Abstract
Childhood maltreatment is associated with attention deficits. We examined the effect of childhood abuse and abuse-by-gene (5-HTTLPR, MAOA, FKBP5) interaction on functional brain connectivity during sustained attention in medication/drug-free adolescents. Functional connectivity was compared, using generalised psychophysiological interaction (gPPI) analysis of functional magnetic resonance imaging (fMRI) data, between 21 age-and gender-matched adolescents exposed to severe childhood abuse and 27 healthy controls, while they performed a parametrically modulated vigilance task requiring target detection with a progressively increasing load of sustained attention. Behaviourally, participants exposed to childhood abuse had increased omission errors compared to healthy controls. During the most challenging attention condition abused participants relative to controls exhibited reduced connectivity, with a left-hemispheric bias, in typical fronto-parietal attention networks, including dorsolateral, rostromedial and inferior prefrontal and inferior parietal regions. Abuse-related connectivity abnormalities were exacerbated in individuals homozygous for the risky C-allele of the single nucleotide polymorphism rs3800373 of the FK506 Binding Protein 5 (FKBP5) gene. Findings suggest that childhood abuse is associated with decreased functional connectivity in fronto-parietal attention networks and that the FKBP5 genotype moderates neurobiological vulnerability to abuse. These findings represent a first step towards the delineation of abuse-related neurofunctional connectivity abnormalities, which hopefully will facilitate the development of specific treatment strategies for victims of childhood maltreatment.
Introduction
Child abuse is, regrettably, common with twenty-two percent of adolescents in the UK reporting lifetime physical, emotional, sexual abuse or neglect [1]. Childhood maltreatment causes extreme stress which, via physiological, neurochemical, and hormonal changes, can lead to alterations in brain structure, function and connectivity most consistently in fronto-limbic areas and networks [2, 3], but with some evidence for alterations also in temporal and parietal regions [4–6].
Neuropsychological studies of childhood maltreatment have reported auditory [7, 8] and visual [8–12] attention deficits. Sustained attention, the ability to keep one's mind continuously focused on a particular task, is a key dimension of attention control [13] and is important for mature goal-directed behavior, thought to underpin higher-level attention processes such as selective and divided attention as well as general cognitive ability [14]. Children with maltreatment-related PTSD [9] and institutionalized children make more omission errors than healthy controls during sustained attention, which are related to longer institutional care [15, 16]. Sustained attention deficits have also been reported in adults with childhood physical abuse and neglect histories [17].
Despite consistent neuropsychological findings of attention deficits in maltreated children, to date only one functional magnetic resonance imaging (fMRI) study has examined sustained attention in individuals exposed to childhood maltreatment. Previously published data by our group suggest that childhood abuse is associated with reduced activation during the most challenging attention condition in the same sustained attention task used in the current study, compared to healthy controls, in typical dorsal and ventral attention networks including left dorsolateral prefrontal cortex (DLPFC), inferior frontal cortex (IFC) and temporal regions [18]. Other fMRI studies in childhood maltreatment have reported alterations in activation during emotion processing [19–21]; motor response inhibition [22–24] and working memory [25].
Most fMRI studies of childhood maltreatment have concentrated exclusively on functional activation and neglected more sophisticated functional connectivity analyses. Functional communication between brain regions is vital in cognition and the few published functional connectivity studies of child abuse demonstrated altered connectivity of diffuse neural networks, fronto-limbic in particular, during resting state [26, 27], emotion processing [21, 28, 29] and response inhibition [30]. These preliminary findings suggest that it is crucial to better understand the effect of maltreatment on brain networks in addition to isolated regions. This is of particular relevance as childhood trauma has been shown to affect the morphometry and integrity of white matter tracts [31–33] and functional connectivity strength has been shown to correlate with structural connectivity of white matter tracts in the same regions [34].
Although childhood maltreatment is an important risk factor for several psychiatric disorders, it does not invariably lead to dysfunction. It is recognized that genetic differences influence the likelihood that abuse exposure will result in psychopathology [35] so it is important to examine if the abuse-related brain abnormalities are sensitive to gene-by-environment (GxE) interactions. GxE studies on early stress including childhood maltreatment show increased risk for emotional and antisocial behavioural problems in youth with the long (L) allele of the 5-HTTLPR polymorphism of the serotonin transporter gene [36–40] and the low activity variant of the variable number tandem repeat (VNTR) polymorphism of the monoamine oxidase type A (MAOA) gene [41–45]. Risk alleles of four common single nucleotide polymorphisms (SNPs) (rs1360780, rs3800373, rs9470080, rs9296158) of the FK506-binding protein 51 (FKBP5), which regulates glucocorticoid receptor sensitivity, have been reported to interact with childhood trauma to predict PTSD symptomatology [46], limbic irritability, depression and dissociation [47].
This study therefore examined the association between severe childhood maltreatment and functional connectivity of sustained attention networks in medication-naïve, drug-free young people using a parametrically modulated vigilance task requiring target detection with a progressively increasing load of sustained attention. As, during a previous study of the same sample [18], functional activation group differences were found only for the most challenging attention condition, the current paper focuses on functional connectivity for this condition only. As different forms of abuse present differently clinically (e.g., [48]) and likely have different effects on behaviour and neurobiology effects of different maltreatment types should ideally be considered separately. The current study aimed to investigate the effect specific to physical child abuse on the brain. Sexual abuse was excluded due to the known differences in structural, behavioural and psychiatric consequences [48, 49]. Preferably we would also have excluded neglect and emotional abuse but this was not possible as all cases of physical abuse that we identified had also experienced some degree of emotional abuse and/or neglect. This is representative of the abused population as most forms of child abuse do not occur in isolation [50, 51]. Based on evidence of the role of fronto-parieto-temporal regions in sustained attention [52–57], altered structure and function of these regions in individuals with a history of childhood maltreatment [2–4, 22, 25], the fact that they have been shown to develop relatively late in childhood and be progressively more activated with increasing age between childhood and adulthood [53, 58], and in particular our previous findings of decreased activation of dorsal and ventral fronto-temporal sustained attention regions [18], we hypothesized that the abused group, relative to healthy controls, would have abnormal functional connectivity of dorsolateral and inferior fronto-parieto-temporal networks during sustained attention. We also explored if these abnormalities would be moderated by 5-HTTLPR, MAOA or FKBP5 polymorphisms.
Materials and methods
Participants
Fifty (23 maltreated and 27 healthy controls) right-handed, medication-naïve, drug-free and age-matched youths between the ages of 13 and 20 years old were initially assessed using the Development and Well-Being Assessment (DAWBA) [59]. The Strengths and Difficulties Questionnaires (SDQ) [60] and Beck’s Depression Inventory (BDI) [61] were used to provide psychopathology symptom scores. IQ was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI) [62]. The Childhood Trauma Questionnaire (CTQ) [63] was used to measure the severity of childhood physical, emotional and sexual abuse and emotional and physical neglect. Socioeconomic status (SES) was measured by two items from the Family Affluence Scale (FAS) [64] on housing tenure and room occupancy.
