Abstract
Study Design.
A retrospective, single-center study.
Objective.
Investigation of the changes in the treatment outcomes of patients with lung cancer derived metastatic spine tumors.
Summary of Background Data.
Metastatic spine tumors derived from lung cancer had been progressive, and their prognosis is poor. It has recently been reported that the use of molecularly targeted drugs and bone-modifying agents (BMAs) improved the treatment outcomes of patients with lung cancer, but no detailed information about the treatment of metastatic spine tumors has been reported.
Methods.
Two hundred seven patients with lung cancer derived metastatic spine tumors who were examined after 2000 were analyzed. They were divided into 54 patients who were treated in or before 2005 (surgical treatment: 25 patients, conservative treatment: 29 patients) (group B) and 153 patients who were treated from 2006 onwards, when a molecularly targeted drug and BMA were introduced (surgical treatment: 24, conservative treatment: 129) (group A), and the treatment outcomes of the two groups were compared.
Results.
Significant differences in age and the affected vertebral level, paralysis grade, and Tokuhashi score (general condition, the number of vertebral metastases, and the total score) were detected between the groups. Regarding treatment outcomes, the mean duration of the post-treatment survival period was 5.1 and 9.3 months in groups B and A, respectively, that is, it was significantly longer in group A (P < 0.05). No significant intergroup difference in pain improvement was noted, and no significant post-treatment improvement in paralysis was achieved in either group. The post-treatment discharge-to-home eligibility rate did not differ significantly between the groups, but the Barthel Index improved significantly after treatment in group A (P < 0.05).
Conclusion.
After molecularly targeted drugs and BMA were introduced as treatments for lung cancer derived metastatic spine tumors, the survival periods of patients with such tumors increased, and their activity of daily living after treatment improved.
Level of Evidence: 4
Keywords: activities of daily living, Barthel index, bone-modifying agent, chemotherapy, life expectancy, lung cancer, metastatic spine tumor, molecularly targeted drug, multidisciplinary treatment, radiation, surgery
Metastatic spine tumors derived from lung cancer are rapidly progressive and have a poor prognosis, as they are one of the most difficult types of metastatic spine tumor to treat.1 They rapidly cause paralysis in many cases, and the appropriateness of local treatment has to be judged promptly.1,2–5 It has recently been reported that molecularly targeted drugs and bone-modifying agents (BMAs) improved the treatment outcomes of patients with lung cancer or lung cancer derived metastatic spine tumors,6–9 but no detailed information regarding the treatment of lung cancer derived metastatic spine tumors has been published.9,10 In addition, the general approach to the treatment of spinal metastasis has changed over time, that is, it is now actively treated in a multidisciplinary manner by numerous hospital departments, and treatment selection methods have also altered. Thus, it might be necessary to investigate the outcomes of patients with lung cancer derived metastatic spine tumors based on not only their surgical outcomes but also the treatments administered, including conservative treatments.11,12
Since 1990, we have selected treatments for metastatic spine tumors based on predictions of treatment outcomes obtained using the revised version (Table 1)13 of the Tokuhashi score.14 However, the maximum total score did not reach 10 in lung cancer cases, and it was difficult to predict patients’ outcomes at 1 year or more using this method. On the contrary, gefitinib, a molecularly targeted drug, was introduced into Japan in 2002. It has been demonstrated to be effective against primary lung cancer, and its adverse effects are widely known. In addition, it has occasionally been reported to be effective against metastatic spine tumors derived from lung cancer.15 Furthermore, the BMA, zoledronate, and denosumab, were introduced in 2005 and 2012, respectively.
TABLE 1.
