Abstract
Biomarker definitions for preclinical Alzheimer’s disease (AD) have identified individuals with neurodegeneration (ND+) without β-amyloidosis (Aβ-) and labeled them with suspected non-AD pathophysiology (SNAP). We evaluated Apolipoprotein E (APOE) ε2 and ε4 allele frequencies across biomarker definitions—Aβ-/ND- (n = 268), Aβ+/ND- (n = 236), Aβ-/ND+ or SNAP (n = 78), Aβ+/ND+ (n = 204)—hypothesizing that SNAP would have an APOE profile comparable to Aβ-/ND-. Using AD Neuroimaging Initiative data (n = 786, 72±7 years, 48% female), amyloid status (Aβ+ or Aβ-) was defined by cerebrospinal fluid (CSF) Aβ-42 levels, and neurodegeneration status (ND+ or ND-) was defined by hippocampal volume from MRI. Binary logistic regression related biomarker status to APOE ε2 and ε4 allele carrier status, adjusting for age, sex, education, and cognitive diagnosis. Compared to the biomarker negative (Aβ-/ND-) participants, higher proportions of ε4 and lower proportions of ε2 carriers were observed among Aβ+/ND- (ε4: OR = 6.23, p<0.001; ε2: OR = 0.53, p = 0.03) and Aβ+/ND+ participants (ε4: OR = 12.07, p<0.001; ε2: OR = 0.29, p = 0.004). SNAP participants were statistically comparable to biomarker negative participants (p-values>0.30). In supplemental analyses, comparable results were observed when coding SNAP using amyloid imaging and when using CSF tau levels. In contrast to APOE, a polygenic risk score for AD that excluded APOE did not show an association with amyloidosis or neurodegeneration (p-values>0.15), but did show an association with SNAP defined using CSF tau (β = 0.004, p = 0.02). Thus, in a population with low levels of cerebrovascular disease and a lower prevalence of SNAP than the general population, APOE and known genetic drivers of AD do not appear to contribute to the neurodegeneration observed in SNAP. Additional work in population based samples is needed to better elucidate the genetic contributors to various etiological drivers of SNAP.
Introduction
Alzheimer’s disease (AD) is marked by a long preclinical stage in which the pathophysiology has evolved but clinical symptoms are not yet present. The National Institute on Aging and Alzheimer’s Association Workgroup established preclinical AD criteria for research based on biomarkers of β-amyloidosis and neurodegeneration [1]. These criteria led to the subsequent identification of a subset of individuals labeled with suspected non-AD pathophysiology (SNAP) due to biomarker evidence of neurodegeneration without β-amyloidosis. SNAP does not align with the leading theoretical progression of dynamic biomarkers during preclinical AD in which β-amyloidosis evidence appears first, followed by neurodegeneration [2] Among cognitively normal older adults, both SNAP and amyloid positive profiles are associated with worse cognitive trajectories when compared to biomarker negative individuals, but the fastest rate of decline is observed in individuals who are positive for markers of both amyloid and neurodegeneration [3]. Among individuals with mild cognitive impairment (MCI), a prodromal stage of dementia, SNAP is associated with increased risk of progressive cognitive decline [4], smaller cortical volume [5], and higher burden of white matter hyperintensities [5] compared to biomarker negative peers. In contrast, when compared to individuals with evidence of both β-amyloidosis and neurodegeneration, SNAP appears to be less likely to progress to clinical AD [6, 7]. Moreover, there is inconsistent evidence of clinical progression among SNAP individuals who are cognitively normal at baseline, with some studies reporting very low conversion rates [6, 8].
Recent genetic exploration of these biomarker groups suggests the frequency of the APOE ε4 genotype (a genetic susceptibility risk factor for AD) may be lower in SNAP when compared to amyloid positive or amyloid positive/neurodegeneration positive groups [4]. This finding suggests that the neurodegeneration and cognitive decline observed in SNAP may have a unique etiology that is not linked to APOE. In addition to the ε4 effect, it is well known that the APOE ε2 allele is protective against AD [9]. Such a protective role may be due to lower levels of AD neuropathology among carriers of the ε2 allele [10], although work in the oldest old has suggested ε2 is associated with preserved cognition but high levels of neuropathology [11]. Additionally, studies of the possible underlying neuropathologies of SNAP, including tangle predominant dementia [12], cerebrovascular disease [13], and argyrophilic grain disease [14], have highlighted an increase in disease risk among APOE ε2 allele carriers. Biomarker groups based on the preclinical AD criteria thus provide a tremendous opportunity to better understand the association between APOE and biomarkers of AD neuropathology and neurodegeneration.
This study assesses the frequency of all APOE alleles across biomarker groups among individuals diagnosed with normal cognition and MCI. We hypothesize that the APOE effect will be specific to the AD cascade [15], such that when compared to biomarker negative participants, we will observe higher frequencies of the APOE ε4 allele and lower frequencies of the ε2 allele among the amyloid positive participants and among the biomarker (both amyloid and neurodegeneration) positive participants but not among the SNAP participants.