A 10 panel T-cup urine test (http://www.testfield.co.uk) was used to test for substance abuse. Participants who tested positive for any of the 10 substances were excluded resulting in the exclusion of 4 participants (3 maltreated and 1 healthy control). Other exclusion criteria were left-handedness, IQ < 70, current psychoactive medication, sexual abuse (as defined by a score of ≥ 6 on the sexual abuse subscale of the CTQ), neurological disorder, major head injuries, drug and alcohol abuse, literacy problems, learning disability, psychotic illness, bipolar disorder, schizophrenia, current suicidal behaviour or general MRI contraindications. Participants received £40 to compensate for their time and travel. The National Research Ethics Service reviewed and approved the study and informed written consent was obtained from all participants and, if below 18 years old, informed written consent was also obtained from parents or guardians. Participants were recruited and scanned during the period 2011 to 2013.
Twenty-three physically maltreated participants were recruited through Kids Company (http://www.kidsco.org.uk/), Child and Adolescent Mental Health Services (CAMHS) and advertisements. They scored ≥ 13 (i.e. the cut-off for severe/extreme physical abuse) on the CTQ physical abuse subscale and the abuse history was corroborated by social service records and the Childhood Experience of Care and Abuse (CECA) interview [65]. Head motion is a well-known confound of both resting state functional connectivity [66, 67] and task based fMRI data [68]. In order to reduce the likelihood of false positives caused by head movement we therefore excluded participants with root mean square (RMS) realignment estimates exceeding 1 mm. This was calculated from realignment parameters (rotational estimates converted to translational at radius of 50 mm) as described by Siegel et al. [68] and resulted in the exclusion of two maltreated participants, leaving a final sample of 21.
The 27 healthy controls with no history of psychiatric illness and childhood maltreatment (scoring below the same cut-offs as above) were recruited through advertisements in the same geographic areas of South London to ensure similar socioeconomic background (Table 1). All healthy controls had RMS movement < 1mm.
Table 1. Demographic characteristics of 21 young people exposed to severe childhood abuse and 27 healthy controls (CA = childhood abuse; HC = healthy controls; ADHD = attention deficit hyperactivity disorder; PTSD = post-traumatic stress disorder; ODD = oppositional defiant disorder; CD = conduct disorder).
| Childhood Abuse | Healthy Controls | ||||
|---|---|---|---|---|---|
| (N = 21) | (N = 27) | ||||
| Mean | SD | Mean | SD | ||
| Age (years) | 17.5 | 2.32 | 17.5 | 1.63 | |
|
[Age Range: 13–20] Socioeconomic status |
2.77 | 0.69 | 3.22 | 0.75 | |
| IQ | 90 | 12.6 | 105.4 | 10.1 | |
| Strengths and Difficulties Questionnaire: | |||||
| Emotional problems | 4.62 | 2.77 | 1.92 | 1.61 | |
| Conduct problems | 4.43 | 2.01 | 1.68 | 1.6 | |
| Hyperactivity | 5.38 | 2.4 | 2.84 | 2.14 | |
| Peer problems | 3.81 | 1.54 | 1.16 | 1.72 | |
| Prosocial | 7.24 | 1.7 | 8.08 | 1.41 | |
| Total difficulties score | 18.2 | 6.2 | 7.6 | 5.73 | |
| Beck’s Depression Inventory | 16 | 10.6 | 5.92 | 6.09 | |
| Childhood Trauma Questionnaire: | |||||
| Physical abuse | 20.8 | 5.04 | 5.52 | 0.94 | |
| Emotional abuse | 18 | 4.4 | 6.04 | 1.13 | |
| Sexual abuse | 5.14 | 0.65 | 5.11 | 0.42 | |
| Physical neglect | 14 | 5.02 | 5.59 | 1.22 | |
| Emotional neglect | 18.3 | 3.93 | 7.93 | 3.35 | |
| Age at onset of abuse (years) | 4.24 | 2.55 | |||
| Duration of abuse (years) | 8.29 | 3.2 | |||
| N | % | N | % | ||
| Gender (Males) | 15 | 71 | 21 | 77 | |
| Ethnicity: | |||||
| White | 10 | 48 | 13 | 48 | |
| Afro-Caribbean | 8 | 38 | 12 | 44 | |
| Others (Asian/mixed) | 3 | 14 | 2 | 8 | |
| Psychiatric diagnosis: | |||||
| PTSD | 12 | 57 | - | ||
| Depression | 6 | 29 | - | ||
| Anxiety disorders | 4 | 19 | - | ||
| Social phobia | 1 | 5 | - | ||
| ADHD | 1 | 5 | |||
| ODD/CD/Other disruptive behaviors | 4 | 19 | |||
Genotyping
Genotyping of the 5-HTTLPR promoter region polymorphism, the MAOA 30 bp-promoter and four common SNPs (rs1360780, rs3800373, rs9470080, rs9296158) of FKBP5 were carried out using previously described methods [69–71]. Individuals were identified as risk allele carriers or not: i.e., long for 5-HTTLPR, short/low for MAOA, T-allele carriers for rs136078 and rs94700800, A-allele carriers for rs9296158 and C-allele carriers for rs3800373.
fMRI paradigm: Sustained attention task (SAT)
Participants practiced the task once prior to scanning. The 12-min SAT is a variant of psychomotor vigilance and delay tasks [53, 54]. Participants need to respond as quickly as possible to the appearance of a visual timer counting up in milliseconds via a right hand button response within 1s. The visual stimuli appear either after short, predictable consecutive delays of 0.5s, in series of 3–5 stimuli (260 in total), or after unpredictable time delays of 2s, 5s or 8s (20 each), pseudo-randomly interspersed into the blocks of 3–5 0.5s delays. The long, infrequent, unpredictable delays place a higher load on sustained attention/vigilance while the short, predictable 0.5s delays are typically anticipated [72] placing a higher demand on sensorimotor synchronization [53, 54, 73] (S1 Fig).
Performance data analysis
Independent sample t-tests were used to compare the main variables of the sustained attention task performance between the abused and the control group using SPSS 21: mean reaction time (RT), intrasubject standard deviation of mean RT (SDintrasubject), omission and premature errors. T-tests for the short delays (0.5s) were also conducted separately on the same measures.
fMRI image acquisition and analysis
Details of image acquisition, preprocessing and first and second-level functional activation analyses methods and results are published elsewhere [18].
