Abstract
Reference genes have been utilized in estimating gene expression levels using quantitative reverse transcriptase-quantitative polymerase chain reaction (qRT-PCR) analysis. Aphidius gifuensis Ashmaed is one of the most widely used biological control agents for aphids. The biological properties of this species have been studied in detail, and current investigations are focused on elucidating the regulatory mechanisms in its host However, the appropriate reference genes for target gene expression studies have not been identified. In this study, the expression profiles of 12 candidate reference genes were evaluated under different experimental conditions(development stage, sex, tissue type, diet) by using dedicated algorithms, including geNorm, Normfinder, BestKeeper, and ΔCt. In addition, RefFinder was used to rank the overall stability of the candidate genes. Finally, we recommend three optimal reference genes for the normalization of qRT-PCR data in the presence of specific variables, which include ACTB, RPL13, and PPI for different developmental stages; RPS18, ACTB, and RPL13 for sexes; RPL13, PRII3, and RPS18 in different tissue types; and RPL13, RPL27, and ACTB in diverse diets. The present study has identified optimal reference genes that could be used in estimating the expression levels of specific genes under these conditions following the Minimum Information for publication of Quantitative real-time PCR Experiments (MIQE) guidelines, which would facilitate in advancements in functional genomics research on A. gifuensis.
Introduction
Real-time quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) is the most sensitive and accurate method for determining mRNA expression levels of target genes under different experimental conditions and is commonly used to confirm the expression of relevant genes in high-throughput sequencing[1–3]. To accurately estimate gene expression levels, internal reference genes are used to normalize quantitative fluorescence data on the target gene. Most internal reference genes must maintain a high degree of uniform expression at different developmental stages, environments, or experimental conditions [4]. Housekeeping genes are commonly used as internal controls because of their relatively stable expression regardless of changes in the environment, physiological conditions, and cell type, and their levels directly represent the number of cells or genomes present in the sample [5, 6].
Screening for internal reference genes has been extensively performed in various insect species and their natural enemies. Acyrthosiphon pisum, Lipaphis erysimi, Spodoptera litura, Bemisia tabaci, and Coleomegilla maculate are some of the commonly used internal reference genes for insects [5, 7–10]. However, numerous studies have shown that the expression levels of these housekeeping genes significantly vary under certain conditions and are thus not suitable as internal reference genes [11, 12]. Although qRT-PCR can be used as a rapid and reliable method for detecting and quantifying gene expression in different biological processes, some internal reference genes exhibit considerable differences in expression among various treatment settings [11]. Therefore, the evaluation of housekeeping genes under different experimental conditions and the selection of the appropriate gene for normalization of expression is critical.
A. gifuensis is the most abundant aphid parasitoid in the open cotton field. However, to control A. gifuensis, it is necessary to establish a standardized qRT-PCR procedure [13] that follows the Minimum Information for publication of Quantitative real-time PCR Experiments (MIQE) guidelines for A. gifuensis. The objective of the present study was to identify and validate the most suitable reference gene(s) for gene expression profiling in A. gifuensis, particularly those that are stably expressed under different treatments. The following 12 housekeeping genes were selected as candidate reference genes: dimethyladenosine transferase (DIMT), ACTB, 60S ribosomal protein L3 (RPL13), peptidylprolyl isomerase (PPI), TUB, RPL18, 18SrRNA, AK, EF1A, TBP, RNA polymerase II (PRII), and ribosomal protein L27 (RPL27). Specifically, we evaluated the stability of the above candidate reference genes under different experimental conditions, including different developmental stages, sex, tissue types, and diet treatment. The expression stability of these genes was evaluated using five statistical algorithms, geNorm[14], NormFinder[15], BestKeeper[16], deltaCt method [17], and RefFinder[18]. To the best of our knowledge, this is the first report on the comprehensive evaluation of reference genes in A. gifuensis. This report thus recommends different reference genes based on each experimental condition.
Materials and methods
Insect materials
A. gifuensis was collected from the Institute of Cotton Research (IRC) of CAAS (Anyang, Henan, China). The adults were reared in the laboratory on cotton-melon aphids; rearing conditions were 24 ± 1°C, a 14:10 h light/dark (L:D) photoperiod, and 75 ± 5% relative humidity (RH). Different developmental stages, two sexes, different tissues in both adult males and females, and different diets were tested in various A. gifuensis samples to evaluate the stability of the candidate genes. The developmental stages included three-day-old larvae (collected at the third day after parasitized cotton aphids and dissected under a microscope), pupae, and adults (including both females and males). For sex, 30 adult females and males were collected, respectively. The tissue types included the head, thorax, and abdomen, which were dissected from various A. gifuensis adult males and females, and detected respectively. For diet, adults were maintained on pure water and honey, respectively, and then harvested at different time points, namely, 1 d, 2 d, 3 d, and 4 d. All collected samples were preserved in 1.5-mL centrifuge tubes, flash frozen in liquid nitrogen, and then stored at -80°C until RNA extraction. Each treatment was repeated three times independently.
Total RNA extraction and cDNA synthesis
Total RNA was extracted from A. gifuensis using the SV Total RNA Isolation System (Promega, USA). RNA concentration was assessed by measuring ultraviolet absorbance at wavelengths of 260 nm (A260) and 280 nm (A280) using a Nanodrop2000C spectrophotometer (Thermo, USA). The A260/A280 ratio was maintained within the range of 1.80–2.00 to ensure mRNA integrity. The concentration of total RNA was normalized to 500 ng/μL. First-strand cDNA was synthesized using the PrimeScript® RT Master Mix (Takara, Japan). The synthesized cDNA samples were stored at -20°C for later RT-qPCR analysis.
Identification of reference genes in A. gifuensis
Candidate reference genes segments were identified from the A. gifuensis RNA-seq transcriptome dataset that was constructed by our laboratory. Primers were designed using Premier 5.0 (S1 Table) (Premier, USA) and used for cloning cDNA sequences that encoded the open reading frames of the reference genes. The conditions for PCR amplification were as follows: 35 cycles of 98°C for 10 s, 55°C for 15 s, and 72°C for 30 s. The PCR products were cloned into a T blunt simple vector (TransGen Biotech, Beijing, China) and then sequenced. Accession numbers were obtained upon submission to NCBI. A total of 12 candidate reference genes were selected as internal controls for qRT-PCR.
