Abstract
Background
The consumption of trans fatty acids (TFAs) is associated with an increased risk of cardiovascular disease, and reducing their consumption is a major public health objective. Food intake studies have provided estimates for TFA concentrations in the US population; however, there is a need for data on TFA blood concentrations in the population.
Objective
The objective of this study was to determine plasma TFA concentrations in a nationally representative group of fasted adults in the US population in NHANES samples from 1999–2000 and 2009–2010.
Design
Four major TFAs [palmitelaidic acid (C16:1n–7t), trans vaccenic acid (C18:1n–7t), elaidic acid (C18:1n–9t), and linoelaidic acid (C18:2n–6t,9t)] were measured in plasma in 1613 subjects from NHANES 1999–2000 and 2462 subjects from NHANES 2009–2010 by gas chromatography–mass spectrometry. Geometric means and distribution percentiles were calculated for each TFA and their sum by age, sex, and race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American), and covariate-adjusted geometric means were computed by using a model that included these demographic and other dietary factors, as well as survey year and any significant interaction terms.
Results
These nationally representative data for the adult US population show that TFA concentrations were 54% lower in NHANES 2009–2010 than in NHANES 1999–2000. Covariate-adjusted geometric means for the sum of the 4 TFAs were 81.4 μmol/L (95% CI: 77.3, 85.6 μmol/L) and 37.8 μmol/L (95% CI: 36.4, 39.4 μmol/L) in NHANES 1999–2000 and 2009–2010, respectively. Even with the large decline in TFA concentrations, differences between demographic subgroups were comparable in the 2 surveys.
Conclusion
The results indicate an overall reduction in TFA concentrations in the US population and provide a valuable baseline to evaluate the impact of the recent regulation categorizing TFAs as food additives.
Keywords: trans-fatty acids, NHANES, gas chromatography, mass spectrometry, cardiovascular disease
INTRODUCTION
trans Fatty acids (TFAs)7 are unsaturated fatty acids that contain ≥1 double bond in the trans configuration. The consumption of TFAs is associated with an increased risk of cardiovascular and other chronic diseases (1). Reducing the risk for coronary artery diseases by reducing the consumption of TFAs is a major public health objective (2–6). In 2003, the Food and Drug Administration (FDA) amended its regulations on nutrition labeling, requiring TFAs to be declared on the nutrition label of conventional foods and dietary supplements (7). In 2015, the FDA determined that TFAs are no longer considered a “generally recognized as safe” substance. This implies that any TFAs intentionally added to food are subject to approval by the FDA (8).
Studies that investigated the TFA contents of foods and estimated TFA intake in the US population reported a decrease in TFA intake over the past decade (9). However, these estimates did not provide information on actual changes in TFA blood concentrations in the general population. An initial study that assessed TFA blood concentrations in a subset of fasting non-Hispanic white (NHW) adults participating in the NHANES reported an average 58% decrease in TFAs between 2000 and 2009 (10). Because these data were limited to a specific population subgroup and the sample size was insufficient for detailed analysis by age and sex, additional data were needed to assess TFA changes in the US population.
The aim of this study was to determine nationally representative estimates for selected plasma TFA concentrations in the adult (aged ≥20 y) US population by analyzing NHANES data from the 1999–2000 and 2009–2010 survey periods and to create a baseline for assessing the impact of the recent FDA regulation.
METHODS
Participants
The NHANES is a cross-sectional study that uses a stratified, multistage, probability cluster sample designed to represent the US population on the basis of age, sex, and race/ethnicity (11). Since 1999, the NHANES has been a continuous survey with data being released every 2 y, representing a survey cycle. All of the participants in the survey gave written informed consent. Morning fasting (≥8 h since last meal) blood samples from persons aged ≥20 y who participated in the 1999–2000 and 2009–2010 survey cycles were used in this study. Samples from 1678 participants in the 1999–2000 cycle and from 2578 participants in the 2009–2010 cycle were obtained. The NHANES protocol was reviewed and approved by the National Center for Health Statistics Research Ethics Review Board.