Functional connectivity analysis
As functional group differences were found only for the 8s delay condition [18], the current paper focuses on functional connectivity group differences for this condition only by conduction of a generalised psychophysiological interaction (gPPI) analysis using SPM8. Ten seed regions were selected: 1,2) Left and right anterior insula (-38,26,16; +38,26,16); 3,4) Left and right dorsolateral prefrontal cortices (DLPFC) (-35,35,39; +37,37,38); 5,6) Left and right inferior frontal cortices (IFC) (-47,31,13; +49,31,13); 7,8) Left and right inferior parietal lobes (IPL) (-41,-47,48; +45,-46,48); 9,10) Left and right superior temporal gyri (STG) (-48, -14,2; +54, -8, -2). These seed regions were chosen based on independent data from previous studies which have demonstrated consistent evidence for their involvement in sustained attention [55, 74–79], in the current task in particular [53, 54, 56] and are brain regions which have also been implicated in previous studies of childhood maltreatment (For a review see [2]). Co-ordinates for all seed regions were selected as the centroids of the region of interest (ROI) as defined using wfupickatlas [80] and aal [81]. For each seed region, at the individual subject level, an average time course was extracted defined as an 8 mm sphere around the abovementioned coordinates for use in the gPPI analysis.
The gPPI toolbox (http://www.nitrc.org/projects/gppi) was used to investigate the interaction effect during our contrast of interest (8s delay vs 0.5s implicit baseline) for all 10 seed regions. The deconvolved time series from the seed region was extracted for each participant to create the physiological variable and condition onset times were separately convolved with the canonical haemodynamic response function for each condition, creating the psychological regressors. Interaction terms (gPPIs) were computed by multiplying physiological and psychological variables and activity within the seed region was regressed on a voxel wise basis against the interaction, with the physiological and psychological variables serving as regressors of interest. Individual gPPI contrast images were entered into separate second level analyses to compare groups. Thus, the resulting activation maps from this analysis correspond to group differences for functional connectivity between the seed region and other brain regions during sustained attention. Results are reported using a cluster threshold of p < 0.05 family-wise error rate (FWER) corrected. Given the limited studies testing brain function differences in physically abused populations, and to control for the false positive rate (using p<0.05 family-wise error rate-corrected cluster statistics) while limiting potential type II errors, we chose an a priori cluster-forming threshold of P<0.001 for significant between-group differences.
Finally, significant clusters were extracted for exploratory correlational analysis with the performance measures for the 8s delay condition within both groups (mean RT, SDintrasubject, omission errors, premature errors) and abuse measures within the maltreated group only (onset, duration, CTQ score). Preliminary analysis of GxE effect on the significant clusters was conducted using ANOVAs with group and genotype (5-HTTLPR, MAOA, rs1360780, rs3800373, rs9470080, rs9296158) as between-subject factors.
Results
Subject characteristics
Groups did not differ significantly on age (t(46) = 0.03, p = 0.97), gender (t(46) = 0.08, p = 0.94), ethnicity (t(47) = 0.48, p = 0.51) nor socioeconomic status (t(47) = 1.49, p = 0.14) but differed on IQ as expected (t(47) = 4.70, p<0.001) (Table 1). Since lower IQ is associated with childhood maltreatment [11], artificially matching groups on IQ is inappropriate as it creates unrepresentative groups; either the abused group will have higher IQs than the abused population or the control group will have IQs below normative expectations [82]. Also, it is misguided to control for IQ differences by covarying IQ when groups are not randomly selected and the covariate is a pre-existing group difference as ANCOVA would lead to potentially spurious results [82, 83]. The primary data are therefore presented without matching or covarying IQ. However, to explore and rule out any potential influence of IQ, an analysis of covariance (ANCOVA) covarying for IQ was conducted.
Although we selected participants with severe childhood physical abuse, they also experienced marked/severe childhood emotional abuse and neglect (Table 1) which typically co-occur with physical abuse, and hence are a representative group of the abused population [50, 51]. Healthy controls scored significantly lower on BDI (p < 0.01) and all SDQ difficulties subscales (p < 0.001) than the abused group (Table 1).
Task performance
Mean performance values are reported in Table 2. There was no significant group effect on mean reaction time (t(46) = 1.03; p = 0.31) but there was a significant group effect on intrasubject variability of mean reaction times (t(46) = 3.57, p < 0.001), with the maltreated group having greater intrasubject variability for all long delay conditions. There was also a significant group effect on omission (t(46) = 2.55, p < 0.05) and premature errors (t(46) = 2.58, p < 0.05), due to the abused group making more omission and premature errors than healthy controls (Table 2).
Table 2. Performance measures for the sustained attention task during 2s, 5s and 8s delays for 21 abused young people and 27 healthy controls.
MRT = mean reaction time (in ms); SDintrasubject = intrasubject variability of mean reaction times (in ms); corr = Bonferroni corrected; CA = childhood abuse; HC = healthy control.
| Childhood Abuse (N = 21) | Healthy Controls (N = 27) | ||||
|---|---|---|---|---|---|
| Delay | Mean | SD | Mean | SD | |
| MRT | 2s | 446 | 64 | 411 | 59 |
| 5s | 450 | 78 | 414 | 74 | |
| 8s | 449 | 87 | 408 | 80 | |
| SDintrasubject | 2s | 101 | 50 | 74 | 38 |
| 5s | 93 | 50 | 85 | 61 | |
| 8s | 84 | 43 | 77 | 43 | |
| Omission errors | 2s | 0.33 | 0.73 | 0.11 | 0.42 |
| 5s | 0.57 | 0.93 | 0.19 | 0.48 | |
| 8s | 0.62 | 1.2 | 0.04 | 0.19 | |
| Premature errors | 2s | 6.43 | 3.93 | 4 | 3.16 |
| 5s | 7.38 | 4.65 | 4.3 | 3.74 | |
| 8s | 6.95 | 4.23 | 5.15 | 3.92 | |
Brain activation
Movement
Multivariate analyses of variance (MANOVAs) showed no significant group effects in the extent of 3-dimensional motion as measured by maximum displacement for x, y, and z axes (F(3,44) = 1.67; p = 0.14).
Functional activation
Within and between group functional brain activation is reported elsewhere [18]. Maltreated participants, relative to healthy controls, displayed significantly reduced activation during the most challenging attention condition only in typical dorsal and ventral attention networks including left dorsolateral and inferior prefrontal and temporal areas (Peak MNI coordinates: -38,26,16; -40,-54,-14). This was due to a significant linear trend of decreasing activation with increasing attention load in these regions in the abused group.
Functional connectivity
Within group connectivity maps
S2 Fig shows within group functional connectivity maps for the different seed regions for the 8s delay vs the 0.5s implicit baseline.
Between group functional connectivity differences
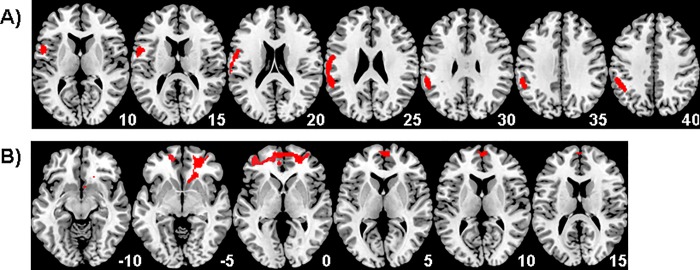
A significant reduction in connectivity in the abused group relative to healthy controls was revealed for the left DLPFC seed region with left IPL, supramarginal gyrus, IFC, postcentral and precentral gyri (BA 40/44/3/6) during the 8s delay condition (F(1,46) = 16.91; p<0.001), (Table 3, Fig 1).