Candidate reference genes and primer design
Upon confirmation of the sequences of each of the 12 candidate reference genes, primers for the subsequent RT-qPCR analyses were then designed by using the Beacon Designer 7 software (Table 1). qRT-PCR was performed using Mastercycle ep realplex (Eppendorf) following the manufacturer’s instructions. Each 20-μL reaction in every well consisted of the following: 1 μL of cDNA, 0.8 μL of each the forward and reverse primers (10 μM), 10 μL of 2× SYBR Green Premix (Promega, USA), and 7.4 μL of ddH2O. RT-qPCR reactions were conducted at the following conditions: 95°C for 2 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. Every template was amplified in triplicate. To verify the specificity of the gene amplifications melting curve analysis was performed. The relative standard curves of the transcripts were generated using a five-fold serial dilution of the cDNA. The corresponding qRT-PCR efficiencies (E) were calculated according to the following formula: E = (10[-1/slope] - 1) × 100.
Table 1. Primers used for studying reference gene expression in A. gifuensis by qRT-PCR.
| Gene | Primer sequence (5’-3’) | Product length (bp) | Primer efficiency (%E) | Regression coefficient (R2) |
|---|---|---|---|---|
| DIMT |
F:AAGCAGCAATAAGACCATC R:TCTAATTCAGCAACCATACG |
133 | 91.56 | 0.9950 |
| ACTB |
F:GCTGTTGTGGTGAATGAG R:CAATCTATGAAGGTTATGCTCTT |
121 | 93.04 | 0.9943 |
| RPL3 |
F:TGATTGATGTTATTGGTGTTAC R:GAATGATACTCTGCTTGGAT |
146 | 92.63 | 0.9941 |
| PPI |
F:CAAGACGTGAACCAGAAGA R:TGCTGTATGTATTATTGCTGTATC |
143 | 93.61 | 0.9803 |
| TBP |
F:CAATAATGCCGCTTCATC R:ACTTCATCCAGGTGTTAC |
184 | 93.04 | 0.9711 |
| RPII3 |
F:CTTGTGAGGCTCTTGATTC R:GAGGCGAGGTAAAGTGTA |
75 | 88.96 | 0.9952 |
| 18SrRNA |
F:CTATGAGTCTGGTAATTGGAAT R:GCAACAACTTAAATATACGCTAT |
123 | 95.76 | 0.9880 |
| RPS18 |
F:GGTTAGCGATGATAGTTACAAT R:CAACATAGATGGCAACAGAA |
167 | 92.63 | 0.9921 |
| AK |
F:CTTGTCTGTCTTGCTGAA R:CGATTCTGGTGTTGGTATT |
112 | 96.2 | 0.9893 |
| EF1A |
F:AACAACCAACACCAACAC R:TAGGCATACCACGACTTC |
153 | 90.37 | 0.9773 |
| RPL27 |
F:CTGATACCAATGTCCACAA R:CCACCACAGAATCAACTT |
134 | 92.62 | 0.9911 |
| RPL29 |
F:GGAATCAAGAAGCCAACAC R:AATCTCTGGTTACGAAGGAAT |
77 | 92.21 | 0.9896 |
Data mining and statistical analysis
In order to select the most stable reference gene for RT-qPCR in A. gifuensis, four Microsoft Excel software Add-Ins: geNorm, NormFinder, BestKeeper, ΔCt method, and one web statistical tool, RefFinder, were used for analysis of expression profiles of 12 candidate genes using various experimental conditions. GeNorm uses the gene-stability measure M to calculate mean pairwise variations between an individual gene and the remaining candidate genes, the value of which is inversely proportional to its stability, the recommended M value is M < 0.5 for homogeneous samples, and for heterogeneous samples is M < 1. Pairwise variation (Vn/n+1) is generally used for the accurate normalization of geNorm to determine the optimal number of internal reference genes. A Vn/n+1 < 0.15 indicates that the additional reference gene will not significantly increase the standardization, The first V-value, 0.15, was after V2/3, indicates that the two reference genes are sufficient to be normalized. NormFinder uses a model-based approach to assess the overall variations in the expression of the candidate reference genes, and a more stable expression of the candidate gene shows a lower stability value. The standard deviation (SD) of the geometric mean of the Ct values of the candidate reference genes were used by BestKeeper, and the lower the index scores, the more stable the reference genes. The delta Ct method calculates the relative expression levels of the gene pairs between one and the other, and the candidate reference genes with smaller SD values are more stable. Finally, RefFinder, a web-based analysis tool, was used to compare and ranke the candidate genes, which provides an overall ranking of the stability of the candidate genes.
Validation of the selected reference genes
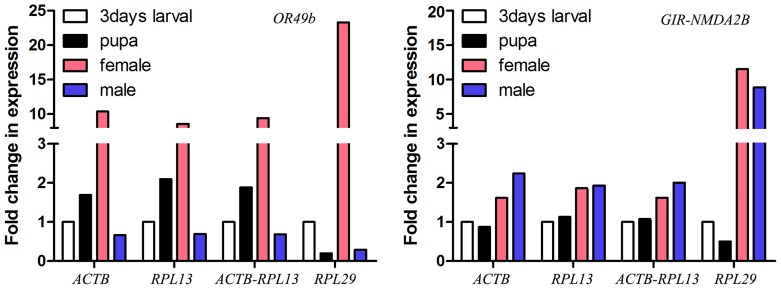
To assess the effects of various treatments on the gene expression profile of the samples, the relative expression profile of OR49b (odorant receptor 49b) and GIR-NMDA2B (glutamate receptor ionotropic, NMDA 2B) were evaluated at three life stages (larva, pupa, and adult). The primers used to identify and amplify OR49b and GIR-NMDA2B were listed in S2 Table. The expression of OR49b and GIR-NMDA2B were normalized with the two most stable reference genes (ACTB, RPL13) and the most unstable gene (RPL29). The 2-ΔΔCt method was used to calculate the relative quantification of OR49b and GIR-NMDA2B expression [19].
Results
PCR amplification and expression profiling of candidate reference genes in A. gifuensis
Twelve candidate reference genes were initially studied by reverse transcription polymerase chain reaction (RT-PCR). All amplicons were sequenced and identical to their corresponding transcripts, and all gene candidates were detected on a 2% agarose gel as a single amplicon with the desired size.