Biomarker measurements and self-reported variables
Four TFAs were analyzed in plasma by using gas chromatography coupled with mass spectrometry: palmitelaidic acid (C16:1n–7t), trans vaccenic acid (C18:1n–7t), elaidic acid (C18:1n–9t), and linoelaidic acid (C18:2n–6t,9t). In brief, 100 μL plasma plus 100 μL internal standard solution containing stable isotope–labeled TFAs (11 μmol/L 13C5-palmitelaidic acid, 30 μmol/L 13C5-trans-vaccenic acid, 30 μmol/L 13C5-elaidic acid, and 2.0 μmol/L 13C5-linoelaidic acid) were successively hydrolyzed with 2 mL 10% (vol:vol) 6 N HCl in acetonitrile and 2 mL 10% (vol:vol) 10 N NaOH in methanol at 104°C for 45 min each. Free fatty acids were extracted from the hydrolysis solution with hexane and derivatized as described by Lagerstedt et al. (12). The derivatized fatty acids were analyzed by gas chromatography coupled with mass spectrometry (Agilent Technologies). The mass spectrometer was operated in negative chemical ionization mode (reagent gas: methane). Chromatographic separation was carried out over 100 min with an Agilent Select FAME column (200 m × 250 μm × 0.25 μm) and with hydrogen as the carrier gas at 3 mL/min. A sample volume of 1 μL was injected (injector spilt mode: 20:1 split ratio). The fatty acids were separated by using a temperature program starting at 130°C and ending at 260°C. The within-day and between-day precision expressed as percentage CV (%CV) determined with 3 different levels of plasma pools ranged from 2–9% to 8–9% for palmitelaidic acid, 3–8% to 7–9% for trans vaccenic acid, 1–7% to 6–10% for elaidic acid, and 2–11% to 9–15% for linoelaidic acid, respectively. The limits of detection determined by using Taylor’s method (13) were 0.07 μmol/L for palmitelaidic acid, 0.43 μmol/L for trans vaccenic acid, 0.29 μmol/L for elaidic acid, and 0.02 μmol/L for linoelaidic acid, respectively. The mean accuracy for all 4 TFAs was 102% (95% CI: 98%, 107%).
Total cholesterol (TC), HDL cholesterol, and triglycerides were measured by using a Roche Modular P chemistry analyzer (Roche Diagnostics) in a laboratory standardized by the CDC Lipids Standardization Program with a maximum imprecision of 1.5% and 3.2% for TC and HDL cholesterol, respectively. Glycated hemoglobin (HbA1c) concentrations were determined by using HbA1c (Tosoh Automated Glycohemoglobin Analyzer HLC-723G8; standardized by the National Glycohemoglobin Standardization Program) with a maximum imprecision of 1.2% (14). Diabetic status was categorized by using the HbA1c concentrations into nondiabetic (HbA1c ≤5.6%) and prediabetic or diabetic (HbA1c >5.6%) categories. Serum cotinine, a bio-marker for tobacco smoke exposure, was measured by HPLC–tandem mass spectrometry with the use of a method previously described (15).
We categorized BMI (kg/m2) by using the WHO criteria (normal or underweight: BMI ≤25; overweight: 25 < BMI ≤ 30; obese: BMI >30) (16). Average daily alcohol consumption was derived from the alcohol-use questionnaires and was categorized on the basis of the daily number of drinks: 0, >0 to <2, or ≥2 drinks/d for men and 0, >0 to <1, or ≥1 drinks/d for women. Lipid-altering medication use was categorized on the basis of self-reported use or nonuse. Physical activity was categorized according to 2 basic levels (self-reported vigorous activity: ≥10 min/d; self-reported moderate physical activity: <10 min/d) because of substantial changes in questionnaires from NHANES 1999–2000 to 2009–2010. Educational attainment was categorized as ≤12 y of education or >12 y of education for use as a measure of socioeconomic status.
Statistical procedures
Geometric means (GMs) and distribution percentiles were calculated for each TFA and the sum of the 4 TFAs (sumTFAs) by age group, sex, and self-reported race/ethnicity [NHW, non-Hispanic black (NHB), Mexican American (MA)]. Individuals not included in 1 of the 3 main race/ethnicity groups (“other”) were included in the total population estimates, but their estimates are not reported for this group. GMs and percentiles were calculated with SUDAAN version 11.0.1 (Research Triangle Institute). We estimated 95% CIs for GMs on the basis of the Taylor series linearization method (17) and adapted CIs for percentiles from the methods of Korn and Graubard (18) and Woodruff (19).
We calculated covariate-adjusted GMs (CGMs) for selected demographic groups by using least-squares multiple regression adjusted for the categorical variables as defined above—survey (1999–2000 or 2009–2010), sex (male or female), race/ethnicity (NHW, NHB, or MA), education, alcohol use, lipid-altering medication use, diabetic status, BMI, and physical activity—and for the continuous variables cotinine, age, and age squared. To arrive at the final model, any variable that was significant for 1 TFA or sumTFAs was retained in regression models to preserve the interpretation of the statistical properties of β-coefficients, P values, and CIs and to keep the interpretation consistent across the set of TFAs and their sum (20). Interaction terms between the covariates and survey cycle that were significant in ≥1 models were also included to allow separate covariate estimates for each survey cycle. The same model was applied to each TFA and the sumTFAs. Significance in CGM concentrations between cycles or population subgroups was determined by assessing whether the ratio of the CGM concentrations was significantly different from a value of 1. The false discovery rate procedure (21) was used to account for multiple comparisons.