Table 3. Regions demonstrating differential functional connectivity with the left dorsolateral prefrontal cortex and left inferior parietal lobe seed regions during the 8s delay versus 0.5s implicit baseline condition for 21 young people exposed to severe childhood abuse and 27 healthy controls.
P-value is <0.05 FWER corrected.
| Cluster Level | Peak | Voxel Level | |||
|---|---|---|---|---|---|
| Seed Region | Comparison and Brain Regions | No. of Voxels | p (corr) | MNI Coordinates | Z |
| L DLPFC | Physically Maltreated < Healthy Controls | ||||
| Left inferior parietal lobe, supramarginal gyrus, pars opercularis, inferior frontal, postcentral gyrus, precentral gyrus (BA 40/44/3/6) | 730 | 0.012 | -56,-42,30 | 4.14 | |
| L IPL | Physically Maltreated < Healthy Controls | ||||
| Bilateral dorsolateral and rostromedial prefrontal cortex (BA 46/10) | 687 | 0.032 | -8,58,-4 | 4.02 | |
Fig 1. Functional connectivity differences between 21 physically maltreated young people and 27 healthy controls for the 8s delay condition vs 0.5s baseline.
Illustrating regions that demonstrated reduced connectivity for maltreated participants compared to healthy controls with A) the seed region of the left dorsolateral prefrontal cortex and B) the left inferior parietal seed region. The threshold is P < 0.05 FWE corrected at the cluster level. Z-coordinates represent distance from the anterior–posterior commissure in mm. The right side of the image corresponds to the right side of the brain.
For the left IPL seed region, a significant group effect for functional connectivity was shown with bilateral DLPFC and rostromedial prefrontal cortex (rmPFC) (BA 46/10) (F(1,46) = 14.55; p<0.001), which was due to reduced connectivity for maltreated compared to healthy adolescents (Table 3, Fig 1). No effect of group was observed for the remaining 8 seed regions.
Exploratory analyses
Correlational analysis
No significant correlations were found between connectivity and performance or abuse measures.
IQ ANCOVA analysis
Given that the maltreated group had a significantly lower mean IQ than the healthy comparison group, data were reanalysed covarying for IQ. All main findings remained significant (S1 Table).
GxE analysis
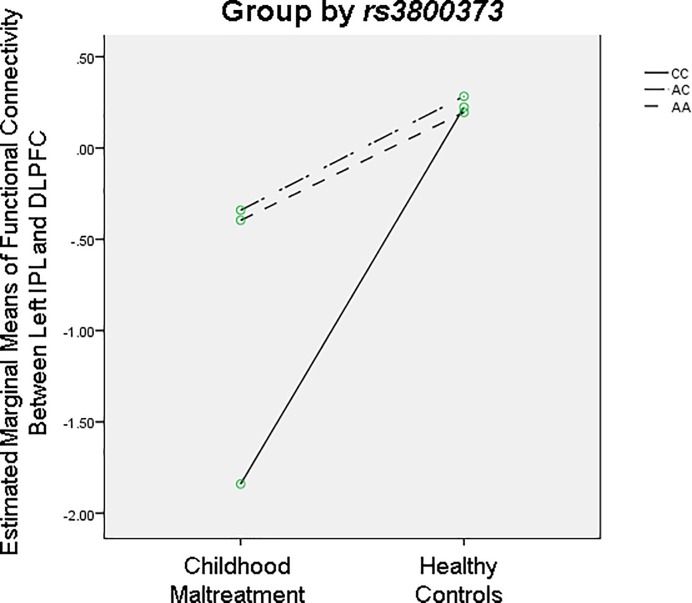
Exploratory GxE analysis was conducted on the brain regions that differed in connectivity between the maltreated and healthy adolescents. ANOVAs with group (maltreated vs. healthy controls) and each genotype as between-subject factors showed a significant group-by-rs3800373 effect on connectivity between left IPL and left DLPFC (F (1,44) = 5.50, p < 0.05), due to a greater deficit in C-allele homozygotes exposed to abuse than A-allele carriers (Fig 2).
Fig 2. Significant GxE interaction effect between group (childhood abuse vs. healthy controls) and rs3800373 genotype (CC vs AC/AA) on functional connectivity between left IPL and DLPFC, p < 0.05.
No significant group-by-genotype effects were observed for 5-HTTLPR, MAOA, rs1360780, rs9470080, or rs9296158.
Discussion
This is the first study examining the association between severe childhood abuse and functional connectivity of brain networks during sustained attention in medication-naïve, drug-free young people. Furthermore, the exploration of GxE effects on maltreatment-related connectivity abnormalities is novel. Behaviorally, maltreated individuals exhibited increased intrasubject variability, premature and omission errors, the main attention measure of the task. Abused participants relative to healthy controls exhibited significantly reduced functional connectivity between left DLPFC and left IPL, supramarginal gyrus, IFC, post- and precentral gyri and between left IPL and bilateral DLPFC and rmPFC during sustained attention. No correlations were observed between functional connectivity deficits and abuse onset, duration or severity. Abuse-related deficits in left hemispheric fronto-parietal connectivity were moderated by FKBP5 Genotype, specifically SNP rs3800373.
Young people with a history of severe childhood abuse showed reduced connectivity relative to healthy controls during the most challenging attention condition in predominantly left hemispheric dorsal and ventral fronto-parietal networks that are known to be important for sustained attention. The fact that extremely similar prefrontal-parietal networks were shown to be affected in connectivity analyses of both the left DLPFC and the left IPL seed regions corroborates and reinforces the finding of fronto-parietal network dysfunctions during attention. DLPFC (BA 46) plays a crucial role in top-down attention and is activated during visuospatial information processing and orienting of attention [58, 74, 79], rmPFC (BA 10) has been implicated in attention during prospective memory paradigms, i.e. carrying out an intended action after a delay [84, 85] and IPL is also a key region in the control of sustained attention [77, 86, 87]. DLPFC, rmPFC and IPL have been associated with sustained attention during this particular task version [53, 54]. The well-established role of fronto-parietal networks in sustained attention [88, 89] is consistent with the theory that decreased connectivity of these networks in maltreated individuals contributes to behavioural attention deficits observed in the current study and the neuropsychological literature in the form of increased omission errors [7, 9–11, 15]. The lack of a correlation between attention measures and connectivity findings in the current study may be related to relatively low power for correlation analyses.