Materials and methods
Participants were selected from the AD Neuroimaging Initiative (ADNI). ADNI launched in 2003 as a public-private partnership. The original ADNI study enrolled approximately 800 participants, aged 55–90 years, excluding serious neurological disease (other than AD), history of brain lesion or head trauma, and history of psychoactive medication use (for full inclusion/exclusion criteria see http://www.adni-info.org). Informed written consent was obtained from all participants at each site, and analysis of ADNI’s publically available database was approved by our local Institutional Review Board prior to data analysis.
Participants
Data were accessed on 10/10/2017. ADNI participants were selected for this study if they had cerebrospinal fluid (CSF) biomarker data, an MRI measure of hippocampal volume, and APOE genotype data available for analysis. Participants were excluded if there MRI visit and CSF visit were more than 6 months apart. These criteria resulted in 786 participants, including 305 with NC and 481 with MCI. Participants with AD were excluded.
APOE genotyping
APOE genotyping in ADNI has been outlined previously [16]. Briefly, PCR was followed by HhaI restriction enzyme digestion and results were visualized using ethidium bromide staining.
Hippocampal volume
The ADNI brain MRI protocol has been reported in detail [17]. The current study included 1.5T T1-weighted structural imaging data from ADNI-1 and 3T data from ADNI-2/GO. Cortical reconstruction and volumetric segmentation were performed with the FreeSurfer image analysis suite version 4.3 in ADNI-1 and 5.1 in ADNI-2/GO (http://surfer.nmr.mgh.harvard.edu/; [18–20]. FreeSurfer processing in ADNI, including quality control (QC) procedures, have been described elsewhere [21]. We only included participants who passed QC procedures. We used left hippocampal volume, right hippocampal volume, and estimated intracranial volume (ICV) as defined by FreeSurfer [22].
Biomarker groups
We used CSF amyloid positivity and MRI hippocampal volume neurodegeneration (ND) to create four biomarker groups: Biomarker Negative (Aβ-/ND-), Amyloid Positive (Aβ+/ND-), Neurodegeneration Positive (or SNAP; Aβ-/ND+), and Biomarker Positive (Aβ+/ND+).
Amyloid positivity was based on the assay-specific cut-point of Aβ-42≤192 pg/mL [23]. CSF protocols, including the quantification of Aβ-42, have been detailed previously for ADNI [23, 24].
Classification as ND+ requires an adjusted hippocampal volume≤6723 mm3, based on previously published criteria [3]. Briefly, adjusted hippocampal volume (adj.HV) was calculated using the following formula:
where total.HV is the added values of right and left hippocampal volume, a is the regression coefficient when total.HV is regressed against ICV in the NC group, and mean.ICV.norm is the mean ICV for all NC participants.
Statistical analyses
All statistical analyses were performed using R Studio (version 0.99.485, www.rstudio.com). NC and MCI group comparisons were completed using χ2 for categorical variables and t-tests for continuous variables.
Unadjusted differences in APOE genotype frequencies were evaluated across all biomarker groups using χ2. Next, two binary logistic regression models assessed differences between the Aβ-/ND- group and the three remaining biomarker groups (i.e., Aβ+/ND-, Aβ-/ND+ [SNAP], and Aβ+/ND+), adjusting for age, sex, education, and cognitive diagnosis. In the first logistic regression model, APOE ε4 carrier status was set as the binary outcome (carriers = ε2/ε4, ε3/ε4, ε4/ε4 versus non-carriers = ε2/ε2, ε2/ε3, ε3/ε3). In the second logistic regression analysis, APOE ε2 carrier status was set as the binary outcome (carriers = ε2/ε2, ε2/ε3, ε2/ε4 versus non-carriers = ε3/ε3, ε3/ε4, ε4/ε4). Finally, using two separate χ2 tests, post-hoc pairwise comparisons restricted to the groups carrying at least one AD biomarker evaluated differences in APOE frequency between SNAP and each of the other two biomarker positive groups (Aβ+/ND- and Aβ+/ND+). In all analyses, significance was set a priori as α = 0.05. Correction for multiple comparisons was performed using the Bonferroni procedure for the 6 planned statistical comparisons from our primary analyses (corrected-α = 0.008).
Supplemental analyses were run to test the impact of diagnostic status on our results and to evaluate differences when defining SNAP based on CSF tau levels rather than hippocampal volume. First, we evaluated biomarker group x diagnosis interactions on APOE ε2 carrier status and ε4 carrier status separately using the same logistic regression models outlined above. Second, we re-ran all analyses stratified by diagnosis, including logistic models and post-hoc χ2 tests. Third, we re-ran primary analyses removing individuals with the ε2/ε4 genotype to ensure these individuals were not driving group results. Fourth, we re-ran primary analyses using CSF tau biomarker levels based on a previously established cut-point (CSF tau≥93 pg/mL; [23]. Fifth, we repeated primary analyses covarying for white-matter hyperintensity burden quantified from 3D axial T2-weighted fluid-attenuated inversion recovery images collected as part of the ADNI-2 protocol and quantified using an established pipeline that has been reported in detail elsewhere [25]. Total white-matter hyperintensity volume was entered into logistic models as a continuous covariate, and we evaluated whether effects persisted when including this covariate. Finally, given potential concerns over batch effects for CSF and scanner effects for MRI, we chose to recalculate all biomarker groups using previously processed PET florbetapir data [26] to define amyloid positivity and restricted MRI data to 3T images from ADNI2/GO.