The amplification efficiency (E) values of all candidate reference genes ranged from 88.96% to 96.20%, and the correlation coefficient (R2) values ranging from 0.971to 0.995 (Table 1 and S1 Fig),based on five-fold serial dilution of the pooled cDNA and the generated standard curve of each gene.
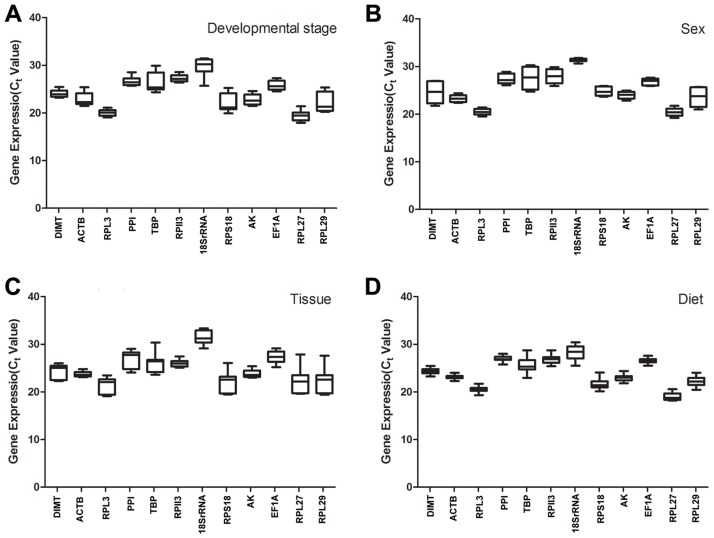
The levels of mRNA and the variable expression of the candidate reference genes were described using their mean values. In qRT-PCR, a variable Ct value for all the reference genes across the four treatments and the overall of all treatments indicated that their gene-expression levels were affected under different conditions (Fig 1). The expression of TBP, 18SrRNA, and RPL29 substantially varied in different development stages and sexes, whereas DIMT, ACTB, and RPL13 and ACTB, RPL13, and RPS18 showed the least variable expression profiles in different development stages and sexes, respectively. Additionally, almost half of the genes showed significant changes in expression levels in different tissues, whereas ACTB, RPII3, and AK showed a narrow range of variable Ct values. Furthermore, except for RPS18 and TBP that displayed highest variations in Ct values, the range of expression values of the other candidate reference genes were very narrow. Regardless of treatments, RPL13 performed the highest, the average Ct value was 20 in A. gifuensis.
Fig 1. Expression profiles of the 12 candidate reference genes in all four experiments.
Expression stability and ranking of the candidate reference genes
The candidate reference genes were ranked by employing four statistical algorithms under four different conditions. Developmental stages included 3-day-old larvae, pupae, and adult females and males. For sex, adult females and males were assessed. For tissues, the head, thorax, and abdomen were dissected from A. gifuensis. For diet, H2O and honey were given to adult males and females.
geNorm
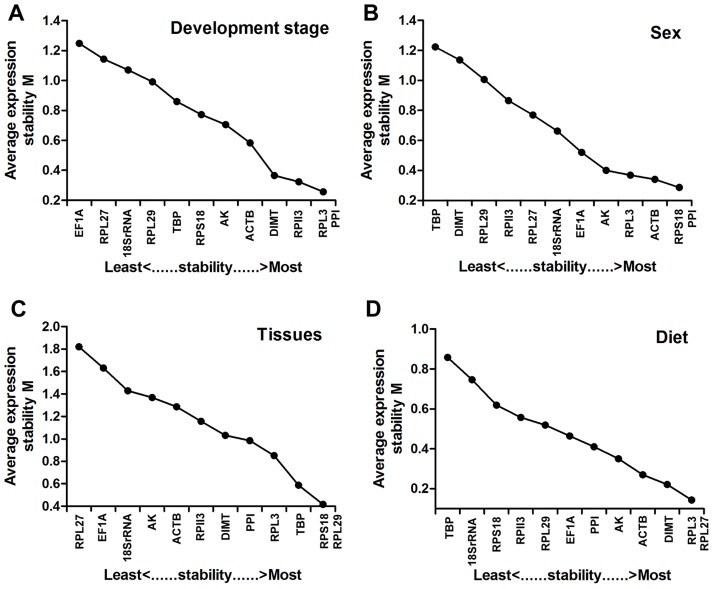
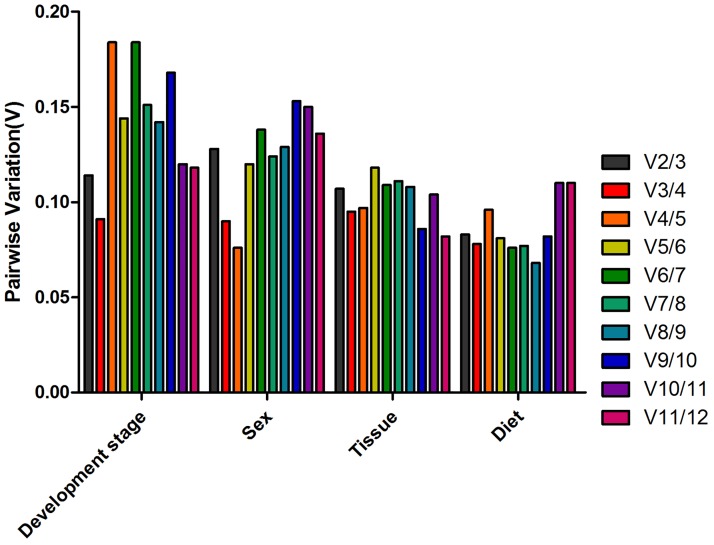
Considering the data obtained from various treatment conditions, all the reference genes showed significant variations in their expression levels across various treatment conditions. Developmental stage analyses identified RPL13 and RPII3 as the most stable reference genes, whereas RPS18 and TBP were the most unstable reference genes, respectively (Fig 2A). For sex, RPS18 and ACTB were co-ranked as the most stable reference genes (Fig 2B). For different tissues, the geNorm demonstrated that the expression of RPS18 and RPL29 were the most stable, whereas RPL27 was the most unstable (Fig 2C). The experiment using diverse diets indicated that RPL13 and PRL27 were the most stably expressed reference genes, followed by DIMT and ACTB (Fig 2D). The overall order based on geNorm from the most stable to the least stable reference genes were shown in Table 2. To determine the minimum number of genes required for normalization, we used geNorm to calculate the V-value. Our study showed that all the pairwise variation V2/3 values were < 0.15 across different conditions. Based on the same principle, in all treatments, two reference genes are needed for the reliable normalization with the first V-value < 0.15 appearing at V2/3 in A. gifuensis samples (Fig 3).
