Abstract
Epidemiological studies show an association between rheumatoid arthritis (RA) and periodontal disease. Porphyromonas gingivalis (P.gingivalis) is a well-known pathogen in periodontitis. This study investigated the pathogenic effects of P.gingivalis on autoimmune arthritis in vivo. Collagen-induced arthritis (CIA) mice were intraperitoneally injected with W83 and 2561 strains of P.gingivalis. Infection with P.gingivalis exacerbated arthritis score in CIA mice. Synovial inflammation and bone destruction in CIA mice infected with P.gingivalis were more severe than in uninfected CIA mice. Both W83 and 2561 strains were more pro-arthritic after arthritis symptom was fully activated. Interestingly, only W83 strain was arthritogenic before autoimmune reaction initiated. Citrullination was detected in synovial tissue of CIA mice and CIA mice inoculated with P.gingivalis, but not in normal control mice. The citrullinated area was greater in P.gingivalis-infected CIA mice than in non-infected CIA mice. This study showed that P.gingivalis exacerbated disease in a mouse model of autoimmune arthritis and increased the expression of citrullinated antigens in the synovium. The arthritogenic effects of P.gingivalis were at least in part, dependent upon the bacterial strain with or without fimbriae expression, route and time of infection. P.gingivalis-mediated citrullination may explain the possible link between periodontal disease and RA.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by joint inflammation and destruction [1]. Production of rheumatoid factor and autoantibodies leads to increased functional disability and morbidity [2]. Although the exact pathophysiology of RA is unclear, it does involve a combination of genetic and environmental factors [3–5].
Citrullination is an important post-translational modification that is ubiquitous in normal physiology. It is a process which arginine is conversed into citrulline in proteins. Peptidylarginine deaminase (PAD) is the enzyme that is responsible for this process [6,7]. PAD enzymes are normally involved in various regulatory processes in human [8,9]. Since PAD involves in biological processes such as epidermal differentiation, hair follicles maturation, and other epigenetic regulations, it is reported to be related to diseases such as psoriasis or multiple sclerosis [10]. PAD is also known to affect the insulation of nerve fibers which induce disease conditions such as alzheimer or prion diseases [8,10,11]. Also, citrullination of chemokines was recently shown to have functional roles in binding, signaling and proteolytic cleavage [12,13]. Citrullination also play a critical role in RA pathogenesis [14,15]. Increased citrullination is observed in the RA synovium, and antibodies against citrullinated peptides are generated by RA-associated autoimmune responses [16–18]. Previous studies suggest that PAD mediates citrullination of proteins in RA as well [6,7,19–21].
PAD is present in most organisms, yet, PAD expressed by Porphyromonas gingivalis (PPAD) is the only active form of bacterial PAD [22]. Porphyromonas gingivalis (P.gingivalis) is a gram-negative, anaerobic bacterium. P.gingivalis is usually found in oral cavities, which induce periodontal disease such as periodontitis [9]. The physiological role of PPAD is yet not known, however, it was suggested to enhance the bacterial survival by producing ammonia during deamination [23]. Ammonia neutralizes the acidic condition in the periodontal pocket and optimizes gingipain and PPAD function which induce ATP production and negatively regulate the neutrophil function [24].
Because of these characteristics, the relation between P.gingivalis, citrullination, and RA was actively investigated [9,25–27]. It is expected that when a host is infected with P.gingivalis, PPAD converts peptidyl-arginine into peptidyl-citrulline and T cells are activated by bacterial lipopolysaccharides. These T cells then activate B cells, leading to production of anti-citrullinated protein/peptide antibodies (ACPA) specific for citrullinated peptides. Citrullinated peptide-ACPA immune complexes are then formed and trigger inflammation in the joints of RA patients. During this process, citrullinated peptides originating from P.gingivalis and host peptides citrullinated by PPAD act as autoantigens that exacerbate autoimmune responses associated with RA [28–31]. Several isotopic forms of PAD in P.gingivalis and humans are reported [22,32,33]. PAD2 and PAD4 expression was shown in the rheumatoid synovium and synovial fluid cells [20,34,35]. PAD4 was present in the synovial fluid of RA patients and patients with spondyloathropathy or OA. On the other hand, PAD2 was expressed in the knee joint of RA patients, but not in OA patients [33]. It was also shown that the PAD expression in the synovium tissue correlated with infiltration of inflammatory cells, synovial thickening, and synovium vascularity [20].
Based on these observations, we aimed to examine the pathogenic role of P.gingivalis in autoimmune arthritis. The arthritogenic effects of P.gingivalis was confirmed by bacterial strain (i.e. W83 and 2561), route, and time point of inoculation. We conducted clinical and histological analyses of a collagen-induced arthritis (CIA) mouse model infected with P.gingivalis. CIA mice infected with P.gingivalis showed increased expression of enolase, fibronectin, and citrullinated antigens in the synovial region. This study may provide a possible link between P.gingivalis and RA.
Materials and methods
Ethical approval
All procedures involving animals were performed in accordance with the Laboratory Animals Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Guidelines and Policies for Rodent Experimentation provided by the Institutional Animal Care and Use Committee of the School of Medicine of The Catholic University of Korea. Experiments were performed according to the ARRIVE guidelines [9]. The study protocol was approved by the Institutional Review Board of The Catholic University of Korea (CUMC-2013-0011-02). Synovial samples from patients with RA were obtained from the Catholic Human Disease Sample Bank. Samples were donated anonymously; therefore, the requirement for consent was waived by the Institutional Review Board.
Induction and assessment of collagen-induced arthritis
Mice were each subdivided into 6 groups. Each group was composed of 5 mice. Non-treated mice were used as normal control group. To induce CIA, mice were immunized intradermally using 2mg/mL Bovine type II collagen (CII; Chondrex, Redmond, WA, USA) emulsified 1:1 with 2mg/mL Complete Freund’s adjuvant (CFA; Chondrex, Redmond, WA, USA). 6-week-old male DBA/1J mice (Orient Bio, Seongnam, Korea) were intradermally injected at day 0 with 100mL of CII/CFA emulsion. CII emulsified 1:1 with Incomplete Freund’s adjuvant (IFA; Chondrex, Redmond, WA, USA) was injected into mice as a booster immunization 21 days after the first immunization.
Development of arthritis was monitored and scored in a blinded manner. Disease severity was scored three times a week. The severity of arthritis in each front and hind paw was scored from 0 to 4 (0, normal; 1, mild swelling confined to the tarsals; 2, swelling of two or more toes or joints, or increased swelling; 3, moderate swelling extending from the ankle to the metatarsal joints; and 4, severe swelling encompassing the ankle, foot, and digits). A representative arthritis score was determined by summing the scores of all four paws [36].
Bacterial infection
Porphyromonas gingivalis W83 (ATCC BAA-308, VA, USA) and Porphyromonas gingivalis 2561 (Kyung Hee University, Seoul, Korea), Fusobacterium nucleatum (ATCC 25586, VA, USA) were used in this experiment. P.gingivalis strains W83, 2561 and Fusobacterium nucleatum (F.n) were grown in anaerobic conditions.