A Revised Tokuhashi Score14
| Predictive Factor | Score (Points) |
| General condition (KPS: Karnofsky performance status) | |
| Poor (KPS 10–40%) | 0 |
| Moderate (KPS 50–70%) | 1 |
| Good (KPS 80–100%) | 2 |
| Number of extraspinal bone metastases foci | |
| ≧3 | 0 |
| 1–2 | 1 |
| 0 | 2 |
| Number of metastases in the vertebral body | |
| ≧3 | 0 |
| 2 | 1 |
| 1 | 2 |
| Metastases to the major internal organs | |
| Unremovable | 0 |
| Removable | 1 |
| No metastases | 2 |
| Primary site of the cancer | |
| Lung, osteosarcoma, stomach, bladder, esophagus, pancreas | 0 |
| Liver, gallbladder, unidentified | 1 |
| Others | 2 |
| Kidney, uterus | 3 |
| Rectum | 4 |
| Thyroid, prostate, breast, carcinoid tumor | 5 |
| Spinal cord palsy | |
| Complete (Frankel A, B) | 0 |
| Incomplete (Frankel C, D) | 1 |
| None (Frankel E) | 2 |
| Total Points | Predicted Prognosis |
| 0–8 | <6 months |
| 9–11 | ≧6 months |
| 12–15 | ≧1 yr |
The treatment outcomes of patients who were treated before the introduction of these new drugs (the patients treated in or before 2005) were compared with those of the patients treated after the introduction of the new drugs (the patients treated from 2006 onwards).
MATERIALS AND METHODS
Patients
Of 215 patients with lung cancer derived metastatic spine tumors who were treated at our department after 2000, 207 patients were included in this study after excluding eight patients who dropped out at ≤6 months after treatment.
They were divided into 54 patients who were treated in or before 2005 (surgical treatment: 25 patients, conservative treatment: 29 patients) (group B) and 153 patients who were treated from 2006 onwards (after molecularly targeted drugs and BMA were included in the treatment strategy for such tumors; surgical treatment: 24, conservative treatment: 129) (group A), and the treatment outcomes of the two groups were compared. Data regarding sex, age, the affected vertebrae, pathology, the Tokuhashi score,13 pain [according to a 100-mm visual analog scale (VAS)], and paralysis state [according to the American Spinal Injury Association (ASIA) classification]16 for the two groups are summarized in Table 2. The treatment methods were selected on the basis of the Tokuhashi score,13 the attending physicians’ opinions, and the patients’ wishes, with the highest priority being given to the predicted prognosis. The use of combinations of treatment methods (adjuvant therapy) was not restricted in either the surgery or conservative treatment group, and the maximum possible multidisciplinary combination treatment was administered as often as possible. Cisplatin and carboplatin were usually used for chemotherapy. In radiotherapy, 20 to 30-Gy fractionated radiation were administered. The treatments administered in the two groups are summarized in Table 3.
TABLE 2.
Background of the 207 Patients
| Group B (2000–2005) | Group A (2006–2016) | |
| Male: female | 38:16 | 98:55 |
| Age, yrs | 45–81, 62.4 ± 8.7 | 36–89, 68.4 ± 10.7 |
| Affected level | ||
| Cervical | 15 | 12 |
| Thoracic | 23 | 96 |
| Lumbosacral | 16 | 45 |
| Pathology | ||
| Adenocarcinoma | 30 | 104 |
| Squamous | 14 | 19 |
| Small cell | 7 | 23 |
| Others | 3 | 7 |
| Tokuhashi score | ||
| General condition | ||
| 0 | 0 | 23 |
| 1 | 5 | 29 |
| 2 | 49 | 101 |
| Number of extraspinal bone metastases foci | ||
| 0 | 24 | 99 |
| 1 | 6 | 17 |
| 2 | 24 | 37 |
| Number of metastases in the vertebral body | ||
| 0 | 18 | 103 |
| 1 | 10 | 19 |
| 2 | 26 | 31 |
| Metastases to the major internal organs | ||
| 0 | 42 | 132 |
| 1 | 0 | 0 |
| 2 | 12 | 21 |
| Total score | 1–10, 5.4 ± 2.0 | 1–10, 4.6 ± 1.9 |
| 0–8 | 51 | 156 |
| 9–10 | 3 | 7 |
| VAS (100 mm) | 35–100, 80.4 ± 10.5 | 29–100, 76.5 ± 11.5 |
| ASIA classification | ||
| A | 1 | 0 |
| B | 5 | 0 |
| C | 14 | 20 |
| D | 17 | 10 |
| E | 17 | 123 |
TABLE 3.