RESULTS
TFA values for all 4 TFAs were obtained for 1613 participants (48% men, 71% NHWs, 10% NHBs, and 6.4% MAs) in the 1999–2000 cycle and for 2462 participants (48% men, 67% NHWs, 11% NHBs, and 8.8% MAs; Table 1) in the 2009–2010 cycle. TFA values for the individual TFAs palmitelaidic acid, trans vaccenic acid, elaidic acid, and linoelaidic acid were obtained for 1678, 1675, 1646, and 1650 participants in the 1999–2000 cycle and for 2574, 2575, 2578, and 2467 individuals in the 2009–2010 cycle, respectively (Supplemental Table 1).
TABLE 1.
Characteristics of fasting adults aged ≥20 y with data for palmitelaidic acid, trans vaccenic acid, elaidic acid, and linoelaidic acid: NHANES 1999–2000 and 2009–20101
| Variable | NHANES 1999–2000 | NHANES 2009–2010 |
|---|---|---|
| Participants with values for all 4 TFAs, n | 1613 | 2462 |
| Weighted proportion of men, % | 48 | 48 |
| Non-Hispanic whites,2 n (%) | 762 (71) | 1165 (67) |
| Non-Hispanic blacks,2 n (%) | 257 (10) | 405 (11) |
| Mexican Americans,2 n (%) | 462 (6.4) | 489 (8.8) |
| Education,3 % | ||
| ≤12 y | 50.3 | 41.2 |
| >12 y | 49.7 | 58.8 |
| Age, y | 42 (31–57)4 | 46 (32–59) |
| BMI, kg/m2 | 26.7 (23.3–30.9) | 27.8 (24.1–32.4) |
| Total cholesterol, mg/dL | 199 (175–228) | 192 (166–221) |
| LDL cholesterol, mg/dL | 123 (101–147) | 114 (92–137) |
| HDL cholesterol, mg/dL | 47 (39–58) | 51 (42–63) |
| Triglycerides, mg/dL | 121 (83–172) | 104 (75–151) |
| HbA1c, % | 5.2 (4.9–5.5) | 5.5 (5.2–5.8) |
| Total kilocalories | 2110 (1528–2734) | 1978 (1492–2646) |
| Healthy Eating Index score | 44.7 (34.6–56.0) | 47.8 (37.7–58.8) |
“Fasting” indicates no meals consumed in the past ≥8 h. HbA1c, glycated hemoglobin; TFA, trans fatty acid.
Values are weighted estimates.
Values are weighted percentages.
Median; IQR in parentheses (all such values).
The GMs of the sumTFAs in the 1999–2000 and 2009–2010 NHANES were 80.9 μmol/L (95% CI: 75.7, 86.5 μmol/L) and 37.4 μmol/L (95% CI: 36.1, 38.8 μmol/L), respectively (Supplemental Table 1); and the 5th and 95th percentiles were 38.2 and 176 μmol/L in 1999–2000 and 17.6 and 84.0 μmol/L in 2009–2010 (Supplemental Table 2). The GMs (95% CIs) for NHWs, NHBs, and MAs were 85.7 μmol/L (78.8, 93.2 μmol/L), 69.5 μmol/L (65.5, 73.7 μmol/L), and 81.1 μmol/L (74.0, 88.8 μmol/L) in the 1999–2000 NHANES and 38.8 μmol/L (37.1, 40.6 μmol/L), 33.3 μmol/L (31.6, 35.1 μmol/L), and 42.7 μmol/L (39.3, 46.4 μmol/L) in the 2009–2010 NHANES (Supplemental Table 1). The 5th and 95th percentiles in the 1999–2000 NHANES ranged between 41.6–185, 33.9–149, and 35.5–188 μmol/L and in the 2009–2010 NHANES between 18.9–88.1, 16.7–66.2, and 20.5–91.9 μmol/L for NHWs, NHBs, and MAs, respectively (Supplemental Table 2).
Among the 4 TFAs measured, trans vaccenic acid was the major TFA, which constituted 46.7% and 48.0% of the sumTFAs in the 1999–2000 NHANES and 2009–2010 NHANES, respectively. Elaidic acid was the second major TFA, which constituted 40.5% and 36.0%, respectively (Supplemental Table 3). The correlation among TFA concentrations, as expressed by the Pearson correlation coefficient, ranged between 0.65 and 0.98 with high-abundant, major TFAs having, in general, higher correlation coefficients than minor, low-abundant linoelaidic acid (Supplemental Table 4).