Finally, additional analyses were run leveraging a polygenic risk score (PGRS) of AD based on the top 21 loci from the Lambert et al. meta-analysis [27]. All ADNI genotype data were imputed to the HRC reference panel and genetic processing was performed using PLINK (version 1.9) [28]. Quality control steps excluded SNPs with a minor allele frequency < 1%, with a genotyping rate < 99%, and out of Hardy Weinberg equilibrium (p<0.000001). Risk scores were calculated using the score function in PLINK. The association between PGRS was regressed on age, sex, diagnostic group, and biomarker group. One analysis was run using biomarker group defined using neurodegeneration definitions, and one analysis was run using CSF tau definitions.
Results
Demographic and clinical characteristics are presented in Table 1. The distribution of biomarker groups differed between NC and MCI participants (χ2(3) = 104, p<0.001). While the frequency of Aβ+/ND- (33% in NC, 28% in MCI) and SNAP (10% in both NC and MCI) were comparable between groups, the NC group showed a higher frequency of Aβ-/ND- (50% in NC, 24% in MCI) and a lower frequency of Aβ+/ND+ (8% in NC, 38% in MCI).
Table 1. Sample characteristics.
| Clinical Diagnosis | Statistical Test | ||
|---|---|---|---|
| Normal Cognition | Mild Cognitive Impairment | ||
| Sample Size, n | 305 | 481 | |
| Age, years | 73±6 | 72±7 | t(520.5) = -3.88, p<0.001 |
| Sex, % female | 53% | 43% | χ2(1) = 7.39, p = 0.007 |
| Education, years | 16±3 | 16±3 | t(520.5) = -1.29, p = 0.196 |
| CSF Aβ-42, pg/mL | 201±52 | 172±52 | t(520.5) = -7.69, p<0.001 |
| CSF Total Tau, pg/mL | 67±30 | 92±58 | t(520.5) = 7.89, p<0.001 |
| Hippocampus Volume, mm3 | 7482±787 | 6769±1172 | t(520.5) = -10.19, p<0.001 |
| Composite Memory Performance, z-score | 1.06±0.55 | 0.20±0.69 | t(520.5) = -19.35, p<0.001 |
| Composite Executive Function Performance, z-score | 0.81±0.72 | 0.26±0.80 | t(520.5) = -9.94, p<0.001 |
| PET Amyloid Burden, SUVR# | 1.12±0.18 | 1.22±0.23 | t(520.5) = 5.81, p<0.001 |
| Biomarker Group, n (%) | χ2(3) = 104.19, p<0.001 | ||
| Aβ-/ND- | 153 (50%) | 115 (24%) | |
| Aβ+/ND- | 100 (33%) | 136 (28%) | |
| Aβ-/ND+ (SNAP) | 29 (10%) | 49 (10%) | |
| Aβ+/ND+ | 23 (8%) | 181 (38%) | |
| APOE Genotype, n (%) | χ2(5) = 52.02, p<0.001 | ||
| ε2/ε2 | 0 (0%) | 1 (0.2%) | |
| ε2/ε3 | 43 (14%) | 27 (6%) | |
| ε2/ε4 | 4 (1%) | 7 (1%) | |
| ε3/ε3 | 178 (58%) | 212 (44%) | |
| ε3/ε4 | 73 (24%) | 184 (38%) | |
| ε4/ε4 | 7 (2%) | 50 (10%) | |
Results are presented as mean ± standard deviation unless otherwise indicated. Hippocampal volume adjusted for intracranial volume.
#PET Amyloid Imaging present at same visit for 212 NC and 339 MCI participants.
