Abstract
Vascular malformation is occasionally complicated by consumptive coagulopathy, known as localized intravascular coagulopathy (LIC), which is characterized by a reduced fibrinogen level, an elevated D-dimer level and a normal platelet count. We report the case of a 17-year-old Japanese girl who presented with LIC secondary to extensive vascular malformations, whose condition had progressed to disseminated intravascular coagulation (DIC). She suddenly presented with severe anaemia, despite the absence of obvious bleeding, and she began to require regular red blood cell (RBC) transfusions. As she was suffering from paroxysmal atrial fibrillation, we treated her with dabigatran, after obtaining informed consent. Immediately after the administration of dabigatran, the results of clotting tests improved dramatically. Seven months later, she has not required any RBC transfusions, and the dabigatran treatment has been well tolerated. The present case report suggests that dabigatran may be a useful treatment option for patients with DIC associated with vascular malformations.
Keywords: dabigatran, disseminated intravascular coagulation, local intravascular coagulopathy, vascular malformation
Introduction
Vascular malformation, which is an embryonic structural anomaly of the vasculature, is a noninheritable congenital disorder; it can be classified as capillary malformation, venous malformation, lymphatic malformation or arteriovenous malformation [1]. Consumptive coagulopathy, known as localized intravascular coagulopathy (LIC), is common in slow-flow venous malformation and occurs frequently in cases with a large lesion size, a lesion location in the trunk, the involvement of the viscera and the presence of phleboliths. LIC is characterized by a reduced fibrinogen level, an elevated D-dimer level and a reduced or normal platelet count [2] and can progress to disseminated intravascular coagulation (DIC) resulting in death in some cases [3]. Kasabach–Merritt phenomenon is characterized by profound thrombocytopenia (less than 5.0 × 1010/l), a reduced fibrinogen level and a slightly elevated D-dimer level with predominant platelet destruction in lesions; this condition is often encountered in infants younger than 1 year of age and is associated with a high mortality rate [4,5]. As the treatments for LIC and Kasabach–Merritt phenomenon are completely different, their differentiation is critical. Treatment for patients with LIC or DIC is usually multifaceted and includes compression therapy, anticoagulation therapy and surgery such as sclerotherapy, endovascular laser and operative excision. Compression therapy, such as the use of compression garments, is strongly recommended to improve LIC safely. Anticoagulation therapy, such as subcutaneous low-molecular weight heparin (LMWH), can be administered to patients with a risk of thromboemboli. Surgery may enable a permanent improvement in patients with LIC by reducing the blood pooling arising from venous malformations; however, such procedures should be performed prior to the development of LIC or DIC [6]. An appropriate treatment for LIC or DIC associated with vascular malformations remains to be determined, as only low-level evidence is available.
Case presentation
A 17-year-old Japanese girl with consumptive coagulopathy and profound thrombocytopenia (5.0 × 1010/l) secondary to extensive venous/lymphatic vascular malformations involving the para-aortic varicosity and lymph nodes of the trunk and the abdominal viscera participated in a clinical trial examining the therapeutic efficiency of oral propranolol for Kasabach–Merritt phenomenon, but normalization of the platelet count was not achieved. She had also undergone anticoagulant therapies with nafamostat mesilate and recombinant thrombomodulin, in addition to the administration of fresh frozen plasma and factor XIII (FXIII) concentrates, at the time of an operation for a venous malformation that was performed for haemostasis and cosmetic purposes, but normalization of the platelet count and the fibrinogen level was not achieved. After her bleeding stopped, she did not take any medicines, such as anticoagulants.
In the spring of 2015, she suddenly presented with general fatigue, palpitations and dizziness and visited our emergency room. As laboratory testing showed severe anaemia (haemoglobin: 5.3 g/dl), she was admitted to the emergency department. She recovered and left the hospital after receiving her first red blood cell (RBC) transfusion. However, she had to receive 4–6 units of RBCs per week despite the absence of obvious bleeding, such as purpura, melena and hypermenorrhea.