In both NHANES cycles, BMI, total cholesterol, LDL cholesterol, triglycerides, total kilocalories, and Healthy Eating Index–2010 scores were higher in individuals with sumTFAs in the third tertile of the distribution compared with those in the first tertile, whereas HDL-cholesterol values were highest in the first tertile compared with the third tertile (Supplemental Table 5).
The variability in the sumTFAs among participants, as expressed by the IQR, was 45.7 μmol/L in 1999–2000 and 22.6 μmol/L in 2009–2010 (Supplemental Table 2). In 1999–2000, the IQR was larger in men than in women, whereas it was similar for both groups in 2009–2010. Among age and race/ethnic groups, the IQR patterns were similar in both NHANES cycles. For individual TFAs, in 1999–2000 the IQR was similar for trans vaccenic acid and elaidic acid (21 μmol/L), whereas in 2009–2010 it was lower for elaidic acid (9 μmol/L) than for trans vaccenic acid (12 μmol/L; Supplemental Tables 6–9).
An evaluation of the variables used in the statistical model to derive the CGMs for sumTFAs and individual TFAs listed in Table 2 showed significant main effects (P < 0.05) for race/ethnicity, BMI, and diabetic status, and except for linoelaidic acid, for alcohol consumption and the use of lipid-altering medication (Supplemental Table 10). Significant interactions were observed between survey and age and education and age for sumTFAs and individual TFAs, except for linoelaidic acid. No other significant interactions were detected. No significant association between TFAs and cotinine or between TFAs and diabetic status was observed (data not shown), but diabetic status acted as a confounder and therefore was retained in the models.
TABLE 2.
Covariate-adjusted geometric means (95% CIs) of plasma TFA concentrations (μmol/L) in fasting adults aged ≥20 y: NHANES 1999–2000 and 2009–20101
| Palmitelaidic acid, n | trans Vaccenic acid, n | Elaidic acid, n | Linoelaidic acid, n | SumTFAs, n | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| 1999–2000 | 2009–2010 | 1999–2000 | 2009–2010 | 1999–2000 | 2009–2010 | 1999–2000 | 2009–2010 | 1999–2000 | 2009–2010 | |
| All | 6.68 (6.42, 6.95) [1522] | 3.81 (3.67, 3.95) [2263] | 37.9 (36.1, 39.8) [1519] | 18.2 (17.5, 19.0) [2265] | 33.4 (31.4, 35.6) [1493] | 13.5 (13.0, 14.0) [2267] | 2.90 (2.73, 3.09) [1498] | 1.61 (1.55, 1.67) [2171] | 81.4 (77.3, 85.6) [1463] | 37.8 (36.4, 39.4) [2167] |
| Sex | ||||||||||
| Male | 6.62 (6.27, 6.99) [732] | 3.78 (3.59, 3.98) [1079] | 38.5 (36.2, 41.0) [729] | 18.5 (17.5, 19.5) [1081] | 32.6 (30.2, 35.1) [724] | 13.1 (12.5, 13.8) [1082] | 2.82 (2.63, 3.04) [718] | 1.57 (1.50, 1.63) [1034] | 80.9 (75.9, 86.1) [706] | 37.6 (35.7, 39.6) [1032] |
| Female | 6.73 (6.49, 6.98) [790] | 3.84 (3.72, 3.97) [1184] | 37.3 (35.6, 39.0) [790] | 17.9 (17.2, 18.6) [1184] | 34.3 (32.3, 36.4) [769] | 13.8 (13.3, 14.3) [1185] | 2.98 (2.81, 3.16) [780] | 1.65 (1.59, 1.72) [1137] | 81.8 (78.0, 85.9) [757] | 38.1 (36.7, 39.5) [1135] |
| Race/ethnicity | ||||||||||
