radiotelemetry probes, habituated to 21 h fluid deprivation, then provided water or 0.30 mg/mL MDPV for 10 consecutive days for 3 h. After 3 h fluid access, bottles were removed, consumption volumes were documented, and locomotor activity was recorded continuously for the entirety of these studies.
2.4 Conditioned Place Preference
Place conditioning was accomplished using Panlab 3-compartment spatial discrimination chambers (Harvard Apparatus, Holliston, MA) consisting of a box with two equally-sized conditioning compartments connected by a corridor. Compartments were differentiated by pattern on the walls, color, floor texture, and shape, providing multiple contextual dimensions across sensory modalities. Time spent in each of the two main compartments was recorded for 30 min by an overhead camera connected to a computer running EthoVision XT software (Noldus Information Technology, Wageningen, Netherlands). The first 15 min were considered exploration and ignored, while the final 15 min were scored to determine initial side preference. For all CPP experiments, any mouse spending >65% of the time in a single compartment during the pre-test was considered to have an initial side bias and was excluded from the study.
For CPP studies involving oral MDPV, mice were divided into two groups (experimental and control), and fluid access was restricted to 3 h/day. Following the initial preference test, all animals underwent 10 days of pairing sessions within the conditioning chambers (1 pairing/day, alternating sides each day). For pairing sessions, the initially less-favored side was paired with the availability of a 0.30 mg/mL MDPV solution (experimental group) or water (control group) while the more-favored side was paired with water for all subjects. Just prior to these pairings, mice were given free access to the condition-appropriate fluid for 15 min in the home cage. While isolated inside the compartment, mice were given additional access to the same fluid for another 165 m (i.e., the appropriate fluid was available throughout the entire conditioning session), thus preserving the 3 h access to MDPV solutions or water used in the locomotor experiments. Following 10 days of pairings (one pairing per day, for a total of 5 water and 5 drug pairings presented on alternate days), mice were placed in the chamber with free access to each compartment and time spent in the two main compartments was recorded as described above.
For IP CPP experiments, mice were divided into two groups (experimental and control). Following the initial preference test, all animals underwent 3 days of pairing sessions within the conditioning chambers (2 pairings per day, alternating sides each pairing). The first pairing of the day was always a saline conditioning session, and the second pairing of the day was always an MDPV conditioning session. Again, the initially less-favored side was paired with the availability of a range of doses (0.10, 0.30, and 1.00 mg/kg) of MDPV (experimental group) or saline (control group) while the initially more-favored side was paired with saline for all subjects. Immediately following IP administration of paired compound, mice were isolated in the appropriate compartment of the chamber for 30 min. Following 3 days of pairings, mice were placed in the chamber with free access to each compartment, and time spent in the two main compartments was recorded as described above.
To determine whether apparent appetitive stimulus effects of MDPV could be pharmacologically attenuated, another round of IP CPP experiments were conducted in which mice were pretreated with αMPT. For these experiments, mice were administered 100 mg/kg αMPT immediately following the pretest and once daily for the following 4 days. On days where administration of αMPT overlapped with pairing sessions, αMPT was administered following the second pairing, and αMPT was not administered on the post-test day. The 5 d αMPT regimen described here is consistent with the αMPT pretreatment regimen utilized in the two bottle-choice experiments outlined above. Pairing sessions for these pretreated mice were carried out in a manner identical to the IP CPP experimental procedure outlined above, using 0.30 mg/kg MDPV as the pairing dose of MDPV.
2.5 Drugs
Racemic MDPV was synthesized by one of us (KCR) at the Laboratory of Medicinal Chemistry at NIDA. Quinine hydrochloride dihydrate was purchased from Sigma-Aldrich chemical. αMPT hydrochloride was synthesized in the laboratory of Dr. Peter Crooks (UAMS College of Pharmacy) and provided as a generous gift to the authors. MDPV and quinine were dissolved in water for oral consumption, while αMPT and MDPV was dissolved in 0.9% physiological saline for IP injection.