Oral infection group mice were infected before and after CIA induction. For oral infection, mice were inoculated with 1×109 colony-forming units (CFU) of bacteria in 30 μl PBS with 2% carboxymethyl cellulose (Sigma-Aldrich, St. Louis, MO, USA). Bacteria were administered to the oral cavity of each mouse through a feeding needle two times a week.
For Intraperitoneal infection, mice were injected with 1×109 CFU bacteria in a total volume of 100 μl. Intraperitoneal infection group mice were infected before and after CIA induction as well.
Assessment of periodontitis
The total anaerobic bacteria count was determined using the McFarland turbidity standard protocol. Bacteria were directly swabbed from the gingival surface of CIA mice using a small sterilized brush, and then soaked in tryptic soy broth medium (Difco Laboratories, Detroit, MI, USA), and incubated in an anaerobic basket with an anaerobic gas pack (AnaeroGen, Oxoid Ltd., Hampshire, UK). Alveolar bone loss was examined morphometrically by measuring the distance between the cementoenamel junction (CEJ) and alveolar bone crest (ABC), as previously described [11,13]. Briefly, skulls were first autoclaved and defleshed, and the jaws were immersed in 3% hydrogen peroxide overnight and then stained with 1% methylene blue for 1 min. The distance from the CEJ to the ABC was assessed at five buccal sites per mouse using the Visiopharm Integrator System (Visiopharm, Horsholm, Denmark). All data were analyzed using GraphPad Prism 5 software (GraphPad, San Diego, CA, USA).
Histological assessment
Mice were sacrificed by CO2 inhalation. The joint tissues from the hind paws were fixed in 4% paraformaldehyde and decalcified in 10% EDTA bone decalcifier prior to embedding in paraffin. Paraffin sections were then prepared (5μm thickness) and stained with hematoxylin and eosin, safranin O, and toluidine blue.
The inflammation score and joint destruction score were measured using the procedure of Huckel et al. by three individual researchers in a blinded manner [37,38]. Inflammation and destruction scores were determined microscopically by blinded examiners. The inflammation score of each group was measured by the severity of infiltration and pannus formation. The destruction score was measured based on the cartilage and bone destruction [39,40].
Tartrate-resistant acid phosphatase (TRAP) staining was performed using a commercial kit (Sigma, MO, USA), with hematoxylin as the counterstain. TRAP-positive cells with three or more nuclei were counted as osteoclasts.
Immunostaining
To confirm the presence of citrullinated proteins, joint tissues were stained with anti-CCP (12G1) antibody (AR13-MA0001, Abfrontier, Seoul, Korea) [41]. Joint tissues were deparaffinized in xylene and then hydrated through a graded series of ethanol solutions. Endogenous peroxidase activity was blocked with 3% H2O2 prepared in PBS. Staining was performed using Mouse on Mouse Basic Kit. Tissue sections were incubated with Mouse on Mouse (M.O.M) mouse IgG blocking agent to block nonspecific antibody binding. Anti-CCP (12G1) antibody (AR13-MA0001, Abfrontier, 1:100) was diluted in M.O.M Diluent buffer and incubated overnight at 4°C. IgG isotype control antibody (5415S, Cell Signaling, MA, USA) was used to confirm nonspecific antibody binding IgG. The sections were then incubated with Biotinylated Anti-Mouse Ig Reagent and an avidin-biotin complex. Chromogenic reactions were visualized with DAB Peroxidase (HRP) Substrate Kit (SK-4100, Vector Lab, CA, USA). Sections were counterstained with hematoxylin.
To detect the synovial proteins (i.e. enolase, vimentin, and fibronectin), anti-vimentin antibody (sc7558, Santa Cruz, CA, USA), anti-fibronectin antibody (ab23750, Abcam, Cambrige, UK), anti-enolase antibody (sc15343, Santa Cruz) were diluted at a concentration of 1: 100 in 5% normal goat serum containing 1% PBA. Slides were incubated at 4°C overnight. The sections were then incubated with Biotinylated Anti-Mouse Ig Reagent and an avidin-biotin complex. Chromogenic reactions were visualized with DAB Peroxidase (HRP) Substrate Kit (SK-4100, Vector Lab, CA, USA). Sections were counterstained with hematoxylin.
The co-localization of vimentin, fibronectin, and enolase was confirmed by fluorescence staining. Staining was performed using M.O.M Fluorescein Kit (BMK-2201, Vector Lab). Tissue sections were incubated with M.O.M mouse IgG blocking Reagent. Anti-CCP (12G1) antibody was diluted at a concentration of 1:100 in M.O.M Diluent buffer and was incubated at 4°C overnight. Fluorescein Avidin DCS was diluted in PBS and incubated at RT for 10 min. For serum blocking, 10% normal goat serum (S-1000, Vector Lab) containing 1% PBA was incubated at RT for 30 minutes. Anti-vimentin antibody (sc7558, Santa Cruz, CA, USA), anti-fibronectin antibody (ab23750, Abcam, Cambrige, UK), anti-enolase antibody (sc15343, Santa Cruz) were diluted at a concentration of 1: 100 in 5% normal goat serum containing 1% PBA. Slides were incubated at 4°C overnight. Alexa Fluor 594 goat anti-rabbit IgG (H+L) antibody (A11037, molecular probe, Oregon, USA) was diluted at a concentration of 1:200 in PBS and incubated for 40 minutes at RT. Nucleus staining was performed using 4',6-diamidino-2-phenylindole (DAPI; 10236276001, ROCHE, Basel, Switzerland).
Quantitative image analysis
Automated image analysis techniques were used to quantify the number of TRAP-positive cells. To quantify TRAP staining and IHC staining, slide labeled with TRAP and ACPA were scanned using the Leica SCN400 slide scanner (Leica SCN400, Wetzlar, Germany). Using TissuemorphDP version software (Visiopharm, Denmark), the percentage of surface area per osteoclast (≥3 nuclei) and then DAB surface area were quantified. Assays were performed in triplicate.
Statistical analysis
All experiments were repeated three or more times. The results are shown as mean and standard error of the mean. Error bars represent the standard error of the mean. Statistical analysis was performed and graphs were drawn using GraphPad Prism 5 (GraphPad). The T-test was applied to analyse non-parametric quantitative datasets, and the one-tailed p-value was calculated. *, P<0.01; **, P<0.005; and ***, P<0.001 indicated statistical significance.