Treatment for the 207 Patients
| Group B (2000–2005) | Group A (2006–2016) | |
| Surgeries (pts.) | 25 | 24 |
| Posterior decompression and stabilization | 11 | 16 |
| Posterior stabilization | 3 | 7 |
| Posterior decompression | 1 | 1 |
| Anterior curettage and anterior stabilization | 8 | 0 |
| Anterior curettage and posterior stabilization | 1 | 0 |
| Total en-bloc spondylectomy | 1 | 0 |
| Conservative treatment (pts.) | 15 | 106 |
| Adjuvant therapy (pts.) | ||
| Chemotherapy | 15 | 81 |
| Radiation therapy | 35 | 67 |
| Molecular targeted drug | 0 | 69 |
| Bone modifying agent | 0 | 56 |
| Only care | 14 | 23 |
Methods
Regarding treatment outcomes, the post-treatment survival period, pain score, paralysis grade, and the ability to perform activities of daily living (ADL) were compared between the groups. In addition, the parameters that exhibited significant changes in each group were analyzed.
Statistical Analyses
In the comparisons of the patient background data and treatment outcomes between the two groups, the t test, Welch method, Chi-square test, or Mann-Whitney U test was used. Survival analysis was also performed in both groups. Survival rates were calculated via the Kaplan-Meier method, and univariate analysis was conducted using the log-rank test. All statistical analyses were carried out using StatMate V (Atoms Co., Tokyo, Japan).
RESULTS
Survival Period
The mean duration of the post-treatment survival period was 5.1 ± 5.3 (0.2–29) and 9.3 ± 13.09 (0.2–114) months in groups B and A, respectively (P < 0.01). The survival rate was calculated using the Kaplan-Meier method and was subjected to univariate analysis using the log-rank test. The survival rate also differed significantly between the two groups (P < 0.05) (Figure 1). Regarding the consistency of the Tokuhashi score criteria, the concordance rate between a Tokuhashi score of 0 to 8 and life expectancy of < 6 months was 82.4% in group B, whereas it was only 60.5% in group A, and the survival period was ≥6 months in 39.5% of cases in group A (Figure 2), which clearly reflected the extension of the survival period seen after 2006. The overall concordance rate of the Tokuhashi score criteria was 85.2% in group B and 62.1% in group A, showing that the concordance rate between the Tokuhashi score criteria and the actual survival period decreased after 2006.
Figure 1.
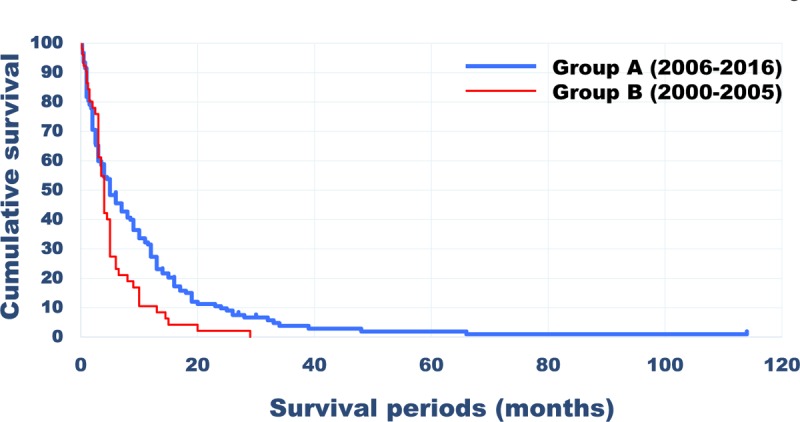
Survival rates calculated using the Kaplan-Meier method in Groups B and A.
Figure 2.