2.6 Data Analysis
Graphical presentation of all data depicts mean±SEM. For two-bottle choice studies, indifference was said to occur if the observed MDPV preference score was ~50%, MDPV solution consumption greater than 75% of total consumption was considered MDPV preference, and MDPV solution consumption less than 25% of total consumption was interpretted as a preference for the control fluid. Fluid consumption and preferences were statistically compared by repeated measures one-way analysis of variance (ANOVA) followed by Holm-Sidak post-hoc tests for all pairwise comparisons, and the effects of saline and αMPT treatment on MDPV preference over quinine were compared using a Student’s t-test. A repeated measures two-way ANOVA followed by Student-Newman-Keuls post-hoc test for all pairwise comparisons was used for statistical comparisons of MDPV consumption versus a water alternative as a function of MDPV concentration and of MDPV history in a forced consumption setting. For radiotelemetry studies, total locomotor counts over 6 h (3 h during fluid access plus 3 h after bottle removal), total fluid consumption (mL/3 h) and MDPV intake (mg/3 h) were statistically analyzed. Locomotor counts and fluid consumption were compared by repeated measures one-way ANOVA followed by Holm-Sidak post-hoc tests for all pairwise comparisons, but MDPV intakes failed a test of equal variance and were therefore compared by Friedman’s repeated measures one-way ANOVA on ranks, followed by Student-Newman-Keuls post-hoc tests for all pairwise comparisons. For experiments involving chronic (10 day) access to water or 0.30 mg/mL MDPV, total fluid consumption, MDPV intake and locomotor activity were compared by repeated measures two-way ANOVA followed by Tukey’s HSD post-hoc tests for all pairwise comparisons using group (water vs. MDPV) and day (water baseline and days 1–10) as main factors. For conditioned place preference tests, additional time spent in the MDPV- or saline-paired compartment (as compared to the initial test) was considered a preference, and changes in time spent in relevant compartments were compared by a Student’s t-test, or a one-way ANOVA and a Holm-Sidak post-hoc analysis in the IP CPP experiments.
3.0 Results
3.1 Establishment of Control Fluids
Prior to choice experiments, 48 h fluid intake was quantified when 0 (water, Figure 1, open bar), 0.01, 0.03, 0.10 and 0.30 mg/mL quinine solutions (Figure 1, filled bars) were the only fluids available to drink. When fluid consumption across all conditions was statistically compared, there was an overall significant effect (F=7.77; df=4; p<0.05) which was due to significantly less consumption of the highest quinine concentration (0.30 mg/mL) as compared to water (t=4.89; p<0.05). Therefore, 0.10 mg/mL quinine was used as a control solution for all further two-bottle choice studies since this represented the highest quinine concentration that did not alter daily fluid consumption.
3.2 Radiotelemetry of Locomotor Activity
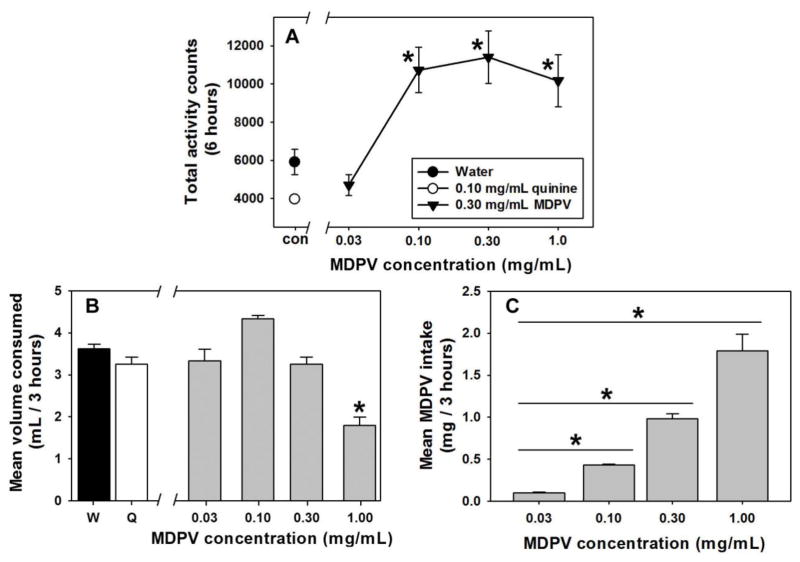
Following 21 h of fluid restriction, 3 h of access to water (Figure 2A, filled circle) or 0.10 mg/mL quinine (Figure 2A, open circle) resulted in low levels of locomotor activity, similar to those typically observed in our laboratory under control (non-injection) conditions. There was no significant difference between locomotor activity following consumption of either of the control solutions or 0.03 mg/mL MDPV (Figure 2A, first filled triangle). Consumption of concentrations of 0.10, 0.30 and 1.00 mg/mL elicited significantly more locomotor counts than observed following consumption of 0.03 mg/mL MDPV (t=4.28, 4.76 and 3.88, respectively; p<0.05), but there were no significant differences in locomotor effects elicited among these three concentrations (p>0.05 for all comparisons). Consumption of 0.10, 0.30 or 1.00 mg/mL MDPV elicited significantly more locomotor activity than consumption of water (t=3.42, 3.90 and 3.03, respectively; p<0.05) or quinine (t=4.80, 5.28 and 4.40; p<0.05). Forced consumption of 1.00 mg/mL MDPV also produced self-injurious behavior (skin picking and self-biting) in some mice, and therefore higher concentrations were not assessed.
Figure 2.