Results
Effects of P.gingivalis on severity of collagen-induced arthritis (CIA)
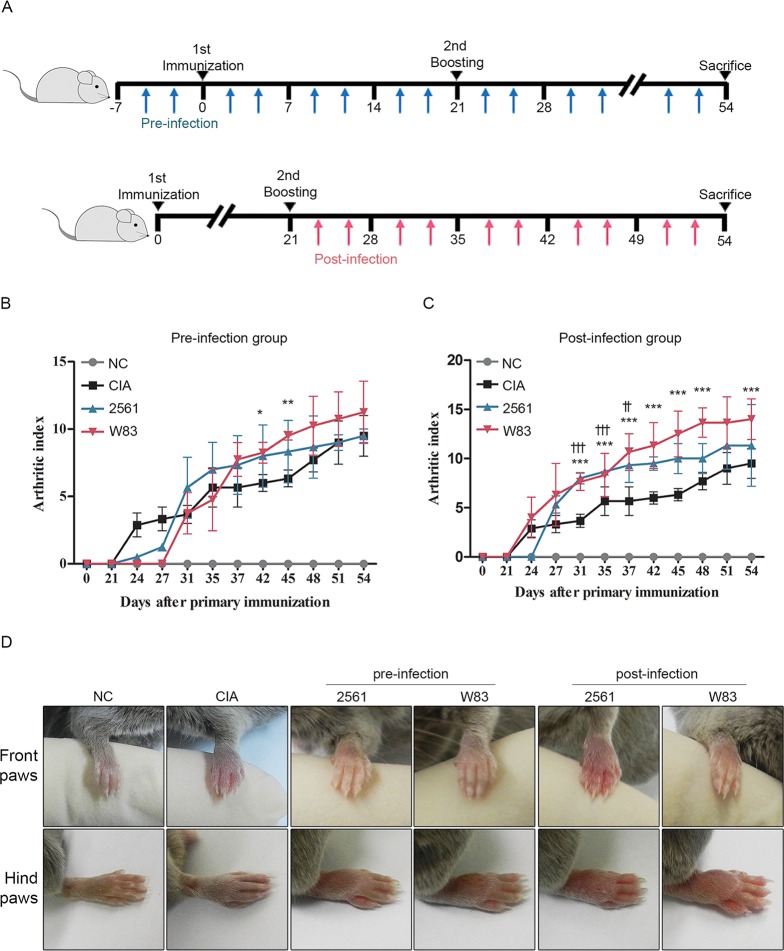
CIA mice model is the most commonly studied autoimmune model of RA that show similar symptoms such as cartilage and bone destruction [42,43]. We investigated whether P.gingivalis infection affects the incidence or severity of CIA. P.gingivalis was inoculated orally and intraperitoneally with both W83 and 2561 strain. W83 is a strain lacking fimbriae expression, while 2561 is known to have several types of fimbriae on its membrane. To determine whether the time of infection affects CIA, we examined two different time points: before the first CII immunization (Pre-infection) and after the boosting CII immunization (Post-infection). Each group was infected twice a week with different strains of P.gingivalis (Fig 1A). When delivered orally, W83 showed increased arthritic index in the pre-infected time point (S 1A). The difference, however, was not observed in the post-infected group when orally delivered (S 1B). The inoculated mice showed defected gum regions than the normal control (NC) or CIA control (S 1C). This destruction was evaluated by methylene blue staining (S 1D). The length of the destructed cemantoenamel junction (CEJ)-alveolar bone crest (ABC) distance was increased in inoculated mice in both time points, which indicates successful induction of periodontitis (S 1E).
Fig 1. Effects of P.gingivalis on severity of collagen-induced arthritis (CIA).
(A) Two P.gingivalis strains (W83 and 2561) were injected intraperitoneally into mice twice per week, starting either 1 week before the first immunization with collagen (Pre-infection group) or immediately after the second booster immunization (Post-infection group). Arthritis score of (B) pre-infected group and (C) post-infected group is shown. Arthritis scores were evaluated twice per week throughout the experimental period. The score for each paw ranged from 0 (no swelling) to 4 (erythema and severe swelling encompassing the ankle and foot); the scores for all four paws were summed to generate a representative arthritis score. Data represent the mean arthritis score (± standard error of the mean; SEM). ** P < 0.05 Pre-infection with W83 versus non-infected CIA; *** P < 0.01 Post-infection with W83 versus non-infected CIA; †† P < 0.05 Post-infection with 2561 versus non-infected CIA; ††† P < 0.01 Post-infection with 2561 versus non-infected CIA. (D) Representative images of front paws and hind paws from mice in each group.
In the case of intraperitoneally inoculated mice, the W83 strain resulted in a higher arthritis score than 2561 in the Pre-infection setting (Fig 1B). Whereas in a Post-infection setting, the arthritis scores in mice infected with W83 or 2561 were higher than those in the non-infected CIA group (Fig 1C). After 1 week, all of the paws of CIA mice (infected and non-infected) were swollen, suggesting successful induction of arthritis. However, there was no difference between the two P.gingivalis strains in terms of severity of swelling (Fig 1D). To confirm if the symptoms are caused by PPAD, we compared the arthritic score of CIA mice infected with W83 or Fusobacterium nucleatum (F.n) (S 2). F.n is a bacterial strain with mutant PAD. The W83 infected CIA mice resulted in an increase in arthritis scores whereas the F.n infected CIA mice arthritis score was similar to that of CIA mice. Through this observation we can assume that the increased inflammation in the CIA mice paw was affected by PPAD.