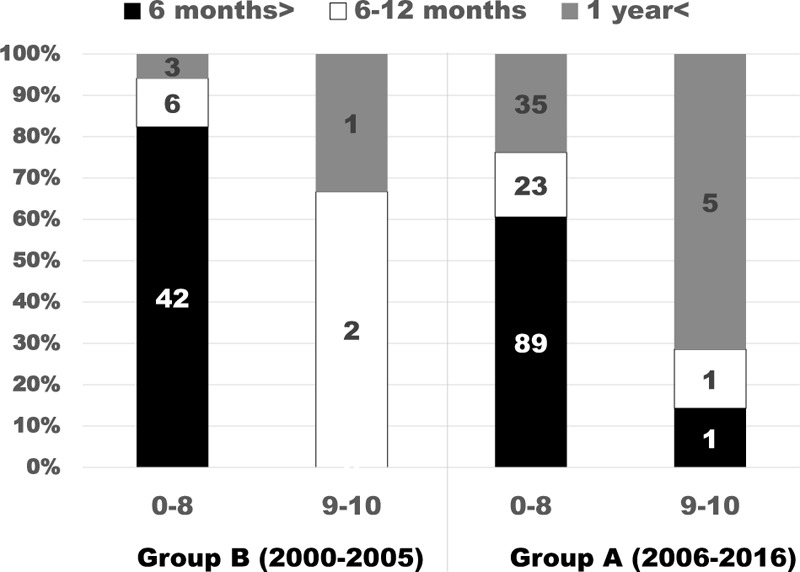
Actual survival time of Groups B and A by the total Tokuhashi score. On the basis of the prognostic criteria in the revised version of the Tokuhashi score (Table 1), the life expectancy of a total score of 0–8 is shorter than 6 months and that of 9–10 is 6 months or longer. The total score was 0–8 in Group A, showing that the survival time extended.
Pain
Pain was evaluated using a VAS. No significant difference was noted between the VAS scores of the two groups before treatment. The VAS tests were repeated at 1 month after treatment. The VAS score had improved in 50 (92.6%) of the 54 patients in group B, and the mean post-treatment VAS score was 25.1 ± 10.5 (0–50). In group A, the VAS score improved in 141 (92.2%) of the 153 patients, and the mean post-treatment VAS score was 26.8 ± 8.5 (0–57). No significant difference was noted between the VAS scores of the two groups, and both groups exhibited favorable outcomes.
Paralysis
Changes in the severity of paralysis were evaluated based on the ASIA grading system. The paralysis grades observed before treatment or at 1 month after treatment were compared with those seen at the final follow-up (paralysis status was evaluated at regular intervals until death). It was difficult to compare the outcomes of the two groups because the pre-treatment ASIA grade distributions of the two groups were significantly different (P < 0.001). In group A, as treatment was initiated before the onset of paralysis in many cases, none of the patients were categorized as ASIA grade A or B, and 123 (80.4%) of 153 patients were classified as grade E. In contrast, the pre-treatment ASIA grade was only E in 17 (31.5%) of the 54 patients in group B. The paralysis grade distributions seen before and after treatment are summarized in Table 4. When the pre-treatment paralysis grade was compared with that observed at 1 month after treatment, a slight post-treatment improvement in the paralysis grade was detected in group B, whereas in group A, some patients’ conditions improved but others’ worsened (no obvious tendency was detected). At the final follow-up examination, the patients’ paralysis was slightly worse than that seen before treatment in both groups. No significant differences were noted between the paralysis grades recorded before treatment and at 1 month after treatment, or between those seen before treatment and at the final follow-up, in either group.
TABLE 4.