Fig 2. Expression stability and relative ranking of the 12 reference genes as predicted by using geNorm.
The mean expression stability (M) was calculated by stepwise exclusion of the least stable gene across all the samples within a particular group set. The mean stability of different genes is plotted; the least stable genes are represented on the left and the most stable on the right side of the plot.
Table 2. Stability of reference gene expression under four experimental conditions.
| Experimentconditions | Reference gene | geNorm | NormFider | BestKeeper | ΔCt | ||||
|---|---|---|---|---|---|---|---|---|---|
| Stability | Rank | Stability | Rank | Stability | Rank | Stability | Rank | ||
| Developmental stage | DIMT | 0.365 | 3 | 0.96 | 7 | 0.611 | 1 | 1.21 | 7 |
| AK | 0.706 | 5 | 0.58 | 3 | 0.912 | 7 | 1.096 | 5 | |
| PPI | 0.323 | 2 | 0.542 | 2 | 0.741 | 4 | 1.007 | 2 | |
| RPII3 | 0.257 | 1 | 0.723 | 6 | 0.621 | 2 | 1.077 | 4 | |
| TBP | 1.07 | 9 | 1.167 | 10 | 1.685 | 11 | 1.413 | 10 | |
| ACTB | 0.583 | 4 | 0.345 | 1 | 1.105 | 8 | 1.001 | 1 | |
| 18SrRNA | 1.248 | 11 | 1.567 | 12 | 1.269 | 9 | 1.775 | 12 | |
| RPS18 | 0.991 | 8 | 1.094 | 9 | 1.561 | 10 | 1.366 | 8 | |
| RPL13 | 0.257 | 1 | 0.665 | 5 | 0.694 | 3 | 1.033 | 3 | |
| RPL27 | 0.859 | 7 | 1.072 | 8 | 0.82 | 6 | 1.375 | 9 | |
| RPL29 | 1.143 | 10 | 1.238 | 11 | 1.707 | 12 | 1.502 | 11 | |
| EF1A | 0.772 | 6 | 0.621 | 4 | 0.764 | 5 | 1.12 | 6 | |
| Sex | DIMT | 1.136 | 10 | 1.474 | 11 | 2.238 | 11 | 1.57 | 11 |
| AK | 0.369 | 3 | 0.428 | 4 | 0.622 | 4 | 0.96 | 4 | |
| PPI | 0.4 | 4 | 0.255 | 2 | 0.96 | 8 | 0.95 | 3 | |
| RPII3 | 0.865 | 8 | 0.569 | 5 | 1.502 | 9 | 1.1 | 6 | |
| TBP | 1.224 | 11 | 1.587 | 12 | 2.327 | 12 | 1.66 | 12 | |
| ACTB | 0.286 | 1 | 0.349 | 3 | 0.708 | 6 | 0.94 | 2 | |
| 18SrRNA | 0.662 | 6 | 1.36 | 9 | 0.289 | 1 | 1.51 | 9 | |
| RPS18 | 0.286 | 1 | 0.148 | 1 | 0.822 | 7 | 0.89 | 1 | |
| RPL13 | 0.34 | 2 | 0.588 | 6 | 0.542 | 2 | 1 | 5 | |
| RPL27 | 0.769 | 7 | 1.371 | 10 | 0.623 | 5 | 1.54 | 10 | |
| L29 | 1.007 | 9 | 1.165 | 8 | 1.923 | 10 | 1.4 | 8 | |
| EF1A | 0.52 | 5 | 0.795 | 7 | 0.62 | 3 | 1.16 | 7 | |
| Tissue | DIMT | 1.032 | 5 | 0.721 | 3 | 1.299 | 5 | 1.502 | 3 |
| AK | 1.369 | 8 | 1.253 | 8 | 0.689 | 2 | 1.803 | 8 | |
| PPI | 0.985 | 4 | 0.824 | 4 | 1.543 | 7 | 1.535 | 4 | |
| RPII3 | 1.157 | 6 | 0.616 | 2 | 0.709 | 3 | 1.48 | 2 | |
| TBP | 0.588 | 2 | 1.17 | 7 | 1.586 | 8 | 1.712 | 7 | |
| ACTB | 1.286 | 7 | 1.105 | 6 | 0.438 | 1 | 1.683 | 6 | |
| 18SrRNA | 1.428 | 9 | 1.321 | 9 | 1.151 | 4 | 1.838 | 10 | |
| RPS18 | 0.416 | 1 | 1.082 | 5 | 1.809 | 10 | 1.625 | 5 | |
| RPL13 | 0.851 | 3 | 0.37 | 1 | 1.416 | 6 | 1.372 | 1 | |
| RPL27 | 1.821 | 11 | 2.556 | 12 | 1.978 | 12 | 2.738 | 12 | |
| L29 | 0.416 | 1 | 1.37 | 10 | 1.953 | 11 | 1.804 | 9 | |
| EF1A | 1.632 | 10 | 2.529 | 11 | 1.757 | 9 | 2.735 | 11 | |
| Diet | DIMT | 0.221 | 2 | 0.219 | 3 | 0.479 | 5 | 0.653 | 3 |
| AK | 0.35 | 4 | 0.334 | 4 | 0.554 | 7 | 0.706 | 5 | |
| PPI | 0.41 | 5 | 0.499 | 7 | 0.518 | 6 | 0.785 | 7 | |
| RPII3 | 0.519 | 7 | 0.46 | 6 | 0.725 | 8 | 0.771 | 6 | |
| TBP | 0.746 | 10 | 1.303 | 11 | 1.168 | 11 | 1.398 | 11 | |
| ACTB | 0.27 | 3 | 0.353 | 5 | 0.399 | 1 | 0.698 | 4 | |
| 18SrRNA | 0.858 | 11 | 1.326 | 12 | 1.201 | 12 | 1.418 | 12 | |
| RPS18 | 0.619 | 9 | 0.772 | 10 | 0.861 | 10 | 0.968 | 10 | |
| RPL13 | 0.143 | 1 | 0.071 | 1 | 0.444 | 3 | 0.61 | 1 | |
| RPL27 | 0.143 | 1 | 0.083 | 2 | 0.422 | 2 | 0.61 | 2 | |
| L29 | 0.557 | 8 | 0.51 | 8 | 0.8 | 9 | 0.817 | 8 | |
| EF1A | 0.464 | 6 | 0.631 | 9 | 0.466 | 4 | 0.86 | 9 | |
Fig 3. Pairwise variation (V) values in four experimental groups by using geNorm.