A – Locomotor effects of water (filled circle), 0.10 mg/mL quinine solution (open circle) or MDPV solutions (filled triangles) in a forced choice setting after 21-hours of fluid restriction. Abscissa: Fluid available, with water and quinine control solutions presented at the “con” point, and with MDPV concentrations expressed as mg/mL on a log scale. Ordinate: Total locomotor counts summed over 6 hours during and after 3 hour access to each fluid. Asterisks represent significant differences from water control. B – Effects of water (filled bar), 0.10 mg/mL quinine solution (open bar), and each concentration of MDPV solution (gray bars) on fluid consumption during locomotor activity assessments. Abscissa: Fluid available, with water and quinine control solutions presented at the “W” and “Q” points, respectively, and with MDPV concentrations expressed as mg/mL on a log scale. Ordinate: Total volume consumed in mL during the 3 hour access period. Asterisk represents significant difference from water control. C – MDPV intake as a function of concentration of available MDPV solutions. Abscissa: MDPV concentrations expressed as mg/mL on a log scale. Ordinate: Total intake of MDPV, expressed as mg/3 hours. Asterisks indicate significant differences from lower MDPV concentrations.
In this setting of limited access following fluid deprivation, total fluid consumption was relatively stable across all fluids provided (Figure 2B). When daily consumption across all MDPV concentrations and control solutions were statistically compared, the overall ANOVA was significant (F=21.35; df=4; p<0.05), however, only the highest tested MDPV concentration of 1.00 mg/mL engendered significantly less fluid consumption than the water control solution (t=7.21; p<0.05). Importantly, consumption of different MDPV concentrations produced different levels of drug intake during the limited access interval (Figure 2C). When total intake across all MDPV concentrations was statistically compared, the overall ANOVA was significant (χ2=18.00; df=4; p<0.05), despite relatively stable volumes consumed across increasing concentrations of MDPV. Thus, the 0.10 mg/mL MDPV solution engendered significantly more drug intake than the 0.03 mg/mL concentration (q=3.46; p<0.05), the 0.30 mg/mL MDPV solution engendered significantly more drug intake than the 0.03 and 0.10 mg/mL concentrations (q=4.90 and 3.464, respectively; p<0.05), and the 1.00 mg/mL MDPV solution engendered significantly more drug intake than the 0.03, 0.10, and 0.30 mg/mL concentrations (q=5.69, 4.90 and 3.46, respectively; p<0.05).
3.3 Oral Self-Administration: Two-Bottle Choice
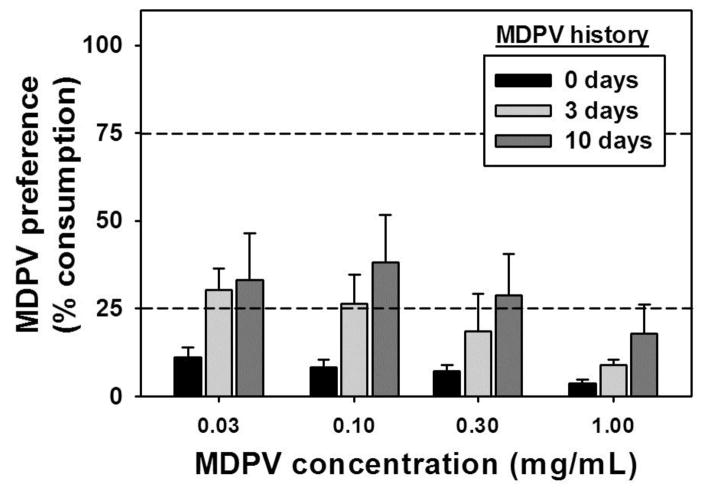
MDPV concentrations were first examined against a water alternative. In this setting, no concentration of MDPV elicited a preference over the water alternative in otherwise MDPV-naïve mice (Figure 3, black bars), and strong preferences for water were observed at every available MDPV concentration. However, interpretation of these data is complicated by the fact that the taste of a given fluid is immediate, while its pharmacological effects (if any) are delayed and only achieved if a sufficient volume is consumed. Therefore, distinct groups of mice received continuous forced-choice access to a single bottle containing 0.30 mg/mL MDPV for 3 days (Figure 3, light gray bars) or for 10 days (Figure 3, dark gray bars) to allow for association of the MDPV solution’s taste and its delayed pharmacological effects. During this period, consumption of the MDPV solution was monitored daily, resulting in mean total intakes of 3.57±0.39 mg for the 3-day history group, and 11.39±0.73 mg for the 10-day history group. Under these conditions, significant main effects of MDPV history (F=8.50; df=2; p<0.05) and concentration (F=3.30; df=3; p<0.05) but not history X concentration (F=0.41; df=3; p>0.05) were revealed by the two-way ANOVA. The overall effect of concentration was driven by preference for the highest MDPV concentration (1.00 mg/mL) differing significantly from preference for the two lowest MDPV concentrations (q=3.92 for 0.03 mg/mL MDPV, q=3.67 for 0.10 mg/mL MDPV; p<0.05), while 3-day and 10-day MDPV consumption histories both significantly differed from 0 days of forced-choice MDPV consumption (q=3.59 and 5.78, respectively; p<0.05). Thus, a history of MDPV consumption in a forced-choice setting significantly increased preference for MDPV over water during subsequent two-bottle choice tests, though preference for MDPV remained low across all MDPV concentrations, regardless of forced-choice history.