Laboratory testing showed increased reticulocytes (10.1%) and reticulated platelets (11.9%); the haematopoietic capacity was enhanced. Schizocytes (10.9%) secondary to excessive coagulation were also observed, whereas her lactase dehydrogenase (LDH) level (127 U/l) and total/indirect bilirubin level (0.4/0.3 mg/dl) were normal, suggesting haemorrhagic anaemia, not microangiopathy. Coagulation testing showed a low platelet count (5.9 × 1010/l), a reduced fibrinogen level (107 mg/dl), a reduced FXIII (7.5%) and an increased FDP (fibrin/fibrinogen degradation products) level (201.5 μg/ml), whereas the prothrombin time (PT: 90%), the activated partial thromboplastin time (APTT: 35.7 s) and the antithrombin level (113%) were all normal. The cause of the severe anaemia seemed to be chronic haemorrhage from the fragile vascular walls of the venous malformations, resulting in severe iron deficiency (Fe, 10 μg/dl; ferritin, 5 ng/ml).
To clarify the bleeding location, a computed tomography (CT) examination was performed; an extensive lymphatic malformation (low-density area) of the para-aorta from the neck to the pelvis and a pudic venous malformation on the surface of the body were observed. After the severe anaemia, new lesions had emerged as multiple cystic-venous malformations with slow-flow in the abdominal para-aorta (Fig. 1a) and an edematous region on the descending colon (Fig. 1b-1). An upper esophagogastroduodenoscopy showed no bleeding, and a colonoscopy showed extensive venous malformation (without bleeding) on the luminal surface of the descending colon (Fig. 1b-2), indicating that the mucosal side of the descending colon was composed of a venous malformation, whereas the edematous wall thickening of the descending colon was composed of a lymphatic malformation. The results of a small-bowel capsule endoscopy showed active bleeding of the small bowel (Fig. 2a); a double-balloon enteroscopy was performed, but the active bleeding had already stopped, leaving scar ulcers (Fig. 2b) [7]. As a result, the LIC was thought to have progressed to DIC in the present case, and the condition was thought to have been exacerbated by the slow-flow cystic-venous malformation in the peritoneal cavity, resulting in active bleeding as a result of the rupture of the venous malformation into the lymphatic malformation in the descending colon. As the patient was also suffering from paroxysmal atrial fibrillation, she was initially treated with dabigatran [110 mg, twice daily (b.i.d.)] during hospitalization, as obvious bleeding was absent; low-dose dabigatran was started to avoid drug-induced bleeding, although her renal function was normal [serum creatinine, 0.42 mg/dl; estimated glomerular filtration rate (GFR), 161.6 ml/min/1.73 m2]. We obtained informed consent from both her and her mother before the administration of dabigatran. This treatment was approved by the Institutional Research Ethics Committee of the Faculty of Medicine, the University of Tokyo.
Fig. 1.
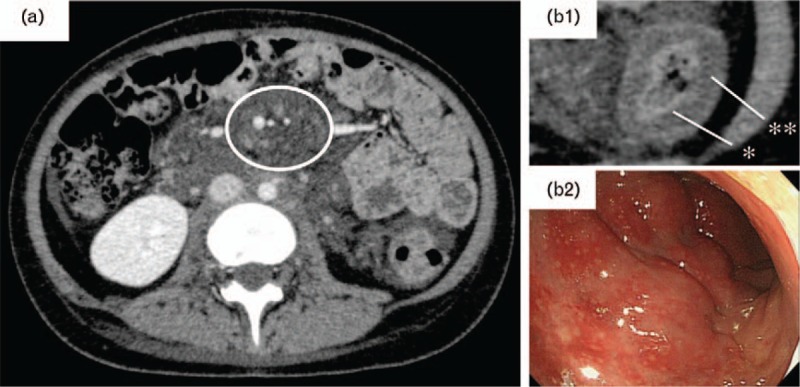
Body distribution of venous malformation and lymphatic malformation. An enhanced computed tomography (CT) image is shown. (a) Transverse section. The multiple, small, high-density areas surrounded by lymphatic malformation (low-density area) in the peritoneal cavity represent cystic-venous malformations with slow-flow. (b-1) Wall thickening of the ascending colon was initially observed. aThe lumen (high-density area) of the descending colon contains a venous malformation, whereas bthe edematous wall thickening (low-density area) of the descending colon contains a lymphatic malformation. (b-2) Colon enteroscopy. A venous malformation under the mucosa of the descending colon was observed, similar to the previous findings.