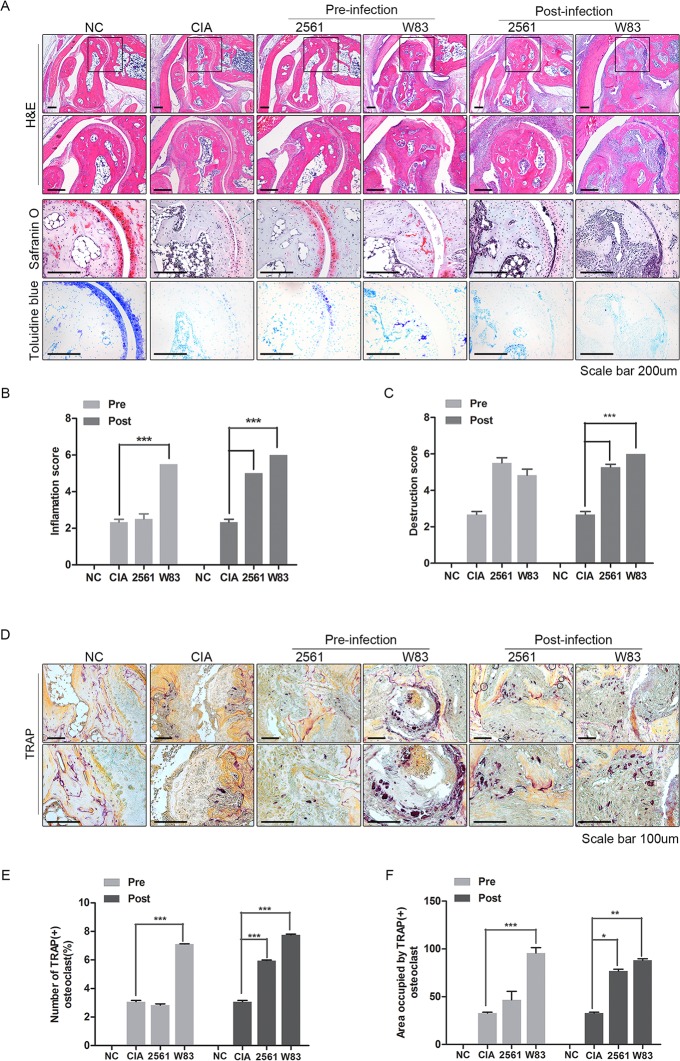
Histological analysis and osteoclastogenesis of arthritic joints from CIA mice treated with P.gingivalis
Since the significance was higher in intraperitoneally infected mice, we further analyzed the joint of mice inoculated by intraperitoneal injections. Infiltration by inflammatory cells, synovial proliferation, cartilage thinning, and joint destruction were observed in all CIA mice. However, P.gingivalis infection aggravated the pathology associated with CIA. Histology revealed that inflammatory cell infiltration and joint destruction in infected CIA mice was more severe than in CIA mice (Fig 2A). Safranin O and toluidine blue staining revealed cartilage thinning and erosion in the joints of all CIA mice. The erosive changes were more severe in infected CIA mice than in non-infected CIA mice. Using a semiquantitative scoring system, histological evaluation was performed. Inflammation (cell exudate or severe infiltration, synovial hyperplasia) and joint destruction (necrosis, erosion and pannus formation) appeared more severe in both the Pre-infected and Post-infected groups compared with non-infected CIA mice (Fig 2B and 2C). While, 2561 failed to affect the symptoms in the Pre-infected group (Fig 2B). The inflammation score was significantly higher in both the Pre-infected and Post-infected groups than in the non-infected CIA group. The destruction score tended to increase in 2561 and W83 Pre-infection groups compared with non-infected CIA group, but did not reach statistical significance. However, the destruction score did increase in the 2561 and W83 Post-infected groups (Fig 2C). Next, we examined the osteoclastogenic activity in each group using tartrate-resistant acid phosphatase (TRAP) staining. Osteoclastogenesis was observed in inflammatory synovium of all CIA mice except for NC mice (Fig 2D). The joint tissues of Pre-infected group and Post-infected group harbored more TRAP-positive cells than those of non-infected CIA mice. TRAP-positive osteoclasts with multinucleated cells were counted using an automated system for quantitative image analysis. TRAP-positive cells were increased in W83 inoculated CIA mice in the Pre-infected group (Fig 2E). Yet, in the Post-infected group, both stains increased the population of osteoclasts. The surface area of stained regions showed similar results (Fig 2F). In conclusion, we confirmed that W83 aggravate the arthritic symptoms in the Pre-infected group, however, both W83 and 2561 provoked arthritis in CIA mice. Also, P.gingivalis infection after immunization seemed to provoke the arthritis symptoms more efficiently.
Fig 2. Histological analysis and osteoclastogenesis of arthritic joints from CIA mice infected with P.gingivalis.
(A) Histological analysis of joints in the hind paws of animals from each group. Tissue sections were stained with hematoxylin and eosin, safranin O, and toluidine blue. Scale bars: 200 μm. (B) Inflammation scores and (C) joint destruction scores were calculated. Histological scores were evaluated by three independent observers in a blinded manner and expressed as the mean ± SEM (*p<0.05, **p<0.01, and ***p<0.001). (D) Osteoclastogenesis in synovial tissues was determined by staining with tartrate-resistant acid phosphatase (TRAP). Scale bar: 200 μm. (E) Area occupied by TRAP-positive osteoclasts. (F) TRAP-positive osteoclasts with multinucleated cells were counted using an automated system for quantitative image analysis. Data represent the mean of three independent counts (± SEM). *P < 0.05.
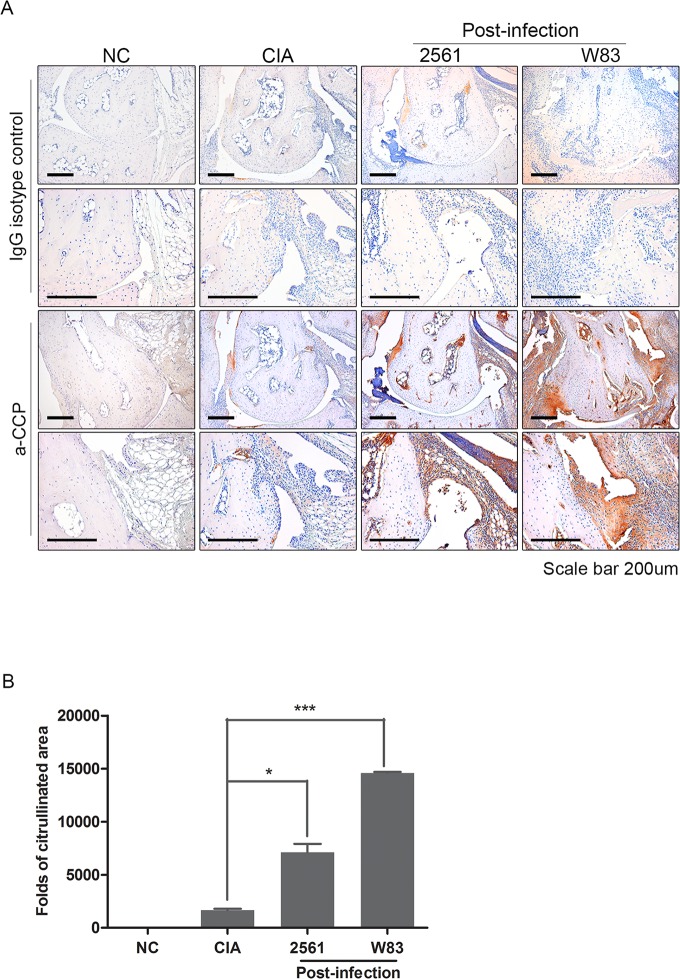
Citrullinated peptides are more common in CIA mice infected with P.gingivalis
To feature the different effect of W83 and 2651 in arthritis, we further examined the Post-infected mice joint which seemed to be more affected by bacterial inoculation. By Immunohistochemical staining with anti-CCP antibody, we confirmed that citrullinated proteins were more extensively distributed in the joints of CIA mice infected with P.gingivalis than in non-infected CIA group (Fig 3A). Sections from the Pre-infected mice also stained positive for anti-CCP, but the intensity was much lower than that in the Post-infection group (data not shown). Automated image analysis was used to calculate the area of citrullination in W83 and 2561 Post-infection groups. The area of citrullination in Post-infection groups was significantly larger than that in non-infected CIA mice (Fig 3B). The staining results were more intense in mice inoculated with W83.
Fig 3. Expression of citrullinated peptides in the arthritic joints of mice with collagen-induced arthritis (CIA).