| NHW | 7.17 (6.88, 7.47) [735] | 4.09 (3.93, 4.26) [1104] | 40.4 (38.3, 42.7) [734] | 19.4 (18.5, 20.3) [1107] | 35.6 (33.3, 38.0) [732] | 14.3 (13.7, 14.9) [1109] | 3.08 (2.87, 3.31) [716] | 1.71 (1.63, 1.79) [1061] | 86.6 (81.9, 91.6) [711] | 40.3 (38.5, 42.1) [1058] |
| NHB | 5.17 (4.80, 5.57) [232] | 2.95 (2.75, 3.17) [364] | 31.3 (29.3, 33.3) [232] | 15.0 (14.0, 16.1) [364] | 27.3 (25.5, 29.2) [224] | 11.0 (10.2, 11.8) [364] | 2.33 (2.18, 2.49) [228] | 1.29 (1.22, 1.37) [345] | 67.1 (63.4, 71.1) [220] | 31.2 (29.4, 33.2) [345] |
| MA | 6.53 (6.12, 6.97) [423] | 3.73 (3.48, 3.99) [436] | 37.4 (34.3, 40.7) [422] | 17.9 (16.5, 19.5) [436] | 33.5 (30.3, 37.0) [423] | 13.5 (12.4, 14.7) [436] | 2.77 (2.55, 3.01) [421] | 1.54 (1.43, 1.65) [428] | 80.5 (74.1, 87.5) [421] | 37.4 (34.7, 40.4) [428] |
| Age | ||||||||||
| 20 y | 6.99 (6.55, 7.47) | 3.78 (3.61, 3.97) | 38.9 (36.5, 41.6) | 17.6 (16.4, 18.8) | 33.4 (31.1, 35.8) | 12.3 (11.5, 13.2) | 2.72 (2.48, 2.99) | 1.50 (1.42, 1.58) | 82.3 (77.1, 87.9) | 35.8 (33.7, 38.1) |
| 50 y | 6.68 (6.42, 6.94) | 3.84 (3.70, 3.98) | 37.6 (35.8, 39.5) | 18.2 (17.5, 19.0) | 33.3 (31.2, 35.6) | 13.6 (13.1, 14.1) | 2.90 (2.73, 3.09) | 1.61 (1.55, 1.68) | 81.1 (76.9, 85.4) | 38.1 (36.7, 39.5) |
| 80 y | 7.04 (6.64, 7.46) | 4.30 (4.05, 4.57) | 34.9 (32.8, 37.2) | 18.2 (16.8, 19.6) | 31.9 (29.8, 34.0) | 14.4 (13.4, 15.4) | 2.67 (2.50, 2.85) | 1.50 (1.38, 1.64) | 77.3 (72.8, 82.2) | 39.2 (36.5, 42.1) |
| Education | ||||||||||
| ≤12 y | 6.67 (6.38, 6.96) [891] | 3.80 (3.64, 3.98) [1145] | 39.0 (37.1, 40.9) [888] | 18.7 (17.8, 19.7) [1147] | 35.5 (33.2, 37.9) [873] | 14.3 (13.7, 14.9) [1148] | 3.02 (2.83, 3.22) [878] | 1.68 (1.60, 1.75) [1098] | 84.7 (80.2, 89.4) [859] | 39.4 (37.5, 41.4) [1096] |
| >12 y | 6.69 (6.40, 6.99) [631] | 3.82 (3.68, 3.96) [1118] | 37.0 (35.0, 39.1) [631] | 17.8 (17.0, 18.5) [1118] | 31.9 (29.7, 34.2) [620] | 12.8 (12.3, 13.4) [1119] | 2.81 (2.63, 3.01) [620] | 1.56 (1.50, 1.62) [1073] | 78.7 (74.4, 83.3) [604] | 36.6 (35.1, 38.2) [1071] |
| BMI | ||||||||||
| Under- or normal weight | 6.05 (5.79, 6.32) [511] | 3.45 (3.31, 3.61) [624] | 34.9 (33.0, 36.8) [510] | 16.7 (15.9, 17.6) [624] | 29.8 (27.9, 31.8) [503] | 12.0 (11.4, 12.6) [625] | 2.52 (2.35, 2.70) [501] | 1.40 (1.35, 1.45) [600] | 73.6 (69.8, 77.6) [492] | 34.2 (32.7, 35.9) [599] |
| Overweight | 6.73 (6.40, 7.08) [516] | 3.84 (3.69, 4.00) [778] | 38.8 (36.7, 41.0) [515] | 18.6 (17.8, 19.5) [778] | 33.9 (31.7, 36.3) [506] | 13.7 (13.1, 14.3) [779] | 3.04 (2.83, 3.26) [510] | 1.69 (1.61, 1.76) [749] | 83.1 (78.3, 88.2) [497] | 38.7 (37.0, 40.4) [746] |
| Obese | 7.35 (7.07, 7.65) [495] | 4.20 (4.02, 4.38) [861 | 40.4 (38.2, 42.8) [494] | 19.4 (18.5, 20.4) [863] | 37.2 (34.5, 40.2) [484] | 15.0 (14.3, 15.7) [863] | 3.22 (3.00, 3.44) [487] | 1.78 (1.69, 1.89) [822] | 88.5 (83.3, 94.0) [474] | 41.2 (39.2, 43.3) [822] |
| Physical activity | ||||||||||
| Active | 6.67 (6.39, 6.96) [787] | 3.81 (3.65, 3.96) [1052] | 37.8 (36.0, 39.8) [787] | 18.2 (17.4, 19.0) [1053] | 32.6 (30.4, 35.0) [775] | 13.2 (12.6, 13.7) [1054] | 2.86 (2.66, 3.07) [774] | 1.59 (1.52, 1.65) [1001] | 80.5 (76.2, 85.1) [756] | 37.5 (35.9, 39.1) [1000] |