Average pairwise variations (V) were calculated between the normalization factors NFn and NFn+1 by geNorm software to indicate the optimum number of reference genes required for qRT-PCR data normalization. A threshold value below 0.15 indicated that the additional reference gene has no significant improvement on normalization in qRT-PCR data.
BestKeeper
Based on the SD of the Ct values in BestKeeper analysis, 18SrRNA was identified as the most stably expressed gene, and RPL29 was the least stably expressed gene in different developmental stages. For sex, 18SrRNA was considered the most stable gene, whereas TBP showed the highest SD. ATCB was the most stable genes in different tissues and diet samples under the algorithmic principle by BestKeeper, whereas RPL27 and RPS18 were the least stable genes in different tissues and diet samples, respectively. The overall order of the most stable reference genes based on BestKeeper is shown in Table 2.
NormFinder
In our study, NormFinder analysis indicated that ACTB was the most stable gene in different development stages. For sex, RPS18 showed the highest expression stability, which was similar to the result of geNorm. Among different tissues and diet, the most stable gene was RPL13; however, in geNorm, RPS18 and RPL29 were the most stable reference genes in different tissues, whereas these were identified as unstable in NormFinder. Based on the results of NormFinder analysis the overall order of the most stable to the most unstable reference gene is shown in Table 2.
DeltaCt method
Table 2 shows that under the inversely proportional (average SD) ΔCt method, ACTB was the most stably expressed reference gene in different development stages, whereas in terms of different sexes, RPS18 was identified as most stably expressed gene. RPL13 was the most stable gene for both different tissues and diet experiments. Based on the results of the ΔCt method, the overall order from the most to least stable expressed reference genes is shown in Table 2.
Comprehensive ranking of reference genes
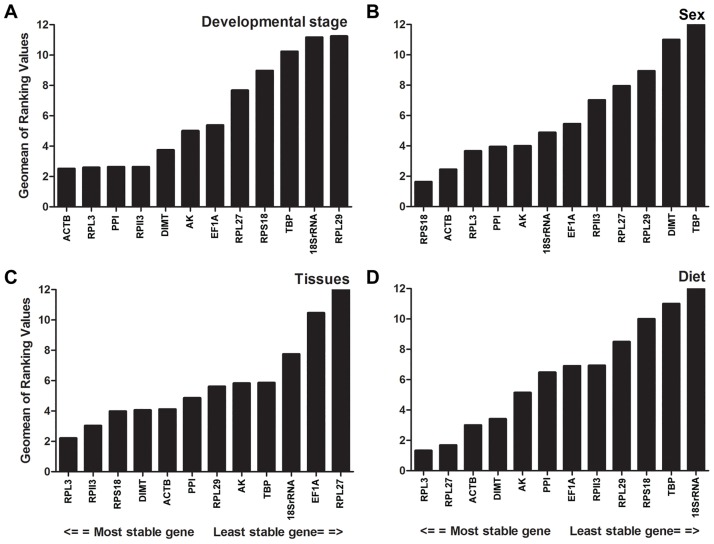
The overall ranking of 12 candidate reference genes under the four treatments was generated, based on the geometric mean (GM) of the rankings obtained from four complementary statistical methods,. The following reference genes are ranked in descending order of expression stability: different development stages: ACTB, RPL13, PPI, PRII3, DIMT, AK, EF1A, RPL27, RPS18, TBP, 18SrRNA, and RPL29 (Fig 4A); for sex: RPS18, ACTB, RPL13, PPI, AK, 18SrRNA, EF1A, PRII3, RPL27, RPL29, DIMT, and TBP (Fig 4B); for tissue type: RPL13, PRII3, RPS18, DIMT, ACTB, PPI, RPL29, AK, TBP, 18SrRNA, EF1A, and RPL27 (Fig 4C); for diet: RPL13, RPL27, ACTB, DIMT, AK, PPI, EF1A, PRII3, RPL29, RPS18, TBP, and 18SrRNA (Fig 4D).
Fig 4. Stability of candidate reference gene expression under different treatments.
A lower Geomean value indicates more stable expression according to RefFinder.
Validation of the recommended reference genes
Analysis of the consolidated data indicated that ACTB and RPL13 were the most stably expressed gene at different developmental stages, whereas RPL29 showed the lowest stability in expression. To examine the validity either as single or in combination of the selected reference genes, the applicability of these reference genes in normalization was tested. Odorant receptor (OR49b) and GIR-NMDA2B were investigated under different developmental stages. In the case of different developmental stages, either ACTB and RPL13 or their combination as normalizer showed more consistent qRT-PCR data compared to that using RPL29 in normalization (Fig 5). Furthermore, the expression profiles of OR49b and GIR-NMDA2B clearly exhibited differences when RPL29 was used in the normalization.
Fig 5. Validation of the recommended reference genes.
Expression profiles of OR49b and GIR-NMDA2 were investigated using different normalization factors. The expression of OR49b and GIR-NMDA2 was normalized using the best reference gene (ACTB), the second best reference gene (RPL13), the top two NF (ACTB—RPL13) and the worst reference gene (RPL29). Bars represent the means and standard deviation of three biological replications.