Fig. 2.
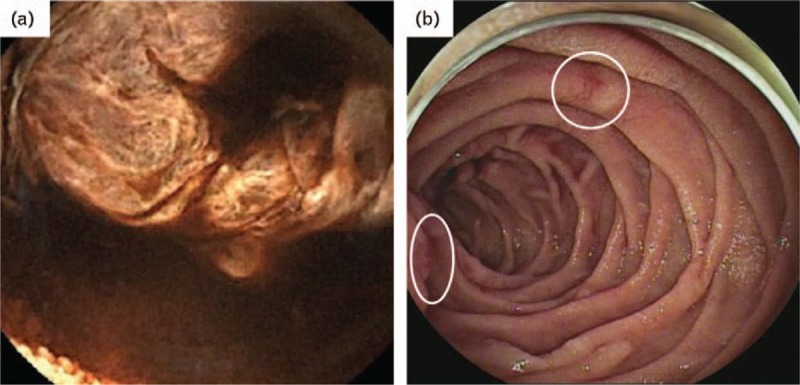
Endoscopic findings for the small bowel. (a) Small-bowel capsule endoscopy. The accumulation of fresh blood was observed. (b) Double-balloon enteroscopy. The active bleeding had already stopped but had left scar ulcers (white circle).
Immediately after the administration of dabigatran, the fibrinogen level rapidly recovered to within the reference value range, and the levels of FDP, D-dimer and thrombin antithrombin complex (TAT) and plasmin-α2 plasmin inhibitor complex (PIC) decreased dramatically. The platelet count was gradually normalized, and only the FXIII level failed to recover completely (Table 1). Therefore, FXIII concentrate was administered, as necessary. Although the results of a clotting test showed an obvious improvement, the anaemia persisted. As an improvement in the schizocytes was not obtained, the dose of dabigatran seemed to be inadequate. Finally, the dose of dabigatran was increased to 150 mg b.i.d. because her renal function remained normal and the regular administration of iron and FXIII concentrate had completely alleviated the need for transfusion. Seven months later, the administration of iron and FXIII concentrate was also stopped, and her FXIII level recovered from less than 10% to about 30% (Table 1). She has remained in good condition, and the dabigatran treatment has been well tolerated (Fig. 3).
Table 1.
Change in parameters from before the onset of anaemia until the present
| Before onset of anaemia | Onset of anaemia | During anaemia with dabigatran treatment | After improvement of anaemia with dabigatran treatment | |
| Platelets (x1010/l) | 4.1 | 5.9 | 15.2 | 9.5 |
| Reticulocytes (%) | 2.3 | 10.1 | 13.1 | 1.9 |
| IPF (%) | Not measured | 11.9 | 4.3 | 5.5 |
| Schizocytes (%) | 1.8 | 10.9 | 6.6 | 1.0 |
| Fibrinogen (mg/dl) | 79 | 107 | 302 | 181 |
| FDP (μg/ml) | 143.3 | 201.5 | 62.7 | 32.8 |
| D-dimer (μg/ml) | 81.4 | 121.8 | 29.2 | 16.7 |
| TAT (ng/ml) | 68.6 | Not measured | 4.3 | 4.7 |
| PIC (μg/ml) | 8.9 | Not measured | 2.7 | 5.2 |
FDP, fibrin/fibrinogen degradation products; IPF, immature platelet fraction; PIC, plasmin-α2 plasmin inhibitor complex; TAT, thrombin antithrombin complex.