The structure and function of the prefrontal cortices, including DLPFC, rmPFC and IFC, are consistently reported to be affected by childhood maltreatment [2, 3, 24], and there is also some evidence for alterations in parietal regions [4]. Our previous fMRI findings using the same task and subjects found abnormally reduced activation in the abused group in typical dorsal and ventral attention networks including left DLPFC, IFC and temporal regions [18]. The finding of diminished functional connectivity for maltreated adolescents, relative to healthy controls, between the prefrontal cortex and parietal lobe extends these previous findings of hypoactivity in DLPFC and IFC to the network level by showing that the functional communication between these regions is disturbed and not just their activation.
The findings also extend previous structural connectivity findings that demonstrate that adolescents exposed to childhood maltreatment have reduced density of bilateral superior longitudinal fasciculi, white-matter tracts that connect prefrontal areas, including the DLPFC, to parietal regions [90]. Interestingly, recent findings, combining diffusion imaging MRI data with magnetoencephalography, implicate the medial branch of the superior longitudinal fasciculus, in top-down control of neuronal synchronisation associated with selective attention [91]. The human brain is plastic and is continually modified by experience across development. Given that prefrontal and parietal regions are among the latest brain regions to develop structurally [78] and functionally [92], developing well into mid-adulthood, their protracted development may render fronto-parietal networks more susceptible to impairment following childhood adversity.
Our preliminary GxE findings in fronto-parietal connectivity are intriguing as they suggest that connectivity deficits in these stress-susceptible error processing brain networks were influenced by the abuse experience and possibly exacerbated in the presence of the risky C-allele of the rs-3800373 SNP of the FKBP5 gene, an effect which seemed only to be present in individuals homozygous for the C-allele. It should, however, be noted that these results are merely exploratory as subject numbers are too small to make any conclusions regarding GxE but it does highlight a possible relationship that warrants further investigation. C-allele carriers of rs-3800373 exposed to childhood maltreatment have been shown to demonstrate increased risk of PTSD [46], limbic irritability, depression, dissociation [47], suicide attempts [93], aggression and violence [94]. No group-by-5-HTTLPR nor MAOA effects were observed suggesting that the specific fronto-parietal functional connectivity deficits observed during sustained attention are not modulated by 5-HTTLPR or MAOA genotype.
Among the strengths of the current study is that all participants were medication-naïve, drug-free and the abuse experience was carefully assessed and corroborated by social service records. It is unclear to what extent pubertal development, malnutrition, prenatal drug exposure and presence of current life stressors may have influenced the findings. The SES measure used is limited, as it does not provide information on parents’ income and education; however, youth often have difficulties in reporting this information [95]. The cross-sectional nature of the study is a further limitation. As the affected fronto-parietal networks develop well into mid-adulthood [78, 92] the true impact of childhood maltreatment on functional connectivity may not be revealed in this adolescent sample. Although we recruited participants exposed to childhood physical abuse, it is unrealistic to separate physical abuse from typically co-occurring emotional abuse and neglect [50, 51]; hence, our abuse group had experienced emotional abuse and neglect as well. An important future direction for research is to investigate the way in which sexual abuse affects functional connectivity during sustained attention to elucidate potential differences in the way distinct abuse types affect neuronal networks. Another limitation is the inclusion of mixed genders as maltreatment may affect the genders differently [96].
Conclusions
In summary, using medication-naïve, drug-free, carefully assessed age-matched groups of young people exposed to severe childhood maltreatment and healthy controls, we found that abused participants had reduced functional connectivity of primarily left hemispheric fronto-parietal networks, including DLPFC and IPL, during sustained attention. Furthermore connectivity deficits were moderated by FKBP5 genotype. Hence, in response to an abusive early environment maltreated individuals may develop a reduction in communication between brain regions involved in sustained attention resulting in attention deficits. These findings represent a first step towards the delineation of abuse-related neurofunctional connectivity abnormalities, which hopefully will facilitate the development of specific treatment strategies for victims of childhood maltreatment.
Supporting information
Subjects are required to press a right-hand button as soon as they see a timer appear on the screen counting seconds. The counter appears after either predictable short delays of 0.5s in blocks of 3–5 stimuli, or after unpredictable long delays of 2s, 5s or 8s, pseudorandomly interspersed into the blocks of 0.5s delays. The long second delays have a progressively higher load on sustained attention than the short 0.5s delays that are typically anticipated and have a higher load on sensorimotor synchronization.
(TIF)
The threshold is P < 0.05 FWE corrected. The right of the image corresponds to the right side of the brain. L = left, R = right, ACC = anterior cingulate cortex, IFC = inferior frontal cortex, SMA = supplementary motor area.
(TIF)
Regions demonstrating differential functional connectivity with the left dorsolateral prefrontal cortex and left inferior parietal lobe seed regions during the 8s delay versus 0.5s implicit baseline condition for 21 young people exposed to severe childhood abuse and 27 healthy controls, when covarying for IQ. P-value is <0.05 FWER corrected.
(DOCX)
Acknowledgments
The authors are grateful to all the individuals and their families who participated in this study. They would also like to thank Dr. Kaylita Chantiluke and Ms. Sinead King for their assistance with data collection, staff at Kids Company for assistance with recruitment of participants and Professor Robert Goodman for supervision and advising on DAWBA diagnoses.
Data Availability
Data are available at doi:10.5061/dryad.3338c.