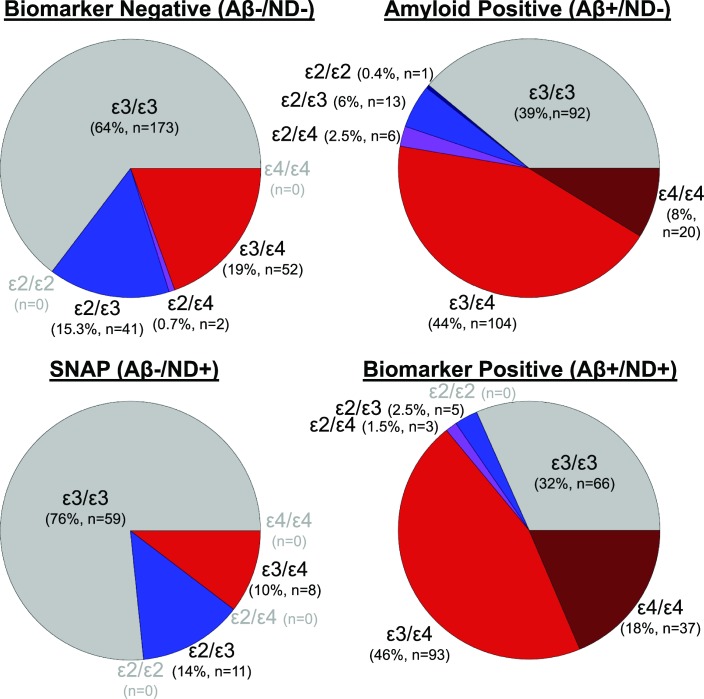
There was an unadjusted difference in the distribution of APOE genotypes across biomarker groups (χ2(15) = 179, p<0.001, see Fig 1), which appears secondary to group differences in both ε4 (χ2(3) = 147, p<0.001) and ε2 allele frequencies (χ2(3) = 20, p<0.001). In fully adjusted models, the Aβ+/ND- and Aβ+/ND+ groups presented less frequently with the APOE ε2 allele compared to the Aβ-/ND- group (p-values<0.03), whereas the ε2 frequency was similar between SNAP and Aβ-/ND- (p = 0.97). Only the difference in ε2 frequency between Aβ+/ND+ and Aβ-/ND- survived correction for multiple comparisons. Similarly, the Aβ+/ND- and Aβ+/ND+ groups presented more frequently with the ε4 allele when compared to the Aβ-/ND- group (p-values<0.001) and survived correction for multiple comparisons, whereas the SNAP and Aβ-/ND- groups were similar in frequency for the ε4 allele (p = 0.30). See Table 2 for details.
Fig 1. APOE genotypes across AD biomarker.
Pie charts are presented by biomarker group based on amyloid status defined using levels of cerebrospinal fluid amyloid-β 42 (Aβ) and neurodegeneration defined using hippocampal volume (ND). Colors represent APOE genotype whereby gray represents homozygous ε3 allele carriers, shades of red represent ε4 allele carriers, shades of blue represent ε2 allele carriers, and purple is used to represent ε2/ε4 carriers. Sample sizes are presented below the segment label for each allele combination. Allele combinations that do not have any participants within a given biomarker group are labeled in light grey font.
Table 2. Associations between biomarker groups and APOE carrier status.
| Odds Ratio (95% CI) | p-value | |
|---|---|---|
| APOE ε2 | ||
| Age | 0.99 (0.96–1.03) | 0.809 |
| Sex (female) | 1.04 (0.65–1.67) | 0.864 |
| Cognitive Diagnosis (MCI) | 0.57 (0.34–0.95) | 0.031 |
| Aβ+/ND-* | 0.53 (0.29–0.92) | 0.028 |
| Aβ-/ND+ (SNAP)* | 0.99 (0.45–2.04) | 0.972 |
| Aβ+/ND+* | 0.29 (0.12–0.65) | 0.004 |
| APOE ε4 | ||
| Age | 0.91 (0.89–0.94) | <0.001 |
| Sex (female) | 0.93 (0.66–1.30) | 0.666 |
| Cognitive Diagnosis (MCI) | 1.36 (0.93–1.98) | 0.111 |
| Aβ+/ND-* | 6.23 (4.09–9.64) | <0.001 |
| Aβ-/ND+ (SNAP)* | 0.65 (0.27–1.41) | 0.301 |
| Aβ+/ND+* | 12.07(7.25–20.53) | <0.001 |
Boldface signifies effects that are significant at p<0.05.
* 4 level categorical variable for biomarker group with Aβ-/ND- set as the referent
Pairwise comparisons between the biomarker-positive groups (i.e., Aβ+/ND-, SNAP, and Aβ+/ND+) suggest only the SNAP group differed from the Aβ+/ND+ group for the ε2 allele (p = 0.005). No other pairwise comparison for the ε2 allele reached statistical significance (p-values>0.08). Regarding ε4 allele frequency, all groups differed from one another (p-values<0.03). See Table A in S1 Supplemental Materials for details.
Supplemental analyses evaluating diagnostic interactions are presented in Table B in S1 Supplemental Materials. We did not observe an interaction between biomarker group and diagnosis on APOE ε4 (p = 0.17), or on ε2 status (p = 0.09). Stratified models are presented in Table C and D in S1 Supplemental Materials.
Supplemental analyses of pairwise comparisons stratified by diagnosis are presented in Table E and F in S1 Supplemental Materials. While ε4 results appeared to be consistent across diagnostic categories, the significant difference between the Aβ+/ND+ positive group and SNAP in the frequency of ε2 was only present among cognitively normal individuals (p = 0.02).
Supplemental analyses using biomarker groups defined with CSF tau are presented in Table G in S1 Supplemental Materials. The results are consistent with the reported findings when using groups defined based on hippocampal volume.
Supplemental analyses removing ε2/ε4 carriers are presented in Table H in S1 Supplemental Materials. Removing these individuals did not change the pattern of results.