| Sedentary | 6.69 (6.40, 6.99) [735] | 3.82 (3.67, 3.97) [1211] | 38.0 (35.9, 40.1) [732] | 18.2 (17.4, 19.1) [1212] | 34.5 (32.4, 36.7) [718] | 13.9 (13.4, 14.4) [1213] | 2.96 (2.79, 3.14) [724] | 1.64 (1.58, 1.71) [1170] | 82.4 (78.2, 86.9) [707] | 38.3 (36.7, 40.0) [1167] |
| Alcohol intake | ||||||||||
| Nondrinker | 7.50 (7.05, 7.97) [494] | 4.28 (4.04, 4.53) [693] | 42.9 (40.3, 45.8) [495] | 20.6 (19.5, 21.7) [693] | 38.1 (35.2, 41.3) [487] | 15.4 (14.5, 16.2) [694] | 2.98 (2.76, 3.21) [484] | 1.65 (1.58, 1.72) [674] | 91.1 (85.4, 97.2) [474] | 42.4 (40.2, 44.7) [673] |
| <X Drinks/d2 | 6.98 (6.68, 7.30) [589] | 3.99 (3.82, 4.16) [834] | 39.5 (37.3, 41.8) [587] | 18.9 (18.0, 20.0) [834] | 33.9 (31.7, 36.3) [577] | 13.7 (13.0, 14.3) [834] | 2.88 (2.70, 3.08) [580] | 1.60 (1.54, 1.67) [795] | 83.9 (79.3, 88.7) [567] | 39.0 (37.2, 40.9) [794] |
| ≥X Drinks/d2 | 5.86 (5.64, 6.10) [439] | 3.35 (3.23, 3.47) [736] | 33.2 (31.5, 34.9) [437] | 15.9 (15.2, 16.7) [738] | 30.0 (28.0, 32.1) [429] | 12.1 (11.6, 12.6) [739] | 2.87 (2.69, 3.06) [434] | 1.59 (1.52, 1.67) [702] | 72.7 (68.6, 76.9) [422] | 33.8 (32.3, 35.3) [700] |
| Lipid-altering medication use | ||||||||||
| Yes | 5.86 (5.52, 6.21) [123] | 3.34 (3.13, 3.57) [436] | 33.9 (30.9, 37.1) [123] | 16.3 (14.7, 17.9) [436] | 31.2 (28.5, 34.1) [119] | 12.6 (11.7, 13.5) [437] | 2.88 (2.64, 3.13) [122] | 1.60 (1.50, 1.70) [417] | 74.5 (68.4, 81.0) [118] | 34.6 (31.8, 37.7) [416] |
| None | 6.81 (6.54, 7.09) [1399] | 3.89 (3.76, 4.02) [1827] | 38.5 (36.7, 40.4) [1396] | 18.5 (17.8, 19.2) [1829] | 33.8 (31.7, 36.0) [1374] | 13.6 (13.1, 14.1) [1830] | 2.91 (2.73, 3.09) [1376] | 1.61 (1.56, 1.67) [1754] | 82.4 (78.4, 86.7) [1345] | 38.3 (37.0, 39.7) [1751] |
| Diabetic status | ||||||||||
| Nondiabetic | 6.55 (6.29, 6.82) [1180] | 3.74 (3.58, 3.90) [1339] | 37.0 (35.1, 38.9) [1178] | 17.7 (17.0, 18.5) [1340] | 32.4 (30.3, 34.6) [1166] | 13.1 (12.6, 13.5) [1342] | 2.83 (2.65, 3.01) [1164] | 1.57 (1.51, 1.63) [1285] | 79.2 (75.0, 83.6) [1145] | 36.8 (35.3, 38.4) [1282] |
| Diabetic or prediabetic | 7.08 (6.63, 7.55) [342] | 4.04 (3.84, 4.25) [924] | 40.8 (38.4, 43.4) [341] | 19.6 (18.4, 20.8) [925] | 36.7 (34.1, 39.6) [327] | 14.8 (14.0, 15.7) [925] | 3.14 (2.94, 3.36) [334] | 1.74 (1.67, 1.82) [886] | 88.2 (82.7, 94.0) [318] | 41.0 (38.8, 43.4) [885] |
n (shown in brackets) is provided for groups but not for point estimates of continuous variables. “Fasting” indicates no meals consumed in the past ≥8 h. Supplemental Table 10 contains P values for the various main effect and interaction terms in the models, and Supplemental Table 11 shows P values for comparisons of subgroups. The model included survey year, sex, race/ethnicity, education, alcohol use, lipid-altering medication use, BMI, diabetic status, physical activity, age, age squared, and education × age and survey × age as interactions. MA, Mexican American; NHB, non-Hispanic black; NHW, non-Hispanic white; sumTFAs, sum of trans fatty acids; TFA, trans fatty acid.