Funding Statement
The research was supported by Kids Company and the Reta Lila Weston Trust for Medical Research to KR. HH was supported by Kids Company, the Reta Lila Weston Trust for Medical Research and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. LL was supported by the National Medical Research Council (Singapore), Kids Company and the Reta Lila Weston Trust for Medical Research. AS and KR have received support from the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Radford L, Corral S, Bradley C, Fisher HL. The prevalence and impact of child maltreatment and other types of victimization in the UK: Findings from a population survey of caregivers, children and young people and young adults. Child Abuse & Neglect. 2013;37(10):801–13. [DOI] [PubMed] [Google Scholar]
- 2.Hart H, Rubia K. Neuroimaging of child abuse: A critical review. Frontiers in Human Neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim L, Radua J, Rubia K. Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. American Journal of Psychiatry. 2014. [DOI] [PubMed] [Google Scholar]
- 4.Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, et al. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience. 2010;30(22):7466–72. doi: 10.1523/JNEUROSCI.0859-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bellis MD, Keshavan MS, Frustaci K, Shifflett H, Iyengar S, Beers SR, et al. Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biological psychiatry. 2002;51(7):544–52. [DOI] [PubMed] [Google Scholar]
- 6.Tomoda A, Sheu Y-S, Rabi K, Suzuki H, Navalta CP, Polcari A, et al. Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage. 2011;54:S280–S6. doi: 10.1016/j.neuroimage.2010.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DePrince AP, Weinzierl KM, Combs MD. Executive function performance and trauma exposure in a community sample of children. Child abuse & neglect. 2009;33(6):353–61. [DOI] [PubMed] [Google Scholar]
- 8.Nolin P, Ethier L. Using neuropsychological profiles to classify neglected children with or without physical abuse. Child abuse & neglect. 2007;31(6):631–43. [DOI] [PubMed] [Google Scholar]
- 9.Beers SR, De Bellis MD. Neuropsychological function in children with maltreatment-related posttraumatic stress disorder. American Journal of Psychiatry. 2002;159(3):483–6. doi: 10.1176/appi.ajp.159.3.483 [DOI] [PubMed] [Google Scholar]
- 10.Bücker J, Kapczinski F, Post R, Ceresér KM, Szobot C, Yatham LN, et al. Cognitive impairment in school-aged children with early trauma. Comprehensive Psychiatry. 2012;53(6):758–64. doi: 10.1016/j.comppsych.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 11.De Bellis MD, Hooper SR, Spratt EG, Woolley DP. Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. Journal of the International Neuropsychological Society. 2009;15(6):868–78. doi: 10.1017/S1355617709990464 PubMed PMID: WOS:000272114500007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, et al. Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child development. 2010;81(1):224–36. doi: 10.1111/j.1467-8624.2009.01391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langner R, Eickhoff SB. Sustaining attention to simple tasks: A meta-analytic review of the neural mechanisms of vigilant attention American Psychological Association; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35(2):146–60. Epub 2001/05/05. doi: S0165017301000443 [pii]. . [DOI] [PubMed] [Google Scholar]
- 15.Loman MM, Johnson AE, Westerlund A, Pollak SD, Nelson CA, Gunnar MR. The effect of early deprivation on executive attention in middle childhood. Journal of Child Psychology and Psychiatry. 2013;54(1):37–45. doi: 10.1111/j.1469-7610.2012.02602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott JM, Troller-Renfree S, Vanderwert R, Nelson CA, Zeanah CH, Fox NA. Psychosocial deprivation, executive functions, and the emergence of socio-emotional behavior problems. 2013. [DOI] [PMC free article] [PubMed]
- 17.Gould F, Clarke J, Heim C, Harvey PD, Majer M, Nemeroff CB. The effects of child abuse and neglect on cognitive functioning in adulthood. Journal of psychiatric research. 2012;46(4):500–6. doi: 10.1016/j.jpsychires.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim L, Hart H, Mehta MA, Simmons A, Mirza K, Rubia K. Neurofunctional Abnormalities during Sustained Attention in Severe Childhood Abuse. PloS one. 2016;11(11):e0165547 Epub 2016/11/11. doi: 10.1371/journal.pone.0165547 ; PubMed Central PMCID: PMCPMC5104469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, et al. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cognitive, Affective, & Behavioral Neuroscience. 2010;10(1):34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological psychiatry. 2012;71(4):286–93. doi: 10.1016/j.biopsych.2011.10.021 [DOI] [PubMed] [Google Scholar]
- 21.Hart H, Lim L, Mehta MA, Simmons A, Mirza K, Rubia K. Altered fear processing in adolescents with a history of severe childhood maltreatment: An fMRI study. Psychological Medicine. 2017;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrion VG, Garrett A, Menon V, Weems CF, Reiss AL. Posttraumatic stress symptoms and brain function during a response‐inhibition task: an fMRI study in youth. Depression and anxiety. 2008;25(6):514–26. doi: 10.1002/da.20346 [DOI] [PubMed] [Google Scholar]
- 23.Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, et al. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48(10):3037–44. doi: 10.1016/j.neuropsychologia.2010.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim L, Hart H, Mehta MA, Simmons A, Mirza K, Rubia K. Neural correlates of error processing in young people with a history of severe childhood abuse: an fMRI study. American Journal of Psychiatry. 2015. [DOI] [PubMed] [Google Scholar]
- 25.Raine A, Park S, Lencz T, Bihrle S, LaCasse L, Widom CS, et al. Reduced right hemisphere activation in severely abused violent offenders during a working memory task: an fMRI study. Aggressive behavior. 2001;27(2):111–29. [Google Scholar]
- 26.Van der Werff S, Pannekoek J, Veer I, van Tol M-J, Aleman A, Veltman D, et al. Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychological medicine. 2013;43(09):1825–36. [DOI] [PubMed] [Google Scholar]
- 27.Williamson PC, Osuch EA, Lanius RA. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. Journal of psychiatry & neuroscience: JPN. 2009;34(3):187. [PMC free article] [PubMed] [Google Scholar]
- 28.Fonzo GA, Flagan TM, Sullivan S, Allard CB, Grimes EM, Simmons AN, et al. Neural functional and structural correlates of childhood maltreatment in women with intimate-partner violence-related posttraumatic stress disorder. Psychiatry Research: Neuroimaging. 2013;211(2):93–103. doi: 10.1016/j.pscychresns.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jedd K, Hunt RH, Cicchetti D, Hunt E, Cowell RA, Rogosch FA, et al. Long-term consequences of childhood maltreatment: Altered amygdala functional connectivity. Development and psychopathology. 2015;27(4pt2):1577–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elton A, Tripathi SP, Mletzko T, Young J, Cisler JM, James GA, et al. Childhood maltreatment is associated with a sex‐dependent functional reorganization of a brain inhibitory control network. Human brain mapping. 2014;35(4):1654–67. doi: 10.1002/hbm.22280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological psychiatry. 2009;65(3):227–34. doi: 10.1016/j.biopsych.2008.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul R, Henry L, Grieve SM, Guilmette TJ, Niaura R, Bryant R, et al. The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatric disease and treatment. 2008;4(1B):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eluvathingal TJ, Chugani HT, Behen ME, Juhász C, Muzik O, Maqbool M, et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117(6):2093–100. doi: 10.1542/peds.2005-1727 [DOI] [PubMed] [Google Scholar]