An anti-citrullinated peptide antibody was produced by a hybridoma cell line derived by fusing myeloma cells with B cells from mice immunized with cyclic citrullinated peptide. (A) Immunohistochemical staining to confirm the presence of citrullinated peptides in the joints of normal control (NC) mice, non-infected CIA mice, and Post-infected CIA mice (n = 5 per group). The bottom row shows magnifications of the boxed areas. Scale bars: 200 μm. (B) Citrullinated regions were examined by quantitative image analysis. The fold increase in the size of the citrullinated area was then calculated relative to the same area in NC mice. Data represent the mean of three independent experiments (± standard error of the mean). *** P <0.001.
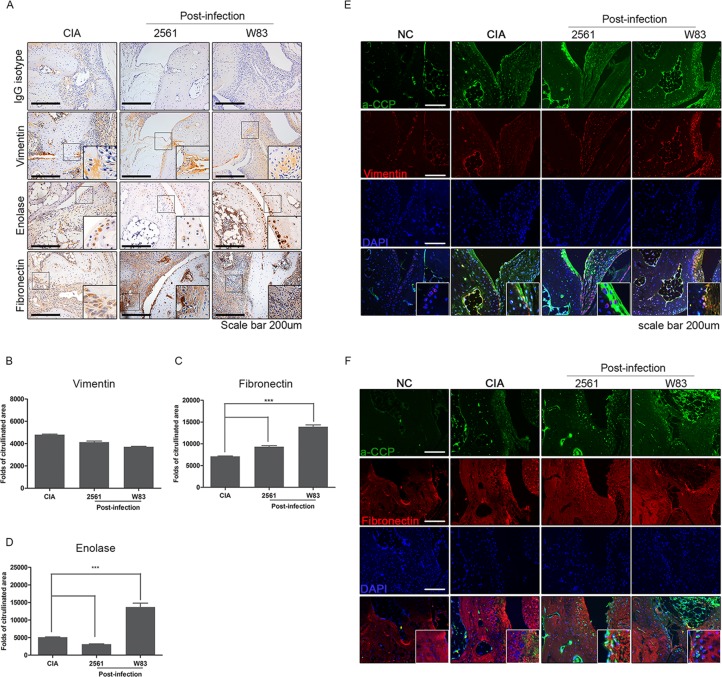
Proteins targeted for citrullination are highly expressed in CIA mice infected with P.gingivalis
Expression of proteins (i.e. vimentin, enolase, and fibronectin) that are prone to citrullination in the joint tissue was examined. The expression of these proteins in the arthritic synovium of Post-infected groups was higher than that in non-infected CIA mice (Fig 4A). The area of these proteins in Post-infection groups was significantly larger than that in non-infected CIA mice. Vimentin, however, did not show any significant difference (Fig 4B). Fibronectin expression was increased in infected mice, especially with W83 (Fig 4C). Enolase was not increased in mice inoculated with 2561, yet, it was significantly increased in W83 infected mice (Fig 4D).
Fig 4. Citrullinated vimentin and fibronectin in synovial tissues of mice with collagen-induced arthritis (CIA) post-infected with P.gingivalis.
(A) Immunohistochemical staining for vimentin, enolase, and fibronectin which are known to be prone to citrullination in the arthritic joints of CIA mice Post-infected with P.gingivalis (n = 3). The number of (B) vimentin-, (C) fibronectin-, and (D) enolase- positive cells was counted. The co-localization of citrulline with (E) vimentin and (F) fibronectin was confirmed. Data represent the mean ± SEM of three independent counts. ***p<0.001.
To confirm if these proteins were citrullinated, we detected the co-localization of citrulline and vimentin or fibronectin. Co-localization of citrulline peptides and vimentin were increased in all CIA induced mice (Fig 4E). The expression seemed to be highest in CIA mice infected with W83. This result was also shown in the case of fibronectin as well (Fig 4F). As a result, we have confirmed increased enolase, and fibronectin expression in the knee joint, and the increased co-localization with citrulline in infected mice.
Discussion
The present study showed that P.gingivalis infection aggravated arthritis and increased citrullination in RA mouse model. Infection with P.gingivalis increased synovial inflammation in CIA mice, which is consistent with the findings of previous studies [44–46]. However, we found it interesting that citrullination of synovial peptides in CIA mice infected with P.gingivalis was higher than that in non-infected CIA mice. In this study, we demonstrated that P.gingivalis affects citrullination in the joint synovium in vivo.
Epidemiological studies consistently report an association between periodontal disease and RA; thus many researchers have attempted to identify a pathophysiological connection between the two diseases [9,25–27,47–49]. P.gingivalis, the causative agent of periodontitis, is an eligible candidate because it citrullinates peptides via bacterial PPAD. Citrullination is a critical process in RA pathogenesis because citrullinated peptides induce pro-inflammatory responses [50,51] and autoantibodies against citrullinated antigens increase osteoclastogenesis in RA [52]. Murine models of RA also exhibit citrullination of synovial proteins [53].
It is unclear how P.gingivalis promotes citrullination in the synovium; however, evidence suggests that the bacterium directly citrullinates the host proteins in RA. P.gingivalis DNA has been detected in the synovial fluid and tissues of patients with RA [54]. Moreover, PPAD targets human proteins in vitro, implying that P.gingivalis generates citrullinated antigens in RA patients. Marsez et al. reported that PPAD plays a critical role in the development and progression of CIA [45]. A recent study suggests that P.gingivalis induces autoimmune experimental arthritis via a mechanism that is independent of citrullination (e.g., induction of a Th17 response via toll-like receptor-2 and interleukin-1 signaling) [3]; thus further research is needed to clarify the arthritogenic role of P.gingivalis.
In this study, we inoculated CIA mice with two strains of P.gingivalis (i.e. 2561 and W83). W83 is afimbriated strain and 2561 is a fimbriated strain of P.gingivalis. The bacteria were delivered through two route; orally or intraperitoneally. Several studies showed significant results through oral infection of P.gingivalis [46,55–58]. Yet, results of intraperitoneally inoculated CIA mice showed more significant difference, especially between pre- and post- infection groups in our study (Fig 1B and 1C). We confirmed that W83 significantly provoked arthritis, yet, 2561 showed no difference compared to CIA control when inoculated before immunization (Fig 1B). When P.gingivalis was delivered after immunization, both strains showed increase arthritis score than that of CIA controls (Fig 1C). Fimbriae are reported to play a critical role in the adherence of P.gingivalis and in triggering the immune responses in the host [59]. The bond between the bacteria and the cell surface are usually mediated by non-specific forces such as covalent bones, Van der Waals forces, based on pH or electrostatics [60–62]. Attachment of fimbriae can be influenced by environmental factors such as pH, salinity, hydrophobicity and charge [63]. Through our observation, we suggest that the changed immune environment in CIA mice before or after immunization, might affect the attachment and pathogenesis of fimbriated strains of P.gingivalis.