Figure 3.
Preference for oral MDPV solutions in a continuous access two-bottle choice procedure where the alternative fluid was water following a 0 day (black bars), 3 day (light gray bars) or 10 day (dark gray bars) history of 0.30 mg/mL MDPV consumption in a forced-choice setting. Mean total MDPV intake for the 3 day history group was 3.57±0.39 mg, and was 11.39±0.73 mg for the 10 day history group. Abscissa: MDPV concentration expressed as mg/mL on a log scale. Ordinate: Mean MDPV preference score (mL MDPV solution consumed/mL total fluid consumption, expressed as a %). Asterisks indicate significant differences from 0 day MDPV history, within dose. Dashed horizontal lines at 25% and 75% indicate thresholds for preference for the alternative fluid and preference for the MDPV solution, respectively.
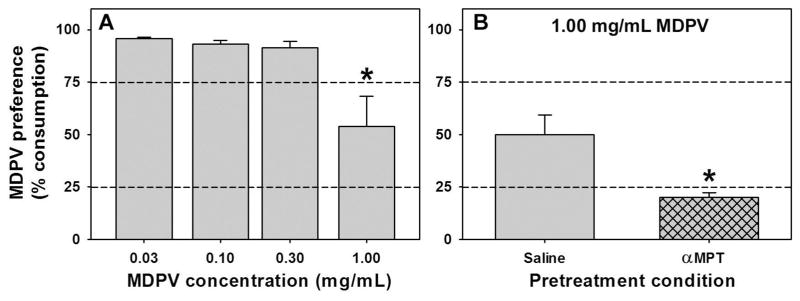
When the alternative fluid was 0.10 mg/mL quinine, MDPV preference depended on concentration. The overall ANOVA comparing consumption across MDPV concentrations was significant (F=8.01; df=3; p<0.05) where preference for 1.00 mg/mL MDPV was significantly different from 0.03, 0.10, and 0.30 mg/mL MDPV (t=4.32, 4.04, and 3.86 respectively; p<0.05), which were consumed almost exclusively over quinine (Figure 4A).
Figure 4.
A – Preference for oral MDPV solutions in a continuous access two-bottle choice procedure where the alternative fluid was 0.10 mg/mL quinine. Abscissa: MDPV concentration expressed as mg/mL on a log scale. Ordinate: Mean MDPV preference score (mL MDPV solution consumed/mL total fluid consumption, expressed as a %). Asterisk indicates significant difference from lower MDPV concentrations. B – Preference for a 1.00 mg/mL MDPV solution in a continuous access two-bottle choice procedure where the alternative fluid was 0.10 mg/mL quinine following pretreatment with saline (gray bar) or the catecholamine synthesis inhibitor MPT (hatched gray bar). Abscissa: Pretreatment regimen (saline or MPT). Ordinate: As described for panel A. Asterisk indicates significant difference from saline treatment. For both panels, dashed horizontal lines at 25% and 75% indicate thresholds for preference for the alternative fluid and preference for the MDPV solution, respectively.
In an attempt to separate the role of the pharmacological effects of MDPV from the taste of the MDPV solutions in the choice procedures, mice treated with saline or αMPT chose between quinine and 1.00 mg/mL MDPV solutions. This MDPV concentration was chosen because it was preferred less than all other tested concentrations, perhaps due to an aversive taste. MDPV preference was significantly lower in mice treated with αMPT (Figure 4B, gray hatched bar) than saline (Figure 4B, gray bar) (t=3.13; df=5; p<0.05). Saline-treated mice thus displayed choice indifference between the quinine and 1.00 mg/mL MDPV solutions, similar to the untreated animals, while αMPT-treated subjects exhibited a strong preference for the quinine solution.