- 34.Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Structure and Function. 2009;213(6):525–33. doi: 10.1007/s00429-009-0208-6 [DOI] [PubMed] [Google Scholar]
- 35.Nugent NR, Tyrka AR, Carpenter LL, Price LH. Gene–environment interactions: early life stress and risk for depressive and anxiety disorders. Psychopharmacology. 2011;214(1):175–96. doi: 10.1007/s00213-010-2151-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chipman P, Jorm AF, Prior M, Sanson A, Smart D, Tan X, et al. No interaction between the serotonin transporter polymorphism (5‐HTTLPR) and childhood adversity or recent stressful life events on symptoms of depression: Results from two community surveys. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144(4):561–5. [DOI] [PubMed] [Google Scholar]
- 37.Chorbov VM, Lobos EA, Todorov AA, Heath AC, Botteron KN, Todd RD. Relationship of 5‐HTTLPR genotypes and depression risk in the presence of trauma in a female twin sample. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144(6):830–3. [DOI] [PubMed] [Google Scholar]
- 38.Laucht M, Treutlein J, Blomeyer D, Buchmann AF, Schmid B, Becker K, et al. Interaction between the 5-HTTLPR serotonin transporter polymorphism and environmental adversity for mood and anxiety psychopathology: evidence from a high-risk community sample of young adults. International Journal of Neuropsychopharmacology. 2009;12(6):737–47. doi: 10.1017/S1461145708009875 [DOI] [PubMed] [Google Scholar]
- 39.Olsson C, Byrnes G, Lotfi-Miri M, Collins V, Williamson R, Patton C, et al. Association between 5-HTTLPR genotypes and persisting patterns of anxiety and alcohol use: results from a 10-year longitudinal study of adolescent mental health. Molecular psychiatry. 2005;10(9):868–76. doi: 10.1038/sj.mp.4001677 [DOI] [PubMed] [Google Scholar]
- 40.Surtees PG, Wainwright NW, Willis-Owen SA, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biological psychiatry. 2006;59(3):224–9. doi: 10.1016/j.biopsych.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 41.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–4. doi: 10.1126/science.1072290 [DOI] [PubMed] [Google Scholar]
- 42.Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptomatology among adolescents from low socioeconomic status backgrounds. Development and psychopathology. 2007;19(04):1161–80. [DOI] [PubMed] [Google Scholar]
- 43.Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Molecular Psychiatry. 2006;11(10):903–13. doi: 10.1038/sj.mp.4001851 PubMed PMID: WOS:000241302100004. [DOI] [PubMed] [Google Scholar]
- 44.Taylor A, Kim-Cohen J. Meta-analysis of gene-environment interactions in developmental psychopathology. Development and Psychopathology. 2007;19(4):1029–37. doi: 10.1017/S095457940700051X PubMed PMID: WOS:000250400300006. [DOI] [PubMed] [Google Scholar]
- 45.Weder N, Yang BZ, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J, et al. MAOA Genotype, Maltreatment, and Aggressive Behavior: The Changing Impact of Genotype at Varying Levels of Trauma. Biological Psychiatry. 2009;65(5):417–24. doi: 10.1016/j.biopsych.2008.09.013 PubMed PMID: WOS:000263455300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama. 2008;299(11):1291–305. doi: 10.1001/jama.299.11.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dackis MN, Rogosch FA, Oshri A, Cicchetti D. The role of limbic system irritability in linking history of childhood maltreatment and psychiatric outcomes in low-income, high-risk women: moderation by FK506 binding protein 5 haplotype. Development and psychopathology. 2012;24(04):1237–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ackerman PT, Newton JE, McPherson WB, Jones JG, Dykman RA. Prevalence of post traumatic stress disorder and other psychiatric diagnoses in three groups of abused children (sexual, physical, and both). Child abuse & neglect. 1998;22(8):759–74. [DOI] [PubMed] [Google Scholar]
- 49.Heim CM, Mayberg HS, Mletzko T, Nemeroff CB, Pruessner JC. Decreased cortical representation of genital somatosensory field after childhood sexual abuse. American Journal of Psychiatry. 2013;170(6):616–23. doi: 10.1176/appi.ajp.2013.12070950 [DOI] [PubMed] [Google Scholar]
- 50.Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. American Journal of Psychiatry. 2003;160(8):1453–60. doi: 10.1176/appi.ajp.160.8.1453 [DOI] [PubMed] [Google Scholar]
- 51.Trickett PK, Kim K, Prindle J. Variations in emotional abuse experiences among multiply maltreated young adolescents and relations with developmental outcomes. Child abuse & neglect. 2011;35(10):876–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. Journal of Cognitive Neuroscience. 2003;15(7):1028–38. PubMed PMID: ISI:000186004600012. doi: 10.1162/089892903770007416 [DOI] [PubMed] [Google Scholar]
- 53.Murphy CM, Christakou A, Daly EM, Ecker C, Giampietro V, Brammer M, et al. Abnormal functional activation and maturation of fronto-striato-temporal and cerebellar regions during sustained attention in autism spectrum disorder. American Journal of Psychiatry. 2014;171(10):1107–16. doi: 10.1176/appi.ajp.2014.12030352 [DOI] [PubMed] [Google Scholar]
- 54.Christakou A, Murphy C, Chantiluke K, Cubillo A, Smith A, Giampietro V, et al. Disorder-specific functional abnormalities during sustained attention in youth with attention deficit hyperactivity disorder (ADHD) and with autism. Molecular psychiatry. 2013;18(2):236–44. doi: 10.1038/mp.2011.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubia K, Halari R, Cubillo A, Mohammad M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a Rewarded Continuous Performance Task. Neuropharmacology. 2009;57 640–52. doi: 10.1016/j.neuropharm.2009.08.013 [DOI] [PubMed] [Google Scholar]
- 56.Rubia K, Smith A, Halari R, Matukura F, Mohammad M, Taylor E, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure Conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure Attention-Deficit/Hyperactivity Disorder during sustained attention. American Journal of Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212 [DOI] [PubMed] [Google Scholar]
- 57.Tana MG, Montin E, Cerutti S, Bianchi AM. Exploring cortical attentional system by using fMRI during a Continuous Perfomance Test. Computational intelligence and neuroscience. 2010;2010:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith A, Halari R, Giampietro V, Brammer M, Rubia K. Developmental effects of reward on sustained attention networks. Neuroimage. 2011;56 (3):1693–704 doi: 10.1016/j.neuroimage.2011.01.072 [DOI] [PubMed] [Google Scholar]
- 59.Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: Description and Initial Validation of an Integrated Assessment of Child and Adolescent Psychopathology. Journal of Child Psychology and Psychiatry. 2000;41(5):645–55. doi: 10.1111/j.1469-7610.2000.tb02345.x [PubMed] [Google Scholar]
- 60.Goodman R, Scott S. Comparing the strengths and difficulties questionnaire and the child behavior checklist: Is small beautiful? Journal of Abnormal Child Psychology. 1999;27(1):17–24. PubMed PMID: ISI:000079225700003. [DOI] [PubMed] [Google Scholar]
- 61.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8(1):77–100. doi: http://dx.doi.org/10.1016/0272-7358(88)90050-5 [Google Scholar]
- 62.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, Texas: The Psychological Corporation; 1999. [Google Scholar]
- 63.Bernstein D, Fink L. Childhood Trauma Questionnaire: A retrospective self-report. Manual.: San Antonio, TX: The Psychological Cooperation; 1998. [Google Scholar]