Supplemental analyses including white-matter hyperintensity burden as a covariate are presented in Table I in S1 Supplemental Materials. Although the sample size is reduced due to the fact that FLAIR images were only acquired as part of the ADNI-2 protocol, the pattern of results remain largely consistent with the unadjusted reports above, suggesting differences in white-matter hyperintensity burden are not contributing to the observed associations between APOE and biomarker groups.
Supplemental analyses leveraging amyloid PET data to define amyloid positivity in biomarker groups are presented in Table J in S1 Supplemental Materials. Again, the pattern of results largely recapitulated the CSF biomarker results, including strong associations between amyloid groups and APOE genotypes.
Results evaluating PGRS associations with biomarker groups are presented in Table K in S1 Supplemental Materials. The PGRS for AD was unrelated to amyloidosis or neurodegeneration, but did show an association with SNAP defined using CSF tau (F(3,518) = 2.76, p = 0.04) whereby the Aβ-42-/Tau+ group (p = 0.02) and the combined Aβ-42+/Tau+ group (p = 0.04) had higher PGRS than the biomarker negative group.
Finally, demographic and clinical characteristics of the sample are presented in Table 3 to provide a more thorough overview of these biomarker groups.
Table 3. Descriptive statistics by biomarker group.
| Biomarker Group | Statistical Test | ||||
|---|---|---|---|---|---|
| Aβ-/ND- | Aβ+/ND- | Aβ-/ND+ | Aβ+/ND+ | ||
| Sample Size, n | 268 | 236 | 78 | 204 | |
| Age, years | 70.11±6.56 | 71.68±6.64 | 75.15±6.64 | 75.12±6.09 | F(3,782) = 29, p<0.001a,b,c,d,e |
| Sex, % female | 50% | 51% | 38% | 42% | χ2(3) = 6.81, p = 0.078 |
| Education, years | 16.26±2.59 | 16.16±2.66 | 16.45±2.91 | 15.94±2.93 | F(3,782) = 1, p = 0.458 |
| CSF Aβ-42, pg/mL | 236.82±26.69 | 146.87±25.04 | 235.64±27.25 | 135.00±25.10 | F(3,782) = 883, p<0.001a,c,d,e,f |
| CSF Total Tau, pg/mL | 57.66±22.82 | 90.56±49.61 | 62.13±25.34 | 112.19±64.42 | F(3,759) = 62, p<0.001a,c,d,e,f |
| Hippocampus Volume, mm3 | 7835±651 | 7559±610 | 5887±699 | 5858±636 | F(3,782) = 505, p<0.001a,b,c,d,e |
| Composite Memory Performance, z-score | 0.92±0.62 | 0.63±0.68 | 0.51±0.77 | -0.07±0.66 | F(3,782) = 88, p<0.001a,b,c,e,f |
| Composite Executive Function Performance, z-score | 0.82±0.77 | 0.49±0.76 | 0.45±0.74 | 0.00±0.72 | F(3,782) = 45, p<0.001a,b,c,e,f |
| PET Amyloid Burden, SUVR# | 1.02±0.07 | 1.27±0.19 | 1.02±0.10 | 1.40±0.22 | F(3,547) = 172, p<0.001a,c,d,e,f |
Significant pairwise statistical comparisons are indicated in the following manner
aAβ-/ND- v. Aβ+/ND-
bAβ-/ND- v. Aβ-/ND+
cAβ-/ND- v. Aβ+/ND+
dAβ+/ND- v. Aβ-/ND+
eAβ+/ND- v. Aβ+/ND+
fAβ-/ND+ v. Aβ+/ND+.
Results are presented as mean ± standard deviation unless otherwise indicated. Hippocampal volume adjusted for intracranial volume.
#PET Amyloid Imaging present at same visit for a subset of participants.
Discussion
We evaluated the distribution of APOE allele frequencies in biomarker groups operationally defined using the classification scheme for preclinical AD. As expected, we observed a lower ε2 allele frequency and a higher ε4 allele frequency in participants with evidence of amyloidosis (i.e., Aβ+/ND- and Aβ+/ND+) compared to biomarker negative (Aβ-/ND-) participants. As hypothesized, we observed comparable APOE allele frequencies among participants with SNAP compared to participants who were biomarker negative (Aβ-/ND-). These results add to growing evidence that SNAP is not driven by an APOE pathway and that the neurodegeneration that defines SNAP likely has a unique etiology that differs from AD.