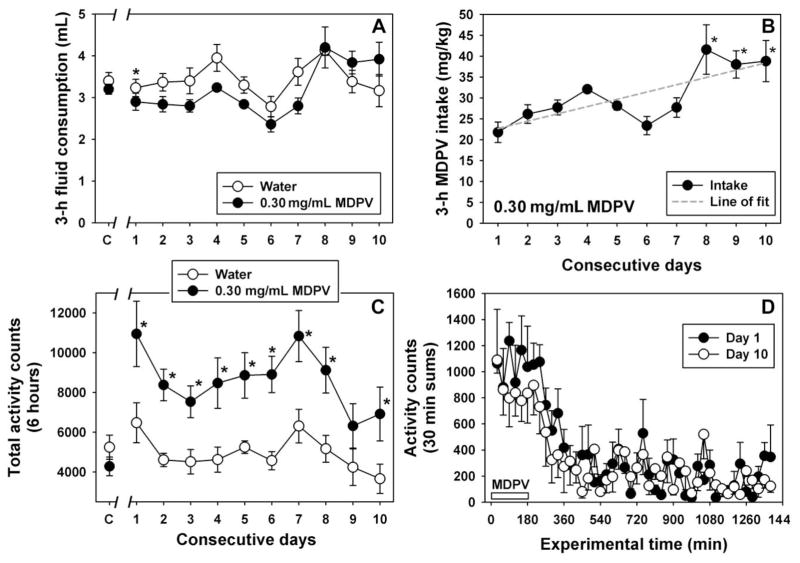
3.4 Chronic (10 day) Access to MDPV
Because 0.30 mg/mL MDPV maintained near exclusive choice over the quinine solution in the two-bottle test, and because consumption of this concentration in a forced-choice setting elicited robust locomotor stimulant effects, it was of interest to determine whether animals would escalate their intakes of drug over days, and whether locomotor effects would progressively increase (consistent with the concept of locomotor sensitization) or decrease (consistent with the concept of tolerance to locomotor stimulant effects.) As previously noted in limited access studies above, 0.30 mg/mL MDPV maintained equivalent consumption as water across the 10 day access period, with the exception of the first day of MDPV availability where MDPV consumption was significantly less than water (q=4.15, p<0.05) (Figure 5A). No systematic differences in consumption between groups or across days were observed. However, MDPV intakes on days 8, 9 and 10 were significantly different from MDPV intake on day 1 (p<0.05 for all comparisons), and the best-fit linear regression for MDPV intake across days (r2=0.60) is a line with a positive slope of 1.74, suggesting greater drug intake as a function of repeated exposure (Figure 5B). Consumption of 0.30 mg/mL solutions stimulated locomotor activity above the levels observed following consumption of water, as indicated by a main effect of group (F=42.26, df=1; p<0.05) and by significant between-group differences on all days except day 9 (p<0.05 for all comparisons except day 9) (Figure 5C). However, despite increasing MDPV intake across the 10-day access period, no systematic trends in locomotor activity were observed. Indeed, the locomotor stimulant effects of MDPV tended to be greater across the timecourse of activity on day 1 (when intake was lower) than on day 10 (Figure 5D), although these differences were not statistically different (F=0.57, df=1; p=0.49). Nevertheless, the fact that drug intake increased across the access period (resulting in a greater MDPV dose) but locomotor activity did not indicates that locomotor sensitization did not occur in these subjects under these access conditions, and may instead suggest that tolerance to locomotor stimulant effects develops with chronic MDPV exposure.
Figure 5.
A – Consumption of water (open circles) or 0.30 mg/ml MDPV solution (filled circles) during 3 hour fluid access period for 10 consecutive days. Points at “C” represent the mean fluid consumption measured over 3 days of 3 hour water access for both groups. Abscissa: consecutive days of water or 0.30 mg/ml MDPV solution availability, with water control solutions presented for both groups at the “C” point. Ordinate: Mean fluid consumption. Consumption of water was significantly greater than MDPV on day 1 (q=4.151, P<0.05), but no other between-group comparisons were significant, nor was there a significant effect of day. B – Escalating intake of MDPV over consecutive days of 0.30 mg/ml MDPV solution access. Abscissa: Consecutive days. Ordinate: Total MDPV intake during the 3 hour access period, expressed as mg/kg. Asterisks represent significant differences from day 1 consumption, and dashed line represents the best fit linear regression (r2=0.60) indicating a significant positive slope. C – Assessment of locomotor activity during 3 hour access to and for 3 hours following consumption of water (open circles) or 0.30 mg/ml MDPV solution (closed circles) over 10 consecutive days. Points at “C” represent locomotor activity measured over 3 days of water access for both groups. Abscissa: as described in panel A. Ordinate: 6 hour activity counts. MDPV consumption elicited significantly more activity than water consumption on all days except for day 9 (P<0.05 for all comparisons except day 9). D – Comparison of locomotor effects following consumption of 0.30 mg/mL MDPV on day 1 (filled circles) and day 10 (open circles) across the 24 hour recording period. The white bar from 0 min to 180 min indicates the period of MDPV availability. Abscissa: experimental time, in minutes. Ordinate: mean activity counts, expressed as 30 min sums.