Discussion
QRT-PCR has become an important tool for gene expression analysis based on its high accuracy, specificity, sensitivity, and repeatability, [20–23]. However, variations in qRT-PCR data may be unavoidable during PCR analysis [6, 7], and this may be due to differences in specific conditions. Therefore, normalization using reference genes has been adapted into qRT-PCR assays to offset confounding variations among extensive experimental datasets [8, 9, 24, 25]. An ideal reference gene should be stably expressed in all test samples [26]. Unfortunately, qRT-PCR analysis is largely influenced by the choice of reference genes, and in response to different experimental conditions, the expression of commonly used reference genes may significantly change[14, 27, 28]. Thus, candidate reference genes should be validated under different conditions before utilized in actual research studies. A growing number of reference genes has been assessed for application to qRT-PCR analyses [5, 8, 10, 29–32]. However, no related studies involving references genes in A. gifuensis have been conducted to date. The present study performed a comprehensive evaluation of 12 commonly used reference genes using geNorm, NormFinder, BestKeeper, ΔCt method, and RefFinder at different developmental stages, sexes, tissue types, and diets.
Unlike previous studies [32, 33], the present study detected significant differences in candidate gene expression levels under specific conditions and based on four independent statistical analyses, which is similar to the results of previous investigations [27, 34, 35]. For example, in terms of developmental stage, RPL13 and ACTB were co-ranked by geNorm as the most stably expressed reference genes, whereas BestKeeper identified DIMT, and NormFinder and ΔCt method indicated ACTB. In terms of different tissues, the four analytical approaches generated similar results, wherein 18SrRNA and RPL29 were the most stably expressed genes by geNorm, BestKeeper identified ACTB, and NormFinder and ΔCt method RPL13. In addition, RPS18 and RPL13 were identified as the optimal reference genes by geNorm, and NormFinder and ΔCt identified the same gene for sexes and diets, respectively, whereas DIMT and ATCB were the more stably expressed genes based on BestKeeper. BestKeeper has been shown to exhibit the highest number of discrepancies [36]. Based on our findings, we conclude that RefFinder generated the most reliable results because it combined the four algorithms. Therefore, the optimal reference genes for the four common variables in A. gifuensis were as follows: ACTB, RPL13, and PPI for developmental stage, RPS18, ACTB, and RPL13 for sex, RPL13, PRII3, and 18SrRNA for tissue type, and RPL13, RPL27, and ACTB for diet.
The most commonly used internal reference genes include actin, tubulin, and 18S ribosomal RNA (rRNA), which are integral components of cells and are thus essential for the maintenance of physiological activities[4, 23, 37–39]. The transcript levels of these reference genes are generally less susceptible to the external environment than other genes [40]. However, certain limitations such as batch to batch variations in output and variable reverse transcription and PCR efficiencies may influence threshold (Ct) values, which are the main data collected during normalization [16, 41, 42]. Collectively, the results of our study have validated the findings of earlier reports that no single reference gene is stably expressed in all treatments [8, 27, 30, 43]. The novel reference gene, DIMT, which encodes a dimethyladenosine transferase that, specifically dimethylates two adjacent adenosines and is situated within a conserved hairpin loop near the 3′-end of the 18S rRNA of the 40S ribosomal subunit[44], is differentially expressed among different developmental stages and sexes. The present study has also determined that RPII3 should be excluded as a stably expressed reference gene, similar to the findings of a previous study involving Raphanus sativus L.[45]. ACTB is constitutively expressed and is a widely used reference gene for insects [27, 46–49]; however, the use of actin genes has recently been challenged [11]. The results of the present study also show that ACTB is stably expressed during different developmental stages and sexes. Ribosomal proteins are involved in translation and protein synthesis. In the present study, geNorm analysis identified RPL13 as the most stably expressed candidate reference gene in different tissue types and diets, whereas RPS18 was the most stably expressed gene for different sexes, and RPL29 in different tissue types. Similar to previous studies, the findings of our study indicate that ribosomal proteins are the best reference genes for investigating expression profiles in insects [37, 50]. However, RPL27 exhibited relatively unstable expression in our study. Furthermore, TUB, EF1A, TBP, and AK, which have been previously reported to be the most stably expressed genes [27, 37, 51], showed relatively unstable in expression using each variable. These results further suggest that the most suitable reference genes do not necessarily meet the requirement that these are expressed at constant levels under different conditions in various species [5, 6, 52]. Therefore, it is a highly recommended that a customized reference gene be selected for specific experimental conditions.
ORs are key components of insect olfaction. Odorant-binding proteins (OBPs) are transported through the infectious lymphids, filling the cavity around the dendrites and activating ORs, which in turn transform external chemical signals into signaling currents. Ionotropic glutamate receptor, which are key players in synaptic plasticity, mediate the transmission of excitatory synapses[53]. The validation of reference genes was confirmed by assessing the expression profile of OR49b and GIR-NMDA2B. The results showed that transcript abundance is strongly influenced by A. gifuensis development. The expression after normalization by RPL29 differed from that of ACTB or RPL13. Thus, the normalized results based on RPL29 cannot reliably reflect the expression level of target genes in A. gifuensis.
In conclusion, functional genomics and gene expression remain essential research topics relating to A. gifuensis. In this study, five algorithms (geNorm, NormFinder, BestKeeper, ΔCt method, and RefFinder) were used to evaluate the expression profiles of 12 candidate reference genes of A. gifuensis across four variable (developmental stage, sex, tissue type, and diet). We have identified the appropriate genes that can be used in the normalization of the qRT-PCR data under each variable. Furthermore, we suggest two reference genes that can be used not only to normalize expression data but also for more conservative estimates of target gene expression levels in A. gifuensis. Our findings may serve as a foundation for future genetics and functional genomics research on this particular insect species.
Supporting information
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We thank Xiao-Li Zhang (Institute of Cotton Research, Chinese Academy of Agricultural Sciences) for helping in sample collection.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Natural Science Foundation (31572015) ZS.