Change of ASIA Classification in the Group B and Group A
| Group B | Before Treatment | After 1 Month | Final |
| Grade A | 1 | 0 | 1 |
| Grade B | 5 | 6 | 15 |
| Grade C | 14 | 14 | 11 |
| Grade D | 17 | 13 | 11 |
| Grade E | 17 | 21 | 17 |
| Group A | Before Treatment | After 1 Month | Final |
| Grade A | 0 | 0 | 1 |
| Grade B | 0 | 2 | 6 |
| Grade C | 20 | 12 | 14 |
| Grade D | 10 | 19 | 13 |
| Grade E | 123 | 120 | 118 |
ADL
The subjects’ ability to perform ADL was investigated using the Barthel Index.17,18 The mean pre-treatment Barthel Index was 62.6 ± 31.6 (0–100) in group B and 60.7 ± 27.6 (0–100) in group A (P > 0.05). The post-treatment discharge-to-home eligibility rates of groups B and A were 29.4% and 46.7%, respectively (P > 0.05). The mean Barthel Index at the final follow-up was 68.1 ± 29.8 (0–100) in group B and 70.2 ± 24.9 (0–100) in group A. It did not change significantly after treatment in group B, but it significantly improved after treatment in group A (P < 0.05) (Figure 3).
Figure 3.
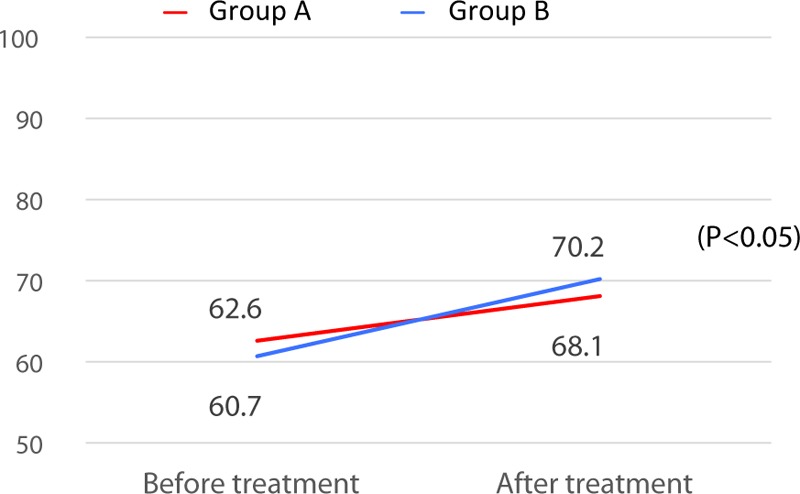
Changes in the Barthel Index on the final follow-up (status of paralysis at regular intervals until death) from that before treatment in Groups B and A. The Barthel Index value significantly improved after treatment in Group A (P < 0.05).
Differences Between Groups B and A
Age, the affected vertebral level, the Tokuhashi score (general condition, the number of vertebral metastases, and the total score), and the paralysis grade distribution (according to the ASIA classification) differed significantly between groups A and B (Table 5). The mean age of the patients treated for lung cancer derived metastatic spine tumors increased after 2006, and the general condition of the patients worsened, but the severity of their paralysis decreased, and the number of patients without paralysis increased.
TABLE 5.
Significant Differences Between B Group and A Group
| Significant Differences Between B Group and A Group | |
| Male: female | NS |
| Age, yrs | P < 0.001 |
| Affected level | P < 0.001 |
| Pathology | NS |
| Tokuhashi score | |
| General condition | P < 0.05 |
| Number of extraspinal bone metastases foci | NS |
| Number of metastases in the vertebral body | P < 0.001 |
| Metastases to the major internal organs | NS |
| Total score | P < 0.05 |
| VAS before treatment | NS |
| ASIA classification before treatment | P < 0.001 |
| Survival periods | P < 0.01 |
| VAS after treatment | NS |
| Change of ASIA classification between before and after treatment | NS |
| Discharge to home | NS |
| Barthel Index before treatment | NS |
| Change of Barthel Index between before and after treatment | P < 0.05 |
ASIA indicates American Spinal Injury Association; NS, no significant difference; VAS, visual analog scale.
Regarding the treatment outcomes, the mean duration of the post-treatment survival period was 5.1 and 9.3 months in groups B and A, respectively, that is, significantly longer survival was seen in group A (P < 0.05). The post-treatment discharge-to-home eligibility rate did not differ significantly between the groups, but the Barthel Index improved significantly after treatment in group A (P < 0.05).