To our knowledge, our results are among the first to report the frequency of the APOE ε2 allele in biomarker groups used to define preclinical AD. We observed lower ε2 frequencies in the amyloidosis (Aβ+/ND- and Aβ+/ND+) groups compared to the biomarker negative (Aβ-/ND-) group, but we did not observe an ε2 frequency difference between the SNAP (Aβ-/ND+) and biomarker negative (Aβ-/ND-) groups. In post-hoc pairwise comparisons, we observed a higher ε2 frequency in SNAP compared the biomarker positive (Aβ+/ND+) groups. As highlighted in the introduction, the APOE ε2 allele is particularly interesting to evaluate in the context of SNAP because it has a well-documented protective effect on AD pathology [10], yet it has been associated with an increased risk of cerebrovascular disease [13]. Similarly, an increased frequency of APOE ε2 have been reported in argyrophilic grain disease [14] and hemorrhage in cerebral amyloid angiopathy [29], suggesting APOE ε2 may play a unique role in the non-AD pathological processes implicated in SNAP [4]. However, our findings suggest that, like the ε4 allele, the frequency of the ε2 allele is comparable to observations in biomarker negative (Aβ-/ND-) individuals. Thus, while ε2 may protect against the AD cascade in SNAP, the similar frequency distribution in biomarker negative (Aβ-/ND-) and SNAP groups suggests ε2 is unlikely to contribute to the neuropathology underlying SNAP. That said, the low ε2 frequency in SNAP emphasizes the need for further exploration in larger datasets where subtle differences in ε2 between SNAP and biomarker negative participants may be more readily detectable.
We also observed expected differences in APOE ε4 frequency among biomarker groups consistent with previous work in both cognitively normal [3] and MCI [4] cohorts, suggesting the ε4 allele frequency is lower in participants with SNAP compared to amyloidosis (Aβ+/ND- or Aβ+/ND+[2, 30] The APOE ε4 allele is thought to promote risk for AD through an amyloid rather than tau pathway [31], and the present results suggest that APOE ε4 does not exert a major effect in the pathophysiology of neurodegeneration in SNAP. It is interesting to note that one of the primary hypothesized pathways of neurodegeneration in SNAP, hippocampal sclerosis (HS), also shows no association with APOE when AD neuropathology is not present in the brain [32]. Similarly, primary age-related tauopathy (PART) appears unrelated to APOE [33, 34], although debate surrounds this issue [35]. The present results suggest that neither APOE ε2 nor ε4 alleles correspond to the neurodegeneration or tauopathy observed in SNAP. In contrast to PART and HS, there is some evidence of an association between arteriolosclerosis and APOE in the presence of amyloidosis [36], and some evidence of an inverse association between APOE ε2 and markers of cerebrovascular disease relative to what is observed in AD [13]. Thus, there is a need in the field to more carefully characterize the underlying neuropathology of SNAP across cohorts to more fully elucidate risk and protective factors. The ADNI participant screening procedures included an exclusion for overt cerebrovascular disease, suggesting it may not be ideally suited to evaluate the contributions of genetic drivers of cerebrovascular disease within SNAP. This feature of the ADNI cohort leaves open the possibility that APOE may contribute to SNAP when the driven by a cerebrovascular etiology.
In supplemental analyses, we observed an association between SNAP groups defined using CSF tau levels and PGRS for AD, suggesting that while APOE does not appear related to SNAP, some of the genetic risk associated with clinical AD is relevant to tau even in the absence of amyloidosis (see Table K in S1 Supplemental Materials). These findings recapitulate the lack of association between CSF amyloid, baseline hippocampal volume, and PGRS in a previous ADNI publication [37], although CSF tau was not evaluated in that manuscript. The patterns of association between PGRS and biomarker groups (that differs from the APOE association with biomarker groups) highlights the need to better understand the genetic architecture of the neuropathological features of AD, and characterize how genetic factors differentially drive the neuropathological and clinical progression in AD. The amount genetic overlap between AD and SNAP likely depends on the various etiologies that underlie SNAP. Regardless of etiology, however, we provide strong evidence that APOE is not associated with SNAP.
Previous reports of these biomarker groups in ADNI, both among individuals with normal cognition at baseline [38] and among individuals with MCI at baseline [4], are consistent with the clinical and cognitive group differences we observed here (Table 3). Specifically, we observed expected group differences in cognition, biomarker status, and hippocampal volume including marked differences in cognition and hippocampal volume among SNAP individuals compared to biomarker negative individuals. Given that some of the group differences between SNAP and biomarker negative individuals depend on the way SNAP is defined (e.g., CSF tau levels), it appears that moving toward a multi-biomarker diagnostic scheme accounting for amyloid, tau, and neurodegeneration (e.g., the A/T/N classification [39]) will more accurately characterize the neuropathology underlying SNAP. Unfortunately, we did not have a large enough sample size to fully evaluate APOE differences among the A/T/N groups. Larger sample sizes with biomarker and imaging data are needed to fully characterize the genetic etiology of these neuropathological processes.