References
- 1.Bustin SA, Benes V, Nolan T, Pfaffl MW. Quantitative real-time RT-PCR—a perspective. Journal of Molecular Endocrinology. 2005;34(3):597 doi: 10.1677/jme.1.01755 [DOI] [PubMed] [Google Scholar]
- 2.Kubista M, Andrade JM, Bengtsson M, Forootan A, Jonák J, Lind K, et al. The real-time polymerase chain reaction. Molecular Aspects of Medicine. 2006;27(2–3):95–125. doi: 10.1016/j.mam.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 3.Vanguilder HD, Vrana KE, Freeman WM. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques. 2008;44(5):619–26. doi: 10.2144/000112776 [DOI] [PubMed] [Google Scholar]
- 4.Radonić A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochemical & Biophysical Research Communications. 2004;313(4):856–62. [DOI] [PubMed] [Google Scholar]
- 5.Li R, Xie W, Wang S, Wu Q, Yang N, Yang X, et al. Reference gene selection for qRT-PCR analysis in the sweetpotato whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Plos One. 2013;8(1):e53006 doi: 10.1371/journal.pone.0053006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu X, Yuan M, Shakeel M, Zhang Y, Wang S, Wang X, et al. Selection and Evaluation of Reference Genes for Expression Analysis Using qRT-PCR in the Beet Armyworm Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). Plos One. 2014;9(1):e84730 doi: 10.1371/journal.pone.0084730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang C, Pan H, Liu Y, Zhou X. Selection of reference genes for expression analysis using quantitative real-time PCR in the pea aphid, Acyrthosiphon pisum (Harris) (Hemiptera, Aphidiae). Plos One. 2014;9(11):e110454–e. doi: 10.1371/journal.pone.0110454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu W, Xie W, Zhang Z, Wang S, Wu Q, Liu Y, et al. Exploring Valid Reference Genes for Quantitative Real-time PCR Analysis in Plutella xylostella (Lepidoptera: Plutellidae). International Journal of Biological Sciences. 2013;9(8):792–802. doi: 10.7150/ijbs.5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang C, Pan H, Liu Y, Zhou X. Stably Expressed Housekeeping Genes across Developmental Stages in the Two-Spotted Spider Mite, Tetranychus urticae. Plos One. 2015;10(3):e0120833 doi: 10.1371/journal.pone.0120833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koramutla MK, Aminedi R, Bhattacharya R. Comprehensive evaluation of candidate reference genes for qRT-PCR studies of gene expression in mustard aphid, Lipaphis erysimi (Kalt). Scientific Reports. 2016;6:25883 doi: 10.1038/srep25883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selvey S, Thompson E, Matthaei K, Lea R, Irving M, Griffiths L. Beta-actin—an unsuitable internal control for RT-PCR. Molecular & Cellular Probes. 2001;15(5):307–11. [DOI] [PubMed] [Google Scholar]
- 12.Provenzano M, Mocellin S. Complementary techniques: validation of gene expression data by quantitative real time PCR. Advances in Experimental Medicine & Biology. 2007;593:66. [DOI] [PubMed] [Google Scholar]
- 13.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry. 2009;55(4):611–22. doi: 10.1373/clinchem.2008.112797 [DOI] [PubMed] [Google Scholar]
- 14.Vandesompele J, Preter KD, Pattyn F, Poppe B, Roy NV, Paepe AD, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen CL, Jensen JL, Ørntoft TF. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. 2004;64(15):5245–50. [DOI] [PubMed] [Google Scholar]
- 16.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnology Letters. 2004;26(6):509–15. [DOI] [PubMed] [Google Scholar]
- 17.Silver N, Best S, Jiang J, Thein SL. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Molecular Biology. 2006;7(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie F, Xiao P, Chen D, Xu L, Zhang B. miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs. Plant Molecular Biology. 2012;80(1):75–84. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 20.Willems E, Mateizel I, Kemp C, Cauffman G, Sermon K, Leyns L. Selection of reference genes in mouse embryos and in differentiating human and mouse ES cells. International Journal of Developmental Biology. 2006;50(7):627–35. doi: 10.1387/ijdb.052130ew [DOI] [PubMed] [Google Scholar]
- 21.Hoogewijs D, Houthoofd K, Matthijssens F, Vandesompele J, Vanfleteren JR. Selection and validation of a set of reliable reference genes for quantitative sod gene expression analysis in C. elegans. BMC Molecular Biology. 2008;9(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langnaese K, John R, Schweizer H, Ebmeyer U, Keilhoff G. Selection of reference genes for quantitative real-time PCR in a rat asphyxial cardiac arrest model. BMC Molecular Biology. 2008;9(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatsumi K, Ohashi K, Taminishi S, Okano T, Yoshioka A, Shima M. Reference gene selection for real-time RT-PCR in regenerating mouse livers. Biochemical & Biophysical Research Communications. 2008;374(1):106–10. [DOI] [PubMed] [Google Scholar]
- 24.Dheilly NM, Maure F, Ravallec M, Galinier R, Doyon J, Duval D, et al. Who is the puppet master? Replication of a parasitic wasp-associated virus correlates with host behaviour manipulation. Proceedings of the Royal Society B Biological Sciences. 2015;282(1803):20142773 doi: 10.1098/rspb.2014.2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan H, Yang X, Siegfried BD, Zhou X. A Comprehensive Selection of Reference Genes for RT-qPCR Analysis in a Predatory Lady Beetle, Hippodamia convergens (Coleoptera: Coccinellidae). Plos One. 2015;10(4):e0125868 doi: 10.1371/journal.pone.0125868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teng X, Zhang Z, He G, Yang L, Li F. Validation of Reference Genes for Quantitative Expression Analysis by Real-Time RT-PCR in Four Lepidopteran Insects. Journal of Insect Science. 2015;12(60):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponton F, Chapuis MP, Pernice M, Sword GA, Simpson SJ. Evaluation of potential reference genes for reverse transcription-qPCR studies of physiological responses in Drosophila melanogaster. Journal of Insect Physiology. 2011;57(6):840–50. doi: 10.1016/j.jinsphys.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 28.Shen GM. Evaluation of endogenous references for gene expression profiling in different tissues of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae). BMC Molecular Biology. 2010;11(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi XQ, Guo WC, Wan PJ, Zhou LT, Ren XL, Ahmat T, et al. Validation of reference genes for expression analysis by quantitative real-time PCR in Leptinotarsa decemlineata (Say). BMC Research Notes. 