Through our results, we confirmed increased expression of proteins prone to citrullination, and citrullinated peptides in these proteins. While the levels of vimentin was not changed, the expression of fibronectin and enolase was increased in W83 infected CIA mice (Fig 4B–4D). 2561 only affected the expression of fibronectin (Fig 4C). The increased citrullination of enolase by P.gingivalis agreed with the previous study reported by Wegner et al [22]. Increased expression of enolase is reported to be related to the progression of various diseases such as neuroblastoma and lung cancer [64–70]. Fibronectin is also reported as an agent associated with disease severity of both RA and periodontitis. Ghosh and colleagues reported that a proapoptotic fibronectin matrix increased the levels of nitric oxide and inducible nitric oxide synthase dose in the inflamed sites caused by bacteria [71]. This might suggest a clue to explain the increased expression of enolase and fibronectin with the provoked arthritic symptoms in infected CIA mice. Also this report can suggest a new connection between P.gingivalis, synovial proteins (i.e. enolase and fibronectin, and RA.
Conclusions
The purpose of this experiment is to investigate the relationship between the severity of arthritis and the route or time point of infection with two strains of P.gingivalis. PAD expressed by P.gingivalis aggravated the disease symptoms in experimental arthritis model.
W83 showed more severe arthritis symptoms in Pre-treatment group compared to 2561 bacteria. However, in Post-treatment group, both of bacteria aggravated arthritis.
Bacterium infection in CIA mice increased several synovial protein (i.e. enolase and fibronectin) expression in the knee joint. Also, citrullinated forms of these proteins were increased in infected mice compared to that of CIA control mice.
Here, we showed that infection of CIA mice with the periodontal pathogen. P.gingivalis induced production of citrullinated antigens and aggravated both the clinical and histological signs of arthritis. Therefore, P.gingivalis-mediated citrullination may be the link between periodontal disease and RA.
Supporting information
(A, B) Arthritis severity score of NC mice (n = 5), CIA mice (n = 5), and CIA mice infected with P.gingivalis (n = 5). Pre and Post P.gingivalis were oral infected twice per week throughout the experimental period. The score for each paw ranged from 0 (no swelling) to 4 (erythema and severe swelling encompassing the ankle and foot); the scores for all four paws were summed to generate a representative arthritis score. (*, P<0.01; **, P<0.005; ***, P<0.001) (C) Representative image of jaw bone from mice in each group. (D) Methylene blue staining image of the jaw bone to examine the CEJ-ABC distance. (E) The distance was measured.
(TIF)
The arthritic index was compared between normal, CIA control mice and mice infected with W83 or Fusobacterium nucleatum (F.n).
(TIF)
Experiments were performed according to the ARRIVE guidelines.
(PDF)
Acknowledgments
This work was supported by a grant from the Korea Healthcare Technology R&D project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (HI14C3417), and by a grant from the Advanced Production Technology Development Program, Ministry for Food, Agriculture, Forestry and Fisheries (312037–05).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant from the Korea Healthcare Technology R&D project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (HI14C3417), and by a grant from the Advanced Production Technology Development Program, Ministry for Food, Agriculture, Forestry and Fisheries (312037-05).
References
- 1.McInnes IB, Schett G (2011) The Pathogenesis of Rheumatoid Arthritis. New England Journal of Medicine 365: 2205–2219. doi: 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- 2.Szodoray P, Szabó Z, Kapitány A, Gyetvai Á, Lakos G, Szántó S, et al. (2010) Anti-citrullinated protein/peptide autoantibodies in association with genetic and environmental factors as indicators of disease outcome in rheumatoid arthritis. Autoimmunity Reviews 9: 140–143. doi: 10.1016/j.autrev.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 3.de Aquino SG, Abdollahi-Roodsaz S, Koenders MI, van de Loo FAJ, Pruijn GJM, Marijnissen RJ, et al. (2014) Periodontal Pathogens Directly Promote Autoimmune Experimental Arthritis by Inducing a TLR2- and IL-1–Driven Th17 Response. The Journal of Immunology 192: 4103–4111. doi: 10.4049/jimmunol.1301970 [DOI] [PubMed] [Google Scholar]
- 4.Lundberg K, Kinloch A, Fisher BA, Wegner N, Wait R, Charles P, et al. (2008) Antibodies to citrullinated α-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis & Rheumatism 58: 3009–3019. [DOI] [PubMed] [Google Scholar]
- 5.Imboden JB (2009) The Immunopathogenesis of Rheumatoid Arthritis. Annual Review of Pathology: Mechanisms of Disease 4: 417–434. [DOI] [PubMed] [Google Scholar]
- 6.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ (2003) PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays 25: 1106–1118. doi: 10.1002/bies.10357 [DOI] [PubMed] [Google Scholar]
- 7.Chang X, Han J, Pang L, Zhao Y, Yang Y, Shen Z (2009) Increased PADI4 expression in blood and tissues of patients with malignant tumors. BMC Cancer 9: 40 doi: 10.1186/1471-2407-9-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu JJ, Ruddy MJ, Conti HR, Boonanantanasarn K, Gaffen SL (2008) The interleukin-17 receptor plays a gender-dependent role in host protection against Porphyromonas gingivalis-induced periodontal bone loss. Infect Immun 76: 4206–4213. doi: 10.1128/IAI.01209-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412 doi: 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Li Z, Chen D, Lu X, Feng X, Wright EC, et al. (2008) Inactivation of microbial arginine deiminases by L-canavanine. J Am Chem Soc 130: 1918–1931. doi: 10.1021/ja0760877 [DOI] [PubMed] [Google Scholar]
- 11.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2012) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage 20: 256–260. doi: 10.1016/j.joca.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 12.Kilkenny C, Browne WJ, Cuthi I, Emerson M, Altman DG (2012) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Vet Clin Pathol 41: 27–31. doi: 10.1111/j.1939-165X.2012.00418.x [DOI] [PubMed] [Google Scholar]
- 13.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 1: 94–99. doi: 10.4103/0976-500X.72351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada R, Suzuki A, Chang X, Yamamoto K (2005) Citrullinated proteins in rheumatoid arthritis. Front Biosci 10: 54–64. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y, Lee J (2015) Development of synthetic anti-cyclic citrullinated peptide antibody and its arthritogenic role. 4: e51 doi: 10.1038/cti.2015.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wegner N, Lundberg K, Kinloch A, Fisher B, Malmström V, Feldmann M, et al. (2010) Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunological Reviews 233: 34–54. doi: 10.1111/j.0105-2896.2009.00850.x [DOI] [PubMed] [Google Scholar]