Fig. 3.
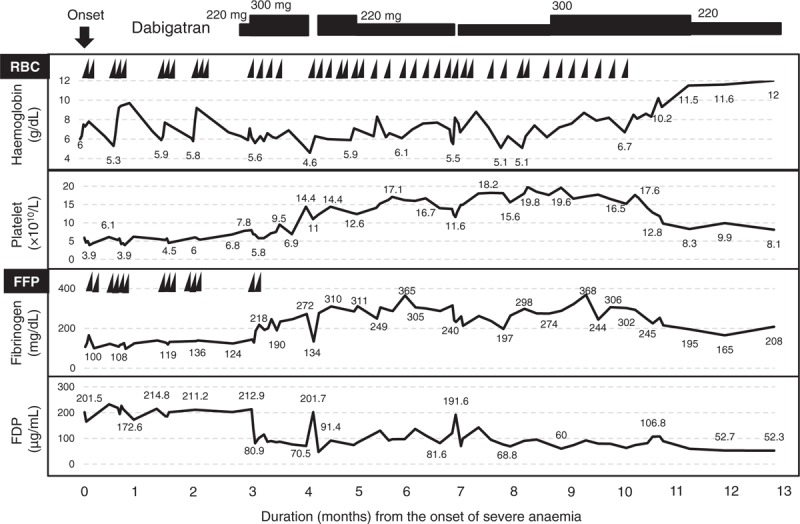
Clinical course of the present patient after the onset of severe anaemia. FDP, fibrin/fibrinogen degradation product; FFP, fresh frozen plasma; RBC, red blood cell concentration.
Discussion
The greatest concern associated with this case was the difficulty in identifying the bleeding location, despite the presence of severe anaemia requiring a massive transfusion. A mild small-intestinal haemorrhage that did not require a massive transfusion was observed, but repeated CT examinations and bleeding scintigraphy did not show active bleeding or bloody ascites in the body cavity. Therefore, multifocal microbleeding was thought to have flowed into the nearby lymphatic malformation through the fragile wall of the venous malformation.
Furthermore, this coagulopathy was shown to have been derived from LIC, which is characteristically associated with slow-flow, cystic and extensive venous malformations in the trunk and viscera. However, profound thrombocytopenia is inconsistent with LIC. The representative causes for consumptive thrombocytopenia associated with vascular malformation are Kasabach–Merritt phenomenon and the progression of LIC to DIC. Kasabach–Merritt phenomenon is known to be associated with an aggressive vascular tumour, such as a kaposiform haemangioendothelioma or tufted angioma, and can be pathologically distinguished from LIC [8]. In this case, the peak age of onset had passed, and the platelet count was not improved by the administration of propranolol [9], hence, a condition suggesting Kasabach–Merritt phenomenon was not observed pathologically. Next, chronic DIC, known as enhanced-fibrinolytic type, is characterized by a normal PT, a normal APTT, a low platelet count, a reduced fibrinogen level, a reduced FXIII and an increased FDP/D-dimer level secondary to aortic aneurysm and giant haemangioma and leads to systemic bleeding diathesis. The cause of reduced FXIII has been reported as consumptive reduction in a lesion [10,11].
In this case, neither an aortic aneurysm nor a giant haemangioma was detected, and systemic bleeding diathesis, such as purpura, was not found; however, she sometimes suffered from severe pain as a result of thrombi in the venous malformation, which is a typical symptom of patients with LIC. Also, the FDP levels, which are typically more than twice as high as the D-dimer levels in enhanced fibrinolytic-type DIC, were relatively low, and the TAT levels were much higher than the PIC levels before treatment (Table 1). On the basis of these findings, this type of coagulopathy was thought to have progressed to balanced fibrinolytic-type DIC. As the FXIII level had remained at less than 10% ever since it was first measured, we did not know whether the production of FXIII was normal. As the FXIII level increased to about 30% without any immunosuppressive therapies after the administration of dabigatran, we hypothesized that the low FXIII level was caused by the consumptive reduction, and not the production of autoantibodies.