These findings must be interpreted in the context of several known limitations of the ADNI dataset. The frequency of SNAP observed in the present analysis (10%) is notably lower than some previous reports of SNAP (25%)[3]. This discrepancy may be due in part to the fact that, as previously mentioned, ADNI underrepresents the burden of cerebrovascular disease among older adults given sample recruitment criteria (Hachinski score<4) and MRI contraindications. Importantly, SNAP is a particularly strong driver of MCI in population-based studies more accurately reflecting the mixed etiologies of age-related cognitive impairment. Thus, it will be critical to further assess the association between APOE and SNAP in more representative cohorts. There is also an over-representation of APOE ε4 carriers in ADNI, highlighting the need to further investigate the full breadth of APOE alleles in additional samples that better represent the frequency of SNAP and each APOE allele. The ADNI cohort is also enriched for White, highly educated individuals who do not reflect the general population of older adults. Larger and more representative samples are required to fully evaluate the role of APOE ε2 in SNAP and will likely require a meta-analysis across multiple datasets. Therefore, it is imperative that future SNAP studies report the full spectrum of APOE genotype to more easily facilitate cross-study evaluations of possible genetic effects. As previously mentioned, there is growing emphasis on biomarker definitions that combine a marker of amyloid, a marker of tau, and a marker of neurodegeneration, called the A/T/N scheme [39]. Additional work focusing on APOE genotype in these smaller sub-groups will require much larger sample sizes, but the present results leave open the possibility that ε2 may be involved in neuropathological processes that underlie a more specific non-amyloid sub group (A-/T+/N+, A-/T+/N-, or A-/T-/N+).
In conclusion, this study is among the first to provide evidence that the APOE ε2 allele frequency is comparable between SNAP and biomarker negative individuals and adds to existing evidence [4] that the frequency of the APOE ε4 allele is lower in participants with SNAP. These results collectively suggest that any genetic etiology of neurodegeneration in SNAP is likely through a non-APOE pathway, further supporting the notion that SNAP represents a heterogeneous subset of older adults experiencing non-AD neurodegeneration.
Supporting information
(DOCX)
Acknowledgments
The authors report no conflicts of interest. This research was supported in part by K01-AG049164 (TJH), K12-HD043483 (TJH, KAG), Alzheimer’s Association IIRG-08-88733 (ALJ), R01-AG034962 (ALJ), R01-HL111516 (ALJ), K24-AG046373 (ALJ), Paul B. Beeson Career Development Award in Aging K23-AG045966 (KAG), UL1-TR000445 (Vanderbilt Clinical Translational Science Award from the National Center for Research Resources and National Institutes of Health), and the Vanderbilt Memory & Alzheimer’s Center.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Data Availability
Data are from the publicly available Alzheimer's disease neuroimaging initiative dataset and can be accessed at http://adni-info.org/.
Funding Statement
This research was supported in part by K01-AG049164 (TJH), K12-HD043483 (TJH, KAG), Alzheimer’s Association IIRG 08 88733 (ALJ), R01-AG034962 (ALJ), R01-HL111516 (ALJ), K24-AG046373 (ALJ), Paul B. Beeson Career Development Award in Aging K23-AG045966 (KAG), UL1-TR000445 (Vanderbilt Clinical Translational Science Award from the National Center for Research Resources and National Institutes of Health), and the Vanderbilt Memory & Alzheimer’s Center. Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's and Dementia. 2011;7(3):280–92. PubMed PMID: 412. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, et al. An operational approach to National Institute on Aging–Alzheimer's Association criteria for preclinical Alzheimer disease. Annals of neurology. 2012;71(6):765–75. doi: 10.1002/ana.22628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mormino EC, Betensky RA, Hedden T, Schultz AP, Amariglio RE, Rentz DM, et al. Synergistic effect of β-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA neurology. 2014;71(11):1379–85. doi: 10.1001/jamaneurol.2014.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caroli A, Prestia A, Galluzzi S, Ferrari C, van der Flier WM, Ossenkoppele R, et al. Mild cognitive impairment with suspected nonamyloid pathology (SNAP) Prediction of progression. Neurology. 2015;84(5):508–15. doi: 10.1212/WNL.0000000000001209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wisse LE, Butala N, Das SR, Davatzikos C, Dickerson BC, Vaishnavi SN, et al. Suspected non-AD pathology in mild cognitive impairment. Neurobiology of aging. 2015;36(12):3152–62. doi: 10.1016/j.neurobiolaging.2015.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knopman DS, Jack C, Wiste H, Weigand S, Vemuri P, Lowe V, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78(20):1576–82. doi: 10.1212/WNL.0b013e3182563bbe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen RC, Aisen P, Boeve BF, Geda YE, Ivnik RJ, Knopman DS, et al. Mild cognitive impairment due to Alzheimer disease in the community. Annals of neurology. 2013;74(2):199–208. doi: 10.1002/ana.23931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. The Lancet Neurology. 2013;12(10):957–65. doi: 10.1016/S1474-4422(13)70194-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West HL, William Rebeck G, Hyman BT. Frequency of the apolipoprotein E ε2 allele is diminished in sporadic Alzheimer disease. Neuroscience Letters. 1994;175(1–2):46–8. doi: http://dx.doi.org/10.1016/0304-3940(94)91074-X [DOI] [PubMed] [Google Scholar]