For males X = 2 drinks and for females X = 1 drink.
The CGM concentration of the sumTFAs was 54% lower in NHANES 2009–2010 (37.8 μmol/L) than in NHANES 1999–2000 (81.4 μmol/L; Table 2). The difference between the 2 survey cycles was highest for elaidic acid (60%), whereas it ranged between 43% and 52% for the other TFAs.
In both NHANES cycles, the CGM of the sumTFAs in NHBs was 23% lower (P < 0.0001; 1999–2000 NHANES: 67.1 μmol/L; 2009–2010 NHANES: 31.2 μmol/L) compared with NHWs (86.6 and 40.3 μmol/L, respectively) and 17% lower (P = 0.0004) compared with MAs (80.5 and 37.4 μmol/L, respectively; Table 2, Supplemental Table 11). In addition, in both cycles, CGMs of the sumTFAs were 7% higher in those with ≤12 y of education compared with those with >12 y of education (84.7 compared with 78.7 μmol/L in the 1999–2000 NHANES and 39.4 compared with 36.6 μmol/L in the 2009–2010 NHANES; P = 0.001). There were no significant differences in sumTFAs between age groups in either cycle.
In both NHANES cycles, persons who reported consuming no alcohol had higher TFA concentrations than those who reported consuming alcohol [8% higher in those having <1 (women) or 2 (men) drinks/d (P = 0.004) and 20% higher for those having >1 (women) or 2 (men) drinks/d (P < 0.0001); Table 2, Supplemental Table 11]. TFA concentrations were higher in overweight (13%) and obese (20%) individuals than in normal-weight and underweight persons in both NHANES cycles (P < 0.0001). Those who reported using lipid-altering medications had slightly lower TFA concentrations (10%) than those who did not report use (P < 0.02), except for linoelaidic acid, for which no significant difference was observed. No measurable differences in individual TFAs or sumTFA were seen between persons who reported being physically active and those who did not, except for elaidic acid (6%; P = 0.006).
DISCUSSION
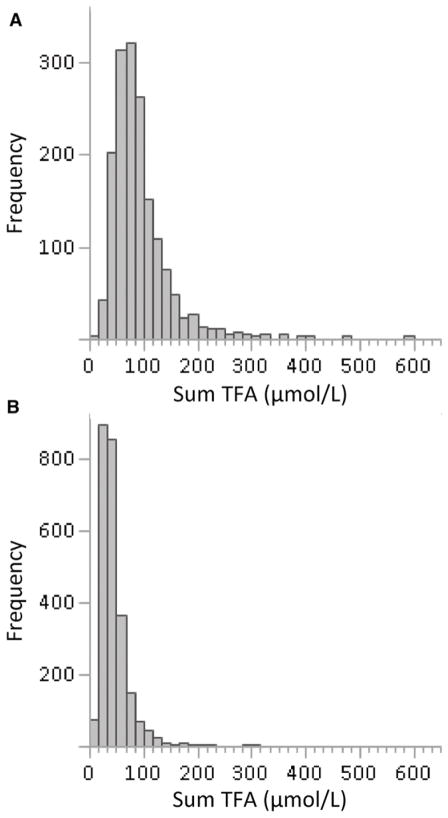
Plasma TFA concentrations in the NHANES populations shifted to lower concentrations and a narrower distribution from NHANES 1999–2000 to 2009–2010. The median of the sumTFAs was 54% lower in 2009–2010 than in 1999–2000 and the IQR was 50% smaller (Figure 1). The magnitude of change remained approximately the same after adjusting TFA concentrations for BMI, age, and other covariates. These findings are consistent with our previous report, which included only a small subset of NHWs (10). Such a notable change can only be explained by an overall reduction in TFA intake in the population, which appears to be consistent with the reported overall reduction in TFAs in food (9, 22).
FIGURE 1.