DISCUSSION
The treatments for metastatic spine tumors have changed over recent years with advances in the treatment of primary cancer. In addition, the general approach to the treatment of spinal metastasis has also changed with numerous hospital departments being involved in the provision of active multidisciplinary treatment, that is, the care provided to such patients is not a simple task that only involves the department in charge of treating the primary cancer; instead, various departments cooperate to provide treatment.
Accordingly, the treatment outcomes of patients with lung cancer derived metastatic spine tumors should not simply be investigated based on surgical outcomes; rather, the details of the treatments, including conservative treatments, provided by various hospital departments should be investigated. Thus, we compared the treatment outcomes of multidisciplinary treatment, including conservative treatment, with those obtained using the previous approach.
The characteristics of patients who are treated for lung cancer derived metastatic spine tumors have also changed markedly. Specifically, the mean age of these patients has risen, the number of patients with cervical spine tumors has decreased (for an unknown reason), the indications for such treatment now include patients with poor general health, and the number of affected vertebrae has increased. Regarding the grade of paralysis, treatment can now be initiated earlier, that is, when the patient's paralysis is mild or upon the discovery of spinal metastasis, which has markedly changed the paralysis grade distribution of patients with lung cancer derived metastatic spine tumors.
The prognosis of metastatic spine tumors varies depending on the type of tumor. In some types of cancer (but not in others), the average survival period for patients with metastatic spine tumors has increased due to advances in treatment. For example, the survival period for patients with lung cancer derived metastatic spine tumors has improved,9 but the median survival period of patients with pulmonary adenocarcinoma-derived metastatic spine tumors was reported to be 3.5 or 5.2 months in recent studies.4,5
We have consistently used the revised version13 of the Tokuhashi score to predict the prognosis of patients with lung cancer derived metastatic spine tumors, but after the introduction of gefitinib, a molecularly targeted drug, and BMA in 2002, the prognosis of such patients clearly improved, and their actual survival periods became longer than their predicted life expectancy according to the Tokuhashi score. Hessler et al.10 also reported that the survival periods of patients with spinal metastasis from lung cancer have increased.
The degree of improvement in a patient's ability to perform ADL is markedly influenced by the severity of their paralysis, and the early initiation of treatment has a significant impact on this.19 One of the aims of treatment for lung cancer derived metastatic spine tumors is to allow the patient to be treated as an outpatient, but no significant improvement in the post-treatment discharge-to-home eligibility rate was detected in this study. This issue remains to be solved together with the lack of increase in the paralysis improvement rate.
The limitations of this study include the fact that it was not a randomized prospective study. Moreover, simple comparisons were not possible because the details of and general approach to treatment changed markedly during the study period, as did the patients’ pre-treatment background data and the treatment selection methods employed. For example, no patients with severe paralysis (corresponding to ASIA grades A and B) were included in group A. This was due to improvements in interdepartmental communication, which led to the patients visiting the orthopedics department before they developed complete motor paralysis. However, the mean duration of the postoperative survival period increased, and the patients’ ability to perform ADL improved after the introduction of molecularly targeted drugs and BMA, although the patients who received treatment for lung cancer derived metastatic spine tumors became older, and their total Tokuhashi scores decreased.
Key Points
The treatment outcomes of 207 patients with lung cancer derived metastatic spinal tumors were compared between the patients who were treated before and after the introduction of a molecularly targeted drug and bone-modifying agents.
After the introduction of the molecularly targeted drug and bone-modifying agents, the background data of the treated patients changed.
After the introduction of the molecularly targeted drug and bone-modifying agents, the patients’ survival periods increased significantly, and their activities of daily living after treatment improved.
Footnotes
The device(s)/drug(s) is/are FDA-approved or approved by corresponding national agency for this indication.
No funds were received in support of this work.
No relevant financial activities outside the submitted work.