- 17.Jansen LMA, van Schaardenburg D, van der Horst-Bruinsma I, van der Stadt RJ, de Koning MHMT, Dijkmans BAC(2003) The predictive value of anti-cyclic citrullinated peptide antibodies in early arthritis. The Journal of Rheumatology 30: 1691–1695. [PubMed] [Google Scholar]
- 18.Fox DA (2015) Citrullination: A Specific Target for the Autoimmune Response in Rheumatoid Arthritis. The Journal of Immunology 195: 5–7. doi: 10.4049/jimmunol.1501021 [DOI] [PubMed] [Google Scholar]
- 19.Moscarello MA, Mastronardi FG, Wood DD (2007) The role of citrullinated proteins suggests a novel mechanism in the pathogenesis of multiple sclerosis. Neurochem Res 32: 251–256. doi: 10.1007/s11064-006-9144-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foulquier C, Sebbag M, Clavel C, Chapuy-Regaud S, Al Badine R, Mechin MC, et al. (2007) Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum 56: 3541–3553. doi: 10.1002/art.22983 [DOI] [PubMed] [Google Scholar]
- 21.Chang X, Zhao Y, Sun S, Zhang Y, Zhu Y (2009) The expression of PADI4 in synovium of rheumatoid arthritis. Rheumatol Int 29: 1411–1416. doi: 10.1007/s00296-009-0870-2 [DOI] [PubMed] [Google Scholar]
- 22.Wegner N, Wait R, Sroka A, Eick S, Nguyen K-A, Lundberg K, et al. (2010) Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: Implications for autoimmunity in rheumatoid arthritis. Arthritis and rheumatism 62: 2662–2672. doi: 10.1002/art.27552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGraw WT (1999) Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infect Immun 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niederman R, Brunkhorst B, Smith S, Weinreb RN, Ryder MI (1990) Ammonia as a potential mediator of adult human periodontal infection: inhibition of neutrophil function. Arch Oral Biol 35. [DOI] [PubMed] [Google Scholar]
- 25.Mikuls TR, Payne JB, Yu F, Thiele GM, Reynolds RJ, Cannon GW, et al. (2014) Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol 66: 1090–1100. doi: 10.1002/art.38348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koziel J, Mydel P, Potempa J (2014) The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep 16: 408 doi: 10.1007/s11926-014-0408-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada M, Kobayashi T, Ito S, Yokoyama T, Abe A, Murasawa A, et al. (2013) Periodontal treatment decreases levels of antibodies to Porphyromonas gingivalis and citrulline in patients with rheumatoid arthritis and periodontitis. J Periodontol 84: e74–84. doi: 10.1902/jop.2013.130079 [DOI] [PubMed] [Google Scholar]
- 28.Rutger Persson G (2012) Rheumatoid arthritis and periodontitis–inflammatory and infectious connections. Review of the literature. Journal of Oral Microbiology 4: doi: 10.3402/jom.v3404i3400.11829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pischon N, Pischon T, Kröger J, Gülmez E, Kleber BM, Bernimoulin JP, et al. (2008) Association Among Rheumatoid Arthritis, Oral Hygiene, and Periodontitis. Journal of Periodontology 79: 979–986. doi: 10.1902/jop.2008.070501 [DOI] [PubMed] [Google Scholar]
- 30.Abdullah SN, Farmer EA, Spargo L, Logan R, Gully N (2013) Porphyromonas gingivalis peptidylarginine deiminase substrate specificity. Anaerobe 23: 102–108. doi: 10.1016/j.anaerobe.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 31.Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K, et al. (2010) Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum 62: 2662–2672. doi: 10.1002/art.27552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nachat R, Méchin M-C, Takahara H, Chavanas S, Charveron M, Serre G, et al. (2005) Peptidylarginine Deiminase Isoforms 1–3 Are Expressed in the Epidermis and Involved in the Deimination of K1 and Filaggrin. Journal of Investigative Dermatology 124: 384–393. doi: 10.1111/j.0022-202X.2004.23568.x [DOI] [PubMed] [Google Scholar]
- 33.Mangat P, Wegner N, Venables PJ, Potempa J (2010) Bacterial and human peptidylarginine deiminases: targets for inhibiting the autoimmune response in rheumatoid arthritis? Arthritis Research & Therapy 12: 209–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vossenaar ER, Radstake TR, Heijden Avander, van Mansum MA, Dieteren C, de Rooij DJ, et al. (2004) Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinloch A, Lundberg K, Wait R, Wegner N, Lim NH, Zendman AJ, et al. (2008) Synovial fluid is a site of citrullination of autoantigens in inflammatory arthritis. Arthritis Rheum 58. [DOI] [PubMed] [Google Scholar]
- 36.Wruck CJ, Fragoulis A, Gurzynski A, Brandenburg L-O, Kan YW, Chan K, et al. (2011) Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Annals of the Rheumatic Diseases 70: 844 doi: 10.1136/ard.2010.132720 [DOI] [PubMed] [Google Scholar]
- 37.Hückel M, Schurigt U, Wagner AH, Stöckigt R, Petrow PK, Thoss K, et al. (2005) Attenuation of murine antigen-induced arthritis by treatment with a decoy oligodeoxynucleotide inhibiting signal transducer and activator of transcription-1 (STAT-1). Arthritis Research & Therapy 8: R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebina K, Oshima K, Matsuda M, Fukuhara A, Maeda K, Kihara S, et al. (2009) Adenovirus-mediated gene transfer of adiponectin reduces the severity of collagen-induced arthritis in mice. Biochemical and Biophysical Research Communications 378: 186–191. doi: 10.1016/j.bbrc.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 39.Rim YA, Yi H, Kim Y, Park N, Jung H, Kim J, et al. (2014) Self in vivo production of a synthetic biological drug CTLA4Ig using a minicircle vector. Scientific Reports 4: 6935 doi: 10.1038/srep06935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park N, Rim YA, Jung H, Kim J, Yi H, Kim Y, et al. (2017) Etanercept-Synthesising Mesenchymal Stem Cells Efficiently Ameliorate Collagen-Induced Arthritis. Scientific Reports 7: 39593 doi: 10.1038/srep39593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y, Lee J, Jung H, Yi H, Rim YA, Jung SM, et al. (2015) Development of synthetic anti-cyclic citrullinated peptide antibody and its arthritogenic role. Clin Transl Immunology 4: e51 doi: 10.1038/cti.2015.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandal I, Karydis A, Luo J, Prislovsky A, Whittington KB, Rosloniec EF, et al. (2016) Bone loss and aggravated autoimmune arthritis in HLA-DRβ1-bearing humanized mice following oral challenge with Porphyromonas gingivalis. Arthritis Research & Therapy 18: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brand DD, Latham KA, Rosloniec EF (2007) Collagen-induced arthritis. Nat Protocols 2: 1269–1275. doi: 10.1038/nprot.2007.173 [DOI] [PubMed] [Google Scholar]