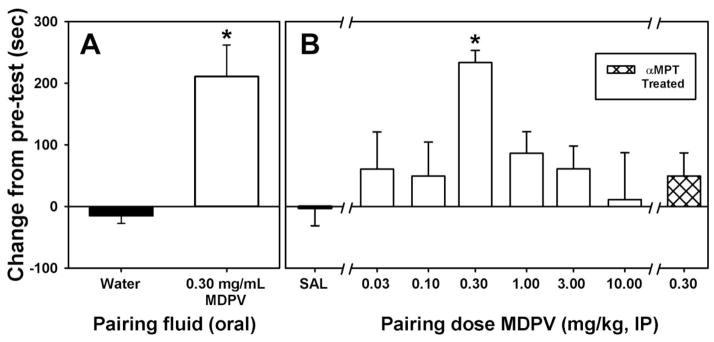
3.5 Conditioned Place Preference
Because 0.30 mg/mL MDPV maintained near exclusive choice over the quinine solution in the two-bottle test, induced escalation of consumption with chronic (10-day) access, and because consumption of this concentration in a forced-choice setting elicited robust locomotor stimulant effects, it was of interest to determine whether consumption of this MDPV concentration would also function as an appetitive stimulus in the CPP test. For mice exposed to water on both sides of the conditioning chamber, no systematic change in side preference was observed when comparing the post-conditioning test to the pre-conditioning test (Figure 6A, filled bar). In contrast, mice given access to a solution of 0.30 mg/mL MDPV on one side of the conditioning chamber and to a water solution on the other side exhibited a robust preference for the MDPV-paired side over the water control side during the post-conditioning test (Figure 6A, open bar), which was significantly different from water-only mice (t=4.316; df=10; p<0.05). Importantly, similar volumes of water and the MDPV solution were consumed during conditioning trials (see Supplemental Figure 22).
Figure 6.
A – Effects of voluntary water or 0.30 mg/mL MDPV solution consumption on place conditioning after 21-hour fluid deprivation. Abscissa: Fluids paired with each side of the two conditioning compartments. Ordinate: Change in time spent in the relevant conditioning compartment during the post-conditioning test. Asterisk indicates significant difference between water-conditioned and MDPV-conditioned groups. B – Effects of IP-administered MDPV on place conditioning. Abscissa: Doses of MDPV paired with each side of the two conditioning compartments. Ordinate: Change in time spent in the relevant conditioning compartment during the post-conditioning test. Asterisk indicates significant difference between saline-conditioned and MDPV-conditioned groups.
To compare the degree of apparent appetitive stimulus effects of oral MDPV consumption to IP-administered MDPV, a full dose-response curve of IP-administered MDPV (Figure 6B, open bars) was generated and compared with data from a saline-only group (Figure 6B, filled bar) (t=2.479; df=7; p<0.05). Only one dose of IP-administered MDPV (0.30 mg/kg) elicited preference for the MDPV-paired side over the saline control side during the post-conditioning test (t=3.63; p<0.001), and this preference score was not different from the orally administered MDPV group (t=-0.41; p=0.687). Therefore, 0.30 mg/kg MDPV was also used in pairing sessions for mice treated with αMPT. We found that αMPT pretreatment (Figure 6B, hatched bar) abolished the apparent rewarding effects of 0.30 mg/kg MDPV such that side preference was not different from mice receiving no pretreatment and subsequently paired with saline alone (t=0.80; p=0.430).
4.0 Discussion
The aims of this study were to (1) determine whether concentrations of oral MDPV would elicit locomotor stimulant effects, (2) determine whether oral consumption of these solutions of MDPV with locomotor stimulant effects would also produce reinforcing effects in a 2-bottle choice paradigm, (3) use CPP to determine whether voluntary consumption of an MDPV concentration with apparent reinforcing and locomotor stimulant effects would also induce appetitive stimulus effects, and (4) determine whether any observed appetitive stimulus effects were similar, in degree, to those observed following IP administration of MDPV. First, mice were prepared with radiotelemetry probes and locomotor activity was quantified before, during, and after consumption of water, 0.10 mg/mL quinine or MDPV solutions. Water, quinine, and 0.03 mg/mL MDPV produced similar low levels of locomotor activity. In contrast to this lowest MDPV concentration, consumption of all other MDPV concentrations induced profound locomotor stimulant effects. However, despite engendering significantly different MDPV intakes, we did not observe dose-dependent differences in locomotor stimulant effects following oral consumption of these MDPV solutions. This is consistent with our previous findings using intraperitoneal injections (Fantegrossi et al., 2013; Gannon et al., 2016) where we also observed a relatively “flat” dose-effect function for MDPV-induced hyperactivity in the cool (~20°C) ambient temperature of the telemetry laboratory. Similarly, chronic (10 day) access to the 0.30 mg/mL MDPV solution in a forced-choice setting engendered significant locomotor stimulant effects across the access period, but no systematic upward or downward trends in locomotor activity were observed despite escalation of intake resulting in approximately a 2-fold higher MDPV dose on the last three days of the access period than on the first day. Indeed, a comparison of locomotor effects on day 1 (when MDPV intake was low) versus day 10 (when MDPV intake was high) shows that activity tended to be higher on day 1 across the MDPV timecourse, which is not consistent with locomotor sensitization. If anything, similar levels of activity despite a doubling of dose is more consistent with the notion of tolerance to locomotor effects, which mice may have been attempting to compensate for by increasing consumption. Further studies designed to investigate this intriguing possibility are needed.