References
- 1.Ryo H, Sakai H, Yoneda S, et al. Bone metastasis of primary lung cancer. Nihon Kokyuki Gakkai Zasshi 1998; 36:317–322. (Japanese). [PubMed] [Google Scholar]
- 2.Aydinli U, Ozturk C, Bayram S, et al. Evaluation of lung cancer metastases to the spine. Acta Orthop Belg 2006; 72:592–597. [PubMed] [Google Scholar]
- 3.Aydin AL, Emel E, Sasani M, et al. Lung cancer metastasis to the spine. Turk Neurosurg 2016; 26:635–642. [DOI] [PubMed] [Google Scholar]
- 4.Park SJ, Lee CS, Chung SS. Surgical results of metastatic spinal cord compression (MSCC) from non-small cell lung cancer (NSCLC): analysis of functional outcome, survival time, and complication. Spine J 2016; 16:322–328. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin CR, Khattab MH, Sankey EW, et al. Factors associated with life expectancy in patients with metastatic spine disease from adenocarcinoma of the lung. Global Spine J 2015; 5:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr LL, Finigan JH, Kern JA. Evaluation and treatment of patients with non-small cell lung cancer. Med Clin N Am 2011; 95:1041–1054. [DOI] [PubMed] [Google Scholar]
- 7.Gregory TM, Coriat R, Mir O. Prognostic scoring systems for spinal metastases in the era of anti-VEGF therapies. Spine (Phila Pa 1976) 2013; 38:965–966. [DOI] [PubMed] [Google Scholar]
- 8.Cagle PT, Chirieac LR. Advances in treatment of lung cancer with targeted therapy. Arch Pathol Lab Med 2012; 136:504–509. [DOI] [PubMed] [Google Scholar]
- 9.Morgen SS, Lund-Andersen C, Larsen CF, et al. Prognosis in patients with symptomatic metastatic spinal cord compression: survival in different cancer diagnosis in a cohort of 2321 patients. Spine (Phila Pa 1976) 2013; 38:1362–1367. [DOI] [PubMed] [Google Scholar]
- 10.Hessler C, Vettorazzi E, Madert J, et al. Actual and predicted survival time of patients with spinal metastases of lung cancer: evaluation of the robustness of the Tokuhashi score. Spine (Phila Pa 1976) 2011; 36:983–989. [DOI] [PubMed] [Google Scholar]
- 11.Rades D, Hueppe M, Schild SE. A score to identify patients with metastatic spinal cord compression who may be candidates for best supportive care. Cancer 2013; 119:897–903. [DOI] [PubMed] [Google Scholar]
- 12.Rades D, Dahlke M, Janssen S, et al. Radiation therapy for metastatic spinal cord compression in patients with hepatocellular carcinoma. In Vivo 2015; 29:749–752. [PubMed] [Google Scholar]
- 13.Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005; 30:2186–2191. [DOI] [PubMed] [Google Scholar]
- 14.Tokuhashi Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 1990; 15:1110–1113. [DOI] [PubMed] [Google Scholar]
- 15.Katagiri H, Okada R, Takagi T, et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med 2014; 3:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maynard FM, Jr, Bracken MB, Creasey G, et al. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord 1997; 35:266–274. [DOI] [PubMed] [Google Scholar]
- 17.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965; 14:61–65. [PubMed] [Google Scholar]
- 18.Prodinger B, O’Connor RJ, Stucki G, et al. Establishing score equivalence of the Functional Independence Measure motor scale and the Barthel Index, utilising the International Classification of Functioning, Disability and Health and Rasch measurement theory. J Rehab Med 2017; 49:416–422. [DOI] [PubMed] [Google Scholar]
- 19.Lei M, Liu Y, Yan L, et al. A validated preoperative score predicting survival and functional outcome in lung cancer patients operated with posterior decompression and stabilization for metastatic spinal cord compression. Eur Spine J 2016; 25:3971–3978. [DOI] [PubMed] [Google Scholar]