During anaemia, the platelets were overproduced because of the upregulation of haematopoiesis, followed by a normalization of the platelet count. Once the anaemia had improved, the platelet count decreased to 8–9 × 1010/l. As the levels of FDP, D-dimer and TAT remained relatively high despite the administration of dabigatran, the consumptive reduction of FXIII and platelets was thought to exceed the production (Table 1).
This is the first study of the successful use of dabigatran to treat a patient with DIC. The recommended treatment of first choice for LIC associated with vascular malformation is LMWH, mainly enoxaparin [4,12]; on the contrary, the treatment of DIC developing from LIC remains to be determined. In Japan, however, the use of enoxaparin is limited to perioperative use, and only dalteparin can be administered as a continuous infusion. Rivaroxaban was recently reported to be an effective, well tolerated and more convenient alternative to LMWH for the reversal of LIC or DIC [13–17]. Our present patient was a rare case of DIC associated with vascular malformation with severe anaemia who could not be treated surgically. We hypothesized that the severe anaemia could be alleviated if the platelet count, the fibrinogen level and the FXIII level being excessively consumed by the DIC could be allowed to recover. In consideration of her quality of life, the administration of oral medications, such as direct oral anticoagulants (DOACs), seemed appropriate. DOACs include oral anti-Xa inhibitors, such as rivaroxaban, and direct thrombin inhibitors, such as dabigatran, with the crucial difference being that dabigatran can inactivate not only the free thrombin activity in blood but also the thrombin activity involved in thrombi. As thrombin activates both platelets and coagulation factor, dabigatran was expected to be superior to oral anti-Xa inhibitors for patients with chronic DIC with profound thrombocytopenia and a reduced fibrinogen level.
Dabigatran was administered to the currently reported patient, resulting in the alleviation of thrombocytopenia. Thereafter, the schizocytes completely disappeared after treatment with dabigatran at a dose of 150 mg b.i.d., and the patient's anaemia improved. In this regard, however, while dabigatran inhibits the hyperactivation of platelets, compared with warfarin or oral anti-Xa inhibitors, attention to the risk of critical bleeding caused by the excessive inhibition of platelet function is needed, particularly in nonconsumptive thrombocytopenic patients. If patients with DIC despite atrial fibrillation are suffering from profound thrombocytopenia, a reduced fibrinogen level and a reduced FXIII level and if they can take dabigatran, we recommend first obtaining written informed consent from the patients or obtaining the approval of the local ethics committee, in addition to taking precautions to prevent acute bleeding and its side effects and hospitalizing the patient until the platelet count increases.
In conclusion, DOACs might be more effective, safer and more convenient for DIC associated with extensive venous malformation. Dabigatran may be a useful new treatment option for cases with profound thrombocytopenia.