With regards to the second aim, we found that almost no MDPV was consumed at any of the concentrations evaluated when water was the alternative, suggesting that all of the MDPV solutions used in the two-bottle choice procedures had a discriminable taste component. Because taste stimuli are immediate whereas pharmacological effects are delayed after oral administration, it seems reasonable to propose that comparisons of presumably bitter drug solutions with water will always be biased toward water choice. Nevertheless, forced-choice exposure to MDPV may have allowed animals to associate the immediate taste of an MDPV solution with its delayed pharmacological effects, as we found that a history of MDPV consumption significantly increased choice of MDPV solutions versus water in subsequent two-bottle tests, although water was always preferred. To control for the apparently aversive taste of the MDPV solutions, a solution of quinine was instead used as the alternative fluid. Quinine is a commonly-used bitter tastant in studies of oral self-administration, and the function of the quinine solutions here was to control for the presumably aversive taste of MDPV. In contrast to the water-controlled experiments, several concentrations of MDPV supported near-exclusive choice over quinine in these two-bottle tests. Like all choice studies involving oral consumption, preference for one option depends critically on the appetitive properties of the other option, and so use of a different alternative fluid (a lower concentration of quinine, water, a sucrose solution, etc.) would certainly alter MDPV choice. We note that the quinine concentration here used was the highest concentration that did not significantly decrease total fluid consumption, and that we tested MDPV up to a concentration that significantly decreased fluid consumption and yielded “choice indifference” with quinine. Since the lowest MDPV concentration likely had the most “water-like” taste and failed to alter locomotor activity, it seems likely that its near-exclusive choice was driven by the taste contrast between it and the quinine alternative. As such, attention was shifted to MDPV solutions with presumably less “water-like” tastes, with the highest concentration of MDPV likely having the most intense taste component. Preference for this highest MDPV concentration was significantly abolished in αMPT pretreated mice, suggesting that the pharmacological effects of MDPV are more salient contributors to choice over quinine than is taste. It is perhaps possible that treatment with αMPT alters taste reactivity in some way, which could alter choice behavior away from MDPV and towards quinine. However, a study in which αMPT was used to block conditioned taste aversion to a sucrose solution paired with a low dose of ethanol, but not to a sucrose solution paired with a high dose of ethanol (Aragon et al., 1991) seems at odds with this proposition.
In CPP experiments, time spent in the 0.30 mg/mL MDPV-paired side of the chamber was significantly increased in the post-test. However, because mice were fluid deprived in these studies, alleviation of thirst could possibly have induced a preference, and therefore any fluid might appear to have appetitive stimulus properties, but a similar preference was not observed in the water controls, even though similar volumes of fluids were consumed. Importantly, the place preference observed following oral consumption of this MDPV solution was not statistically different from the place preference observed following conditioning with IP-administered MDPV. Because αMPT pretreatment blocked MDPV-induced CPP, and because water was almost exclusively preferred over 0.30 mg/mL MDPV in the previous two-bottle test, it is likely that MDPV-induced place preference is due to the catecholamine-mediated pharmacological effects of the drug solution, rather than to its taste.
The summation of all of these experimental results strongly suggests that oral MDPV is biologically active, inducing psychostimulant-like abuse-related effects in the mouse. Despite a recent study in the rat suggesting that phase I metabolites of MDPV might blunt some of its pharmacological effects (Anizan et al., 2014), we observed robust choice, locomotor stimulant, and appetitive stimulus effects of oral MDPV similar to that observed in IP-administered mice, suggesting that phase I metabolites of MDPV do not significantly impact these endpoints in the mouse. One perhaps interesting distinction between oral MDPV and more traditional psychostimulants is the lack of any evidence of sensitization of locomotor activity observed in the present studies. Indeed, the pattern of effects here obtained are more consistent with the notion of tolerance to locomotor stimulant effects, as they are characterized by similar (if not slightly attenuated) levels of locomotor activity on day 10 as compared to those observed on day 1, despite approximately double the drug intake on day 10. As human abuse of bath salts products containing synthetic cathinone analogues like MDPV continues to burden the public health system, further studies into the abuse-related effects of these novel compounds in laboratory animals are required to better understand the acute and chronic effects of these substances on behavior, and perhaps to develop specific therapies aimed at reducing the harms associated with their use.
Supplementary Material
Highlights.
3,4-methylenedioxypyrovalerone (MDPV) solutions are consumed by mice, and produced profound locomotor stimulant effects.
Consumption of MDPV “escalates” over days, but locomotor effects decrease over days.
History of forced MDPV consumption increased MDPV choice over a water alternative.
MDPV is preferred over quinine; preference is abolished by catecholamine depletion with alpha-methyl-para-tyrosine (αMPT).
Intake of MDPV induced conditioned place preference, relative to injection (IP)-administered MDPV.