- 64.Currie C, Molcho M, Boyce W, Holstein Br, Torsheim Tr, Richter M. Researching health inequalities in adolescents: The development of the Health Behaviour in School-Aged Children (HBSC) Family Affluence Scale. Social Science & Medicine. 2008;66(6):1429–36. doi: http://dx.doi.org/10.1016/j.socscimed.2007.11.024 [DOI] [PubMed] [Google Scholar]
- 65.Bifulco A, Brown GW, Harris TO. Childhood Experience of Care and Abuse (CECA): A Retrospective Interview Measure. Journal of Child Psychology and Psychiatry. 1994;35(8):1419–35. doi: 10.1111/j.1469-7610.1994.tb01284.x [DOI] [PubMed] [Google Scholar]
- 66.Power JD, Barnes K. A., Snyder A. Z., Schlaggar B. L., & Petersen S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–54. doi: 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Dijk KRA, Sabuncu M. R., & Buckner R. L. The Influence of Head Motion on Intrinsic Functional Connectivity MRI. Neuroimage. 2012;59(1):431–8. doi: 10.1016/j.neuroimage.2011.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siegel JS, Power J. D., Dubis J. W., Vogel A. C., Church J. A., Schlaggar B. L., & Petersen S. E. Statistical Improvements in Functional Magnetic Resonance Imaging Analyses Produced by Censoring High-Motion Data Points. Human brain mapping. 2014;35(5):1981–96. doi: 10.1002/hbm.22307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, et al. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Human molecular genetics. 1999;8(4):621–4. [DOI] [PubMed] [Google Scholar]
- 70.Zoroğlu S, Erdal ME, Alaşehirli B, Erdal N, Sivasli E, Tutkun H, et al. Significance of serotonin transporter gene 5-HTTLPR and variable number of tandem repeat polymorphism in attention deficit hyperactivity disorder. Neuropsychobiology. 2002;45(4):176–81. doi: 10.1159/000063667 [DOI] [PubMed] [Google Scholar]
- 71.Roberts S, Keers R, Lester KJ, Coleman JR, Breen G, Arendt K, et al. HPA axis related genes and response to psychological therapies: genetics and epigenetics. Depression and anxiety. 2015;32(12):861–70. doi: 10.1002/da.22430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miyake Y, Onishi Y, Poppel E. Two types of anticipation in synchronization tapping. Acta Neurobiologiae Experimentalis. 2004;64(3):415–26. PubMed PMID: WOS:000222645400012. [DOI] [PubMed] [Google Scholar]
- 73.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams S, Simmons A, et al. Prefrontal involvement in "temporal bridging" and timing movement. Neuropsychologia. 1998;36(12):1283–93. PubMed PMID: ISI:000077340800003. [DOI] [PubMed] [Google Scholar]
- 74.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–15. doi: 10.1038/nrn755 PubMed PMID: WOS:000174207900016. [DOI] [PubMed] [Google Scholar]
- 75.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shapiro K, Hillstrom AP, Husain M. Control of visuotemporal attention by inferior parietal and superior temporal cortex. Current biology: CB. 2002;12(15):1320–5. Epub 2002/08/15. . [DOI] [PubMed] [Google Scholar]
- 78.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28(14):3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Squire RF, Noudoost B, Schafer RJ, Moore T. Prefrontal contributions to visual selective attention. Annual review of neuroscience. 2013;36:451–66. doi: 10.1146/annurev-neuro-062111-150439 [DOI] [PubMed] [Google Scholar]
- 80.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. [DOI] [PubMed] [Google Scholar]
- 81.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 82.Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15(3):331–43. doi: 10.1017/S1355617709090481 PubMed PMID: WOS:000266066700001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller G, Chapman J. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110(1):40–8. [DOI] [PubMed] [Google Scholar]
- 84.Burgess PW, Scott SK, Frith CD. The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia. 2003;41(8):906–18. [DOI] [PubMed] [Google Scholar]
- 85.Benoit RG, Gilbert SJ, Frith CD, Burgess PW. Rostral prefrontal cortex and the focus of attention in prospective memory. Cerebral Cortex. 2011:bhr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coull J, Frith C, Frackowiak RSJ, Grasby P. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia. 1996;34(11):1085–95. [DOI] [PubMed] [Google Scholar]
- 87.Smith A, Giampietro V, Brammer M, Halari R, Simmons A, Rubia K. Functional development of fronto-striato-parietal networks associated with time perception. Frontiers in Human Neuroscience. 2011;5 doi: 10.3389/fnhum.2011.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Markett S, Reuter M, Montag C, Voigt G, Lachmann B, Rudorf S, et al. Assessing the function of the fronto‐parietal attention network: insights from resting‐state fMRI and the attentional network test. Human brain mapping. 2014;35(4):1700–9. doi: 10.1002/hbm.22285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. science. 2007;315(5820):1860–2. doi: 10.1126/science.1138071 [DOI] [PubMed] [Google Scholar]
- 90.Huang H, Gundapuneedi T, Rao U. White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology. 2012;37(12):2693–701. doi: 10.1038/npp.2012.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marshall TR, Bergmann TO, Jensen O. Frontoparietal structural connectivity mediates the top-down control of neuronal synchronization associated with selective attention. PLoS Biol. 2015;13(10):e1002272 doi: 10.1371/journal.pbio.1002272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rubia K. Functional brain imaging across development. European Child & Adolescent Psychiatry. 2012:1–13. doi: 10.1007/s00787-012-0291-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roy A, Hodgkinson CA, DeLuca V, Goldman D, Enoch M-A. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. Journal of psychiatric research. 2012;46(1):72–9. doi: 10.1016/j.jpsychires.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bevilacqua L, Carli V, Sarchiapone M, George DK, Goldman D, Roy A, et al. Interaction between FKBP5 and childhood trauma and risk of aggressive behavior. Archives of general psychiatry. 2012;69(1):62–70. doi: 10.1001/archgenpsychiatry.2011.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Currie CE, Elton RA, Todd J, Platt S. Indicators of socioeconomic status for adolescents: the WHO Health Behaviour in School-aged Children Survey. Health education research. 1997;12(3):385–97. [DOI] [PubMed] [Google Scholar]
- 96.Cooke BM, Weathington JM. Human and animal research into sex-specific effects of child abuse. Hormones and Behavior. 2014;65(4):416–26. doi: 10.1016/j.yhbeh.2014.03.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subjects are required to press a right-hand button as soon as they see a timer appear on the screen counting seconds. The counter appears after either predictable short delays of 0.5s in blocks of 3–5 stimuli, or after unpredictable long delays of 2s, 5s or 8s, pseudorandomly interspersed into the blocks of 0.5s delays. The long second delays have a progressively higher load on sustained attention than the short 0.5s delays that are typically anticipated and have a higher load on sensorimotor synchronization.
(TIF)
The threshold is P < 0.05 FWE corrected. The right of the image corresponds to the right side of the brain. L = left, R = right, ACC = anterior cingulate cortex, IFC = inferior frontal cortex, SMA = supplementary motor area.
(TIF)
Regions demonstrating differential functional connectivity with the left dorsolateral prefrontal cortex and left inferior parietal lobe seed regions during the 8s delay versus 0.5s implicit baseline condition for 21 young people exposed to severe childhood abuse and 27 healthy controls, when covarying for IQ. P-value is <0.05 FWER corrected.
(DOCX)
Data Availability Statement
Data are available at doi:10.5061/dryad.3338c.