Acknowledgements
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- 1.Dasgupta R, Fishman SJ. ISSVA classification. Semin Pediatr Surg 2014; 23:158–161. [DOI] [PubMed] [Google Scholar]
- 2.Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol 1997; 36:219–225. [DOI] [PubMed] [Google Scholar]
- 3.Bick RL. Hereditary hemorrhagic telangiectasia and disseminated intravascular coagulation: a new clinical syndrome. Ann N Y Acad Sci 1981; 370:851–854. [DOI] [PubMed] [Google Scholar]
- 4.Mazoyer E, Enjolras O, Laurian C, Houdart E, Drouet L. Coagulation abnormalities associated with extensive venous malformations of the limbs: differentiation from Kasabach-Merritt syndrome. Clin Lab Haematol 2002; 24:243–251. [DOI] [PubMed] [Google Scholar]
- 5.Bousema MT, Kramer MH, Steijlen PM. Extensive capillary malformation with a compensated coagulopathy. Clin Exp Dermatol 1999; 24:372–374. [DOI] [PubMed] [Google Scholar]
- 6.Zhuo KY, Russell S, Wargon O, Adams S. Localised intravascular coagulation complicating venous malformations in children: associations and therapeutic options. J Paediatr Child Health 2017; 53:737–741. [DOI] [PubMed] [Google Scholar]
- 7.Greve E, Moussata D, Gaudin JL, Lapalus MG, Giraud S, Dupuis-Girod, et al. High diagnostic and clinical impact of small-bowel capsule endoscopy in patients with hereditary hemorrhagic telangiectasia with overt digestive bleeding and/or severe anemia. Gastrointest Endosc 2010; 71:760–767. [DOI] [PubMed] [Google Scholar]
- 8.Mulliken JB, Anupindi S, Ezekowitz RA, Mihm MC., Jr Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 13-2004. A newborn girl with a large cutaneous lesion, thrombocytopenia, and anemia. N Engl J Med 2004; 350:1764–1775. [DOI] [PubMed] [Google Scholar]
- 9.Sans V, de la Roque ED, Berge J, Grenier N, Boralevi F, Mazereeuw-Hautier J, et al. Propranolol for severe infantile hemangiomas: follow-up report. Pediatrics 2009; 124:e423–e431. [DOI] [PubMed] [Google Scholar]
- 10.Song JW, Choi JR, Song KS, Rhee JH. Plasma factor XIII activity in patients with disseminated intravascular coagulation. Yonsei Med J 2006; 47:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayakawa K, Tamura S, Gima H, Hayakawa T, Kurihara T, Fujimoto T, et al. Successful treatment of chronic disseminated intravascular coagulation using recombinant human soluble thrombomodulin in a dialysis patient with dissecting aortic aneurysm. Rinsho Ketsueki 2014; 55:2300–2305. [PubMed] [Google Scholar]
- 12.Beier UH, Schmidt ML, Hast H, Kecskes S, Valentino LA. Control of disseminated intravascular coagulation in Klippel-Trenaunay-Weber syndrome using enoxaparin and recombinant activated factor VIIa: a case report. J Med Case Rep 2010; 4:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandenbriele C, Vanassche T, Peetermans M, Verhamme P, Peerlinck K. Rivaroxavan for the treatment of consumptive coagulopathy associated with a vascular malformation. J Thromb Thrombolysis 2014; 38:121–123. [DOI] [PubMed] [Google Scholar]
- 14.Randrianarisoa E, Kopp HG, Balletshofer BM, Jaschonek K, Kanz L, Haering HU, et al. Management of disseminated intravascular coagulopathy with direct factor Xa inhibitor rivaroxaban in Klippel-Trenaunay syndrome. Blood Coagul Fibrinolysis 2013; 24:766–770. [DOI] [PubMed] [Google Scholar]
- 15.Ardillon L, Lambert C, Eeckhoudt S, Boon LM, Hermans C. Dabigatran etexilate versus low-molecular weight heparin to control consumptive coagulopathy secondary to diffuse venous vascular malformations. Blood Coagul Fibrinolysis 2016; 27:216–219. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi T, Nakagawa N, Kadohira Y, Morishiya E, Asakura H. Rivaroxaban in a patient with disseminated intravascular coagulation associated with an aortic aneurysm: a case report. Ann Intern Med 2014; 161:158–159. [DOI] [PubMed] [Google Scholar]
- 17.Kawano H, Hata T, Uda A, Maemura K. Use of rivaroxaban for the effective management of disseminated intravascular coagulation associated with abdominal aortic aneurysm. Intern Med 2015; 54:2625–2628. [DOI] [PubMed] [Google Scholar]