- 10.Nicoll J, Savva G, Stewart J, Matthews F, Brayne C, Ince P. Association between APOE genotype, neuropathology and dementia in the older population of England and Wales. Neuropathology and Applied Neurobiology. 2011;37(3):285–94. doi: 10.1111/j.1365-2990.2010.01130.x [DOI] [PubMed] [Google Scholar]
- 11.Berlau DJ, Corrada MM, Head E, Kawas CH. APOE ε2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009;72(9):829–34. doi: 10.1212/01.wnl.0000343853.00346.a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jellinger KA, Bancher C. Senile dementia with tangles (tangle predominant form of senile dementia). Brain pathology (Zurich, Switzerland). 1998;8(2):367–76. Epub 1998/04/18. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schilling S, DeStefano AL, Sachdev PS, Choi SH, Mather KA, DeCarli CD, et al. APOE genotype and MRI markers of cerebrovascular disease Systematic review and meta-analysis. Neurology. 2013;81(3):292–300. doi: 10.1212/WNL.0b013e31829bfda4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghebremedhin E, Schultz C, Botez G, Rub U, Sassin I, Braak E, et al. Argyrophilic grain disease is associated with apolipoprotein E epsilon 2 allele. Acta Neuropathol. 1998;96(3):222–4. [DOI] [PubMed] [Google Scholar]
- 15.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–3. [DOI] [PubMed] [Google Scholar]
- 16.Saykin AJ, Shen L, Foroud TM, Potkin SG, Swaminathan S, Kim S, et al. Alzheimer's Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: Genetics core aims, progress, and plans. Alzheimer's & Dementia. 2010;6(3):265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack CR, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer's disease neuroimaging initiative (ADNI): MRI methods. Journal of Magnetic Resonance Imaging. 2008;27(4):685–91. doi: 10.1002/jmri.21049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. doi: 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- 19.Fischl B, Sereno MI, Dale AM. Cortical Surface-Based Analysis: II: Inflation, Flattening, and a Surface-Based Coordinate System. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396 [DOI] [PubMed] [Google Scholar]
- 20.Fischl B, Sereno MI, Tootell RBH, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8(4):272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(5):1310–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 23.Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73(15):1193–9. doi: 10.1212/WNL.0b013e3181bc010c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw LM, Vanderstichele H, Knapik-Czajka M, Figurski M, Coart E, Blennow K, et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathologica. 2011;121(5):597–609. doi: 10.1007/s00401-011-0808-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott JA, Braskie MN, Tosun D, Thompson PM, Weiner M, DeCarli C, et al. Cerebral Amyloid and Hypertension are Independently Associated with White Matter Lesions in Elderly. Frontiers in aging neuroscience. 2014;7:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Annals of neurology. 2012;72(4):578–86. doi: 10.1002/ana.23650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45(12):1452–8. Epub 2013/10/29. doi: 10.1038/ng.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. The American Journal of Human Genetics. 2007;81(3):559–75. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarron MO, Nicoll JA. Apolipoprotein E genotype and cerebral amyloid angiopathy-related hemorrhage. Ann N Y Acad Sci. 2000;903:176–9. [DOI] [PubMed] [Google Scholar]
- 30.Jack CR, Knopman DS, Chételat G, Dickson D, Fagan AM, Frisoni GB, et al. Suspected non-Alzheimer disease pathophysiology—concept and controversy. Nature Reviews Neurology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Annals of Neurology. 2010;67(1):122–31. doi: 10.1002/ana.21843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134(5):1506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128(6):755–66. doi: 10.1007/s00401-014-1349-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson PT, Trojanowski JQ, Abner EL, Al-Janabi OM, Jicha GA, Schmitt FA, et al. “New Old Pathologies”: AD, PART, and Cerebral Age-Related TDP-43 With Sclerosis (CARTS). Journal of Neuropathology & Experimental Neurology. 2016;75(6):482–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duyckaerts C, Braak H, Brion J-P, Buée L, Del Tredici K, Goedert M, et al. PART is part of Alzheimer disease. Acta Neuropathologica. 2015;129(5):749–56. doi: 10.1007/s00401-015-1390-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yip AG, McKee AC, Green RC, Wells J, Young H, Cupples LA, et al. APOE, vascular pathology, and the AD brain. Neurology. 2005;65(2):259–65. Epub 2005/07/27. doi: 10.1212/01.wnl.0000168863.49053.4d [DOI] [PubMed] [Google Scholar]
- 37.Mormino EC, Sperling RA, Holmes AJ, Buckner RL, De Jager PL, Smoller JW, et al. Polygenic risk of Alzheimer disease is associated with early- and late-life processes. Neurology. 2016;87(5):481–8. doi: 10.1212/WNL.0000000000002922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toledo JB, Weiner MW, Wolk DA, Da X, Chen K, Arnold SE, et al. Neuronal injury biomarkers and prognosis in ADNI subjects with normal cognition. Acta Neuropathologica Communications. 2014;2(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jack CR, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539–47. doi: 10.1212/WNL.0000000000002923 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Data are from the publicly available Alzheimer's disease neuroimaging initiative dataset and can be accessed at http://adni-info.org/.