2013;6(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan M, Lu Y, Zhu X, Hu W, Shakeel M, Zhan S, et al. Selection and Evaluation of Potential Reference Genes for Gene Expression Analysis in the Brown Planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) Using Reverse-Transcription Quantitative PCR. Plos One. 2014;9(1):e86503 doi: 10.1371/journal.pone.0086503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinha DK, Smith CM. Selection of reference genes for expression analysis in Diuraphis noxia (Hemiptera: Aphididae) fed on resistant and susceptible wheat plants. Scientific Reports. 2014;4(3):5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang QL, Zhu QH, Liao X, Wang XQ, Chen T, Xu HT, et al. Selection of reliable reference genes for normalization of quantitative RT-PCR from different developmental stages and tissues in amphioxus. Scientific Reports. 2016;6:37549 doi: 10.1038/srep37549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhai Y, Lin Q, Zhou X, Zhang X, Liu T, Yu Y. Identification and validation of reference genes for quantitative real-time PCR in Drosophila suzukii (Diptera: Drosophilidae). Plos One. 2014;9(9):e106800 doi: 10.1371/journal.pone.0106800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mafra V, Kubo KS, Alvesferreira M, Ribeiroalves M, Stuart RM, Boava LP, et al. Reference Genes for Accurate Transcript Normalization in Citrus Genotypes under Different Experimental Conditions. Plos One. 2012;7(2):e31263 doi: 10.1371/journal.pone.0031263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo JL, Ling H, Wu QB, Xu LP, Que YX. The choice of reference genes for assessing gene expression in sugarcane under salinity and drought stresses. Scientific Reports. 2014;4:7042 doi: 10.1038/srep07042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petriccione M, Mastrobuoni F, Zampella L, Scortichini M. Reference gene selection for normalization of RT-qPCR gene expression data fromActinidia deliciosaleaves infected withPseudomonas syringaepv.actinidiae. Scientific Reports. 2015;5(16961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mamidala Praveen, Rajarapu SP, Jones SC, Mittapalli Omprakash. Identification and Validation of Reference Genes for Quantitative Real-Time Polymerase Chain Reaction in Cimex lectularius. Journal of Medical Entomology. 2011;48(4):947–51. [DOI] [PubMed] [Google Scholar]
- 38.Lu Y, Jiao Z, Wu K. Early season host plants of Apolygus lucorum (Heteroptera: Miridae) in northern China. J Econ Entomol. 2012;105(5):1603 [DOI] [PubMed] [Google Scholar]
- 39.Scharlaken Bieke dG DC, Goossens Karen, Brunain Marleen, Peelman Luc J., Jacobs Frans J.. Reference Gene Selection for Insect Expression Studies Using Quantitative Real-Time PCR: The Head of the Honeybee, Apis mellifera, After a Bacterial Challenge. 2008;8(33):1–10. [Google Scholar]
- 40.TD S, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. Journal of Biochemical & Biophysical Methods. 2000;46(1–2):69–81. [DOI] [PubMed] [Google Scholar]
- 41.Thellin O, Zorzi W, Lakaye B, De BB, Coumans B, Hennen G, et al. Housekeeping genes as internal standards: use and limits. Journal of Biotechnology. 1999;75(2–3):291–5. [DOI] [PubMed] [Google Scholar]
- 42.Tunbridge EM, Eastwood SL, Harrison PJ. Changed relative to what? Housekeeping genes and normalization strategies in human brain gene expression studies. Biological Psychiatry. 2011;69(2):173 doi: 10.1016/j.biopsych.2010.05.023 [DOI] [PubMed] [Google Scholar]
- 43.Svingen T, Jørgensen A, Rajpertde ME. Validation of endogenous normalizing genes for expression analyses in adult human testis and germ cell neoplasms. Molecular Human Reproduction. 2014;20(8):709–18. doi: 10.1093/molehr/gau030 [DOI] [PubMed] [Google Scholar]
- 44.Sánchez M, Revuelta JL, Del RF, Gwilliam R, Skelton J, Churcher C, et al. Analysis of 41 kb of the DNA sequence from the right arm of chromosome II of Schizosaccharomyces pombe. Yeast. 2001;18(12):1111–6. doi: 10.1002/yea.760 [DOI] [PubMed] [Google Scholar]
- 45.Xu Y, Zhu X, Gong Y, Xu L, Wang Y, Liu L. Evaluation of reference genes for gene expression studies in radish (Raphanus sativus L.) using quantitative real-time PCR. Biochemical & Biophysical Research Communications. 2012;424(3):398–403. [DOI] [PubMed] [Google Scholar]
- 46.Paim RM, Pereira MH, Di PR, Rodrigues JO, Guarneri AA, Gontijo NF, et al. Validation of reference genes for expression analysis in the salivary gland and the intestine of Rhodnius prolixus (Hemiptera, Reduviidae) under different experimental conditions by quantitative real-time PCR. 2012;5(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maroniche GA, Sagadín M, Mongelli VC, Truol GA, Vas MD. Reference gene selection for gene expression studies using RT-qPCR in virus-infected planthoppers. Virology Journal. 2011;8(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Hiel MB, Van WP, Temmerman L, Van SS, Vuerinckx K, Huybrechts R, et al. Identification and validation of housekeeping genes in brains of the desert locust Schistocerca gregaria under different developmental conditions. BMC Molecular Biology. 2009;10(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boer MED, Boer TED, Mariën J, Timmermans MJ, Nota B, Straalen NMV, et al. Reference genes for QRT-PCR tested under various stress conditions in Folsomia candida and Orchesella cincta (Insecta, Collembola). BMC Molecular Biology. 2009;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lord JC, Hartzer K, Toutges M, Oppert B. Evaluation of quantitative PCR reference genes for gene expression studies in Tribolium castaneum after fungal challenge. Journal of Microbiological Methods. 2010;80(2):219–21. doi: 10.1016/j.mimet.2009.12.007 [DOI] [PubMed] [Google Scholar]
- 51.Bansal R, Mian MA, Mittapalli O, Michel AP. Characterization of a Chitin Synthase Encoding Gene and Effect of Diflubenzuron in Soybean Aphid, Aphis Glycines. International Journal of Biological Sciences. 2012;8(10):1323–34. doi: 10.7150/ijbs.4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferguson BS, Nam H, Hopkins RG, Morrison RF. Impact of Reference Gene Selection for Target Gene Normalization on Experimental Outcome Using Real-Time qRT-PCR in Adipocytes. Plos One. 2012;5(12):e15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leal WS. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annual Review of Entomology. 2013;58(1):373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.