Acknowledgments
Role of Funding Source
None of the funding sources participated in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Funding source(s)
This work was supported, in part, by the University of Arkansas for Medical Sciences Center for Translational Neuroscience [Grant GM110702], the University of Arkansas for Medical Sciences Translational Research Institute [Grant RR029884], and the National Institutes of Health National Institute on Drug Abuse [Grants DA022981 and DA039195]. A portion of this work was supported by the National Institutes of Health Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism.
We thank the UAMS Division of Laboratory Animal Medicine for their husbandry services, and Dr. Michael Hambuchen for technical help.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors
BMG, LNR and MSM participated in study design and were responsible for data collection and manuscript preparation. KCR synthesized MDPV used in these studies and participated in manuscript preparation. WEF participated in study design, analyzed data, and participated in manuscript preparation. All authors have approved the final article.
Conflict of Interest
No conflict declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: Self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–40. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Creehan KM, Vandewater SA, Taffe MA. In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: Self-administration and locomotor stimulation in male rats. Psychopharmacology (Berl) 2015a;232:3045–3055. doi: 10.1007/s00213-015-3944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA. Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology (Berl) 2015b;232:1867–1877. doi: 10.1007/s00213-014-3819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anizan S, Concheiro M, Lehner KR, Bukhari MO, Suzuki M, Rice KC, Baumann MH, Huestis MA. Linear pharmacokinetics of 3,4-methylenedioxypyrovalerone (MDPV) and its metabolites in the rat: relationship to pharmacodynamic effects. Addict Biol. 2016;21:339–47. doi: 10.1111/adb.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon CM, Abitbol M, Amit Z. Ethanol-induced CTA mediated by acetaldehyde through central catecholamine activity. Psychopharmacol. 1991;103:74–7. doi: 10.1007/BF02244077. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacol. 2013;38:552–62. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of ‘bath salts’ containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60:103–105. doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Collins GT, Abbott M, Galindo K, Rush EL, Rice KC, France CP. Discriminative stimulus effects of binary drug mixtures: Studies with cocaine, MDPV, and caffeine. J Pharmacol Exp Ther. 2016;359:1–10. doi: 10.1124/jpet.116.234252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola M, Mondola R. Synthetic cathinones: Chemistry, pharmacology and toxicology of a new class of designer drugs of abuse marketed as “bath salts” or “plant food”. Toxicol Lett. 2012;211:144–149. doi: 10.1016/j.toxlet.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: Drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacol. 2013;38:563–73. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuwa T, Fukumori N, Tanaka T, Kubo Y, Ogata A, Uehara S. Microdialysis study of drug effects on central nervous system - Changes of dopamine levels in mice striatum after oral administration of methylenedioxypyrovalerone. Ann Rep Tokyo Metr Inst PH. 2007;58:287–292. [Google Scholar]
- Gannon BM, Galindo KI, Rice KC, Collins GT. Individual differences in the relative reinforcing effects of 3,4-methylenedioxypyrovalerone (MDPV) under fixed and progressive ratio schedules of reinforcement in rats. J Pharmacol Exp Ther. 2017;361:181–9. doi: 10.1124/jpet.116.239376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE. Stereoselective effects of abused “bath salt” constituent 3,4-methylenedioxypyrovalerone in mice: Drug discrimination, locomotor activity, and Thermoregulation. J Pharmacol Exp Ther. 2016;356:615–23. doi: 10.1124/jpet.115.229500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24:437–47. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS, Johnson MW. Investigation of “bath salts” use patterns within an online sample of users in the United States. J Psychoactive Drugs. 2014;46:369–78. doi: 10.1080/02791072.2014.962717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle PB, Iverson RB, Gajagowni RG, Spencer L. Illicit bath salts: Not for bathing. J Miss State Med Assoc. 2011;52:375–77. [PubMed] [Google Scholar]
- Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug ‘bath salts’ containing 3,4-Methylenedioxypyrovalerone (MDPV) J Med Toxicol. 2012;8:69–75. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser JM, Nelson LS. The toxicology of bath salts: A review of synthetic cathinones. J Med Toxicol. 2012;8:33–42. doi: 10.1007/s13181-011-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH. Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology (Berl) 2016;233:1981–1990. doi: 10.1007/s00213-015-4057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–70. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of ‘bath salts’ and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. [Accessed 30 Apr 2015];Global Synthetic Drugs Assessment: Amphetamine-type stimulants and new psychoactive substances. 2014 at http://www.unodc.org/documents/scientific/2014_Global_Synthetic_Drugs_Assessment_web.pdf.
- US Drug Enforcement Administration, Office of Diversion Control. National Forensic Laboratory Information System Special Report: Synthetic Cannabinoids and Synthetic Cathinones Reported in NFLIS, 2009–2010. U.S. Drug Enforcement Administration; Springfield, VA: 2011. [Accessed 13 September 2012]. at deadiversion.usdoj.gov/nflis/2010rx_synth.pdf. [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2014;19:165–74. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.