- 44.Marchesan JT, Gerow EA, Schaff R, Taut AD, Shin S-Y, Sugai J, et al. (2013) Porphyromonas gingivalis oral infection exacerbates the development and severity of collagen-induced arthritis. Arthritis Research & Therapy 15: R186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maresz KJ, Hellvard A, Sroka A, Adamowicz K, Bielecka E, Koziel J, et al. (2013) Porphyromonas gingivalis Facilitates the Development and Progression of Destructive Arthritis through Its Unique Bacterial Peptidylarginine Deiminase (PAD). PLOS Pathogens 9: e1003627 doi: 10.1371/journal.ppat.1003627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marchesan JT, Gerow EA, Schaff R, Taut AD, Shin SY, Sugai J, et al. (2013) Porphyromonas gingivalis oral infection exacerbates the development and severity of collagen-induced arthritis. Arthritis Res Ther 15: R186 doi: 10.1186/ar4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Araujo VM, Melo IM, Lima V (2015) Relationship between Periodontitis and Rheumatoid Arthritis: Review of the Literature. Mediators Inflamm 2015: 259074 doi: 10.1155/2015/259074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Detert J, Pischon N, Burmester GR, Buttgereit F (2010) The association between rheumatoid arthritis and periodontal disease. Arthritis Res Ther 12: 218 doi: 10.1186/ar3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bingham CO, 3rd, Moni M (2013) Periodontal disease and rheumatoid arthritis: the evidence accumulates for complex pathobiologic interactions. Curr Opin Rheumatol 25: 345–353. doi: 10.1097/BOR.0b013e32835fb8ec [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez-Pernaute O, Filkova M, Gabucio A, Klein M, Maciejewska-Rodrigues H, Ospelt C, et al. (2013) Citrullination enhances the pro-inflammatory response to fibrin in rheumatoid arthritis synovial fibroblasts. Annals of the Rheumatic Diseases 72: 1400–1406. doi: 10.1136/annrheumdis-2012-201906 [DOI] [PubMed] [Google Scholar]
- 51.Ling S, Cline EN, Haug TS, Fox DA, Holoshitz J (2013) Citrullinated calreticulin potentiates rheumatoid arthritis shared epitope signaling. Arthritis and rheumatism 65: 618–626. doi: 10.1002/art.37814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. The Journal of Clinical Investigation 122: 1791–1802. doi: 10.1172/JCI60975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vossenaar ER, Nijenhuis S, Helsen MMA, van der Heijden A, Senshu T, van den Berg WB, et al. (2003) Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis & Rheumatism 48: 2489–2500. [DOI] [PubMed] [Google Scholar]
- 54.Totaro MC, Cattani P, Ria F, Tolusso B, Gremese E, Fedele AL, et al. (2013) Porphyromonas gingivalis and the pathogenesis of rheumatoid arthritis: analysis of various compartments including the synovial tissue. Arthritis Research & Therapy 15: R66–R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rivera MF, Lee JY, Aneja M, Goswami V, Liu L, Velsko IM, et al. (2013) Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoE(null) mice. PLoS One 8: e57178 doi: 10.1371/journal.pone.0057178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kesavalu L, Sathishkumar S, Bakthavatchalu V, Matthews C, Dawson D, Steffen M, et al. (2007) Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect Immun 75: 1704–1712. doi: 10.1128/IAI.00733-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Velsko IM, Chukkapalli SS, Rivera MF, Lee JY, Chen H, Zheng D, et al. (2014) Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One 9: e97811 doi: 10.1371/journal.pone.0097811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato K, Takahashi N, Kato T, Matsuda Y, Yokoji M, Yamada M, et al. (2017) Aggravation of collagen-induced arthritis by orally administered Porphyromonas gingivalis through modulation of the gut microbiota and gut immune system. Sci Rep 7: 6955 doi: 10.1038/s41598-017-07196-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma A, Nagata H, Hamada N, Sojar HT, Hruby DE, Kuramitsu HK, et al. (1996) Expression of functional Porphyromonas gingivalis fimbrillin polypeptide domains on the surface of Streptococcus gordonii. Appl Environ Microbiol 62: 3933–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dunne WM Jr. (2002) Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev 15: 155–166. doi: 10.1128/CMR.15.2.155-166.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Oss CJ (2003) Long-range and short-range mechanisms of hydrophobic attraction and hydrophilic repulsion in specific and aspecific interactions. J Mol Recognit 16: 177–190. doi: 10.1002/jmr.618 [DOI] [PubMed] [Google Scholar]
- 62.Berne C, Ducret A, Hardy GG, Brun YV (2015) Adhesins Involved in Attachment to Abiotic Surfaces by Gram-Negative Bacteria. Microbiol Spectr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geng J, Henry N (2011) Short time-scale bacterial adhesion dynamics. Adv Exp Med Biol 715: 315–331. doi: 10.1007/978-94-007-0940-9_20 [DOI] [PubMed] [Google Scholar]
- 64.Chang X, Yamada R, Suzuki A, Kochi Y, Sawada T, Yamamoto K (2005) Citrullination of fibronectin in rheumatoid arthritis synovial tissue. Rheumatology 44: 1374–1382. doi: 10.1093/rheumatology/kei023 [DOI] [PubMed] [Google Scholar]
- 65.Voskuyl AE, Emeis JJ, Hazes JM, van Hogezand RA, Biemond I, Breedveld FC (1998) Levels of circulating cellular fibronectin are increased in patients with rheumatoid vasculitis. Clin Exp Rheumatol 16: 429–434. [PubMed] [Google Scholar]
- 66.Diaz-Ramos A, Roig-Borrellas A, Garcia-Melero A, Lopez-Alemany R (2012) alpha-Enolase, a multifunctional protein: its role on pathophysiological situations. J Biomed Biotechnol 2012: 156795 doi: 10.1155/2012/156795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eriksson B, Oberg K, Stridsberg M (2000) Tumor markers in neuroendocrine tumors. Digestion 62 Suppl 1: 33–38. [DOI] [PubMed] [Google Scholar]
- 68.Niklinski J, Furman M (1995) Clinical tumour markers in lung cancer. Eur J Cancer Prev 4: 129–138. [DOI] [PubMed] [Google Scholar]
- 69.Cooper EH (1994) Neuron-specific enolase. Int J Biol Markers 9: 205–210. [DOI] [PubMed] [Google Scholar]
- 70.Ledermann JA (1994) Serum neurone-specific enolase and other neuroendocrine markers in lung cancer. Eur J Cancer 30a: 574–576. [DOI] [PubMed] [Google Scholar]
- 71.Ghosh A, Park JY, Fenno C, Kapila YL (2008) Porphyromonas gingivalis, gamma interferon, and a proapoptotic fibronectin matrix form a synergistic trio that induces c-Jun N-terminal kinase 1-mediated nitric oxide generation and cell death. Infect Immun 76: 5514–5523. doi: 10.1128/IAI.00625-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A, B) Arthritis severity score of NC mice (n = 5), CIA mice (n = 5), and CIA mice infected with P.gingivalis (n = 5). Pre and Post P.gingivalis were oral infected twice per week throughout the experimental period. The score for each paw ranged from 0 (no swelling) to 4 (erythema and severe swelling encompassing the ankle and foot); the scores for all four paws were summed to generate a representative arthritis score. (*, P<0.01; **, P<0.005; ***, P<0.001) (C) Representative image of jaw bone from mice in each group. (D) Methylene blue staining image of the jaw bone to examine the CEJ-ABC distance. (E) The distance was measured.
(TIF)
The arthritic index was compared between normal, CIA control mice and mice infected with W83 or Fusobacterium nucleatum (F.n).
(TIF)
Experiments were performed according to the ARRIVE guidelines.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.