Frequency distribution of sum TFAs in fasting adults in NHANES 1999–2000 (A) and NHANES 2009–2010 (B). Sum TFA, sum of trans fatty acids.
Estimates of TFA intake from partially hydrogenated vegetable oils with the use of food-consumption data and data on TFAs in food describe a decrease in TFA intake from 4.6 to 1.0 g/d per person in the US population (23), which suggests a reduction in TFA intake of 78%. The reduction in TFA blood concentrations observed in this study (54%) is less profound, which might be explained, in part, by the uncertainties associated with food intake estimates. In addition, TFA concentrations in blood derive from TFAs in partially hydrogenated vegetable oils as well as other sources such as meat and milk from ruminant animals, which may explain, in part, the smaller difference observed in this study.
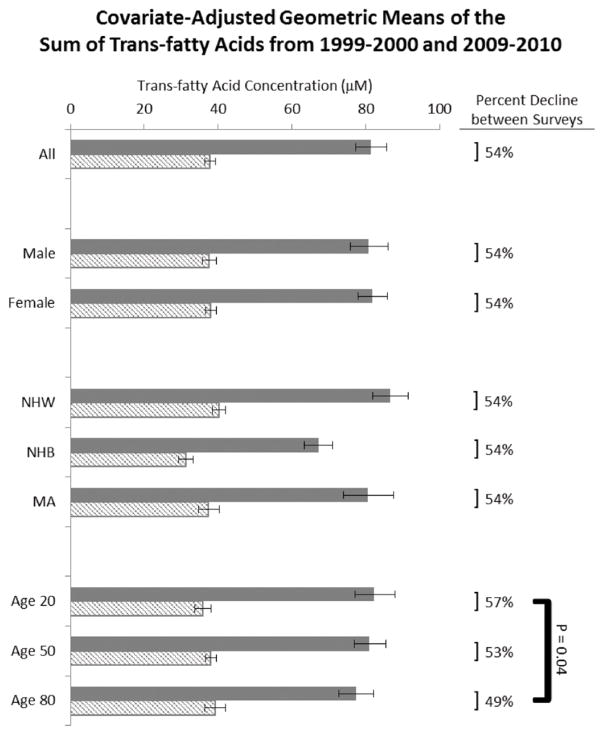
In both NHANES cycles, NHWs had the highest sumTFA values, whereas NHBs had the lowest values. Although the actual sumTFAs changed from NHANES 1999–2000 to NHANES 2009–2010, the percentage difference among NHWs, NHBs, and MAs remained the same. CGMs of sumTFAs in both NHANES cycles were slightly lower in those with >12 y of education than in those with ≤12 y of education. This observation appears to be consistent with a previous report that showed that the Alternate Healthy Eating Index improved in those with a higher education (24). The difference between these 2 subgroups remained the same in both NHANES cycles. Similarly, the differences between other categories, such as alcohol consumption, BMI, and the use of lipid-altering medications, remained the same in both NHANES cycles. This suggests that TFA intake overall declined consistently in these population subgroups, whereas differences between subgroups stayed the same. Only among age groups was the decline in TFA values inconsistent, with individuals aged 20 y showing a higher decline (57%) than those aged 80 y (49%; P = 0.04; Figure 2).
FIGURE 2.
Covariate-adjusted geometric mean concentrations of the sum of trans fatty acids between NHANES 1999–2000 (solid bars) and NHANES 2009–2010 (hatched bars). Error bars represent 95% CIs. MA, Mexican American; NHB, non-Hispanic black; NHW, non-Hispanic white.
The proportion relative to the sumTFAs for elaidic acid, 1 major TFA in partially hydrogenated vegetable oil, changed from 41% in NHANES 1999–2000 to 36% in NHANES 2009–2010, whereas the proportion of trans vaccenic acid, the major fatty acid in fat from ruminant animals, remained the same. Even though these fatty acids are not specific biomarkers for these food sources, the slight differences in changes in these TFAs could be explained with the more profound reduction in intakes of partially hydrogenated vegetable oil (23).
As expected, TFAs are associated with the blood lipids TC, triglycerides, and HDL cholesterol. However, TFA intake is known to change blood lipids and, at the same time, TFAs are part of lipoprotein particles. We chose not to adjust TFA values for cholesterol and other lipids due to the difficulty of interpreting the bidirectional effects of TFAs and blood lipids on one another. Further studies are needed to better understand the associations between TFAs and blood lipids. As expected, triglyceride, TC, and LDL-cholesterol values are higher in individuals with TFA concentrations in the higher tertile than in those in the lower tertile. This observation is consistent for both cycles, even though TFA concentrations in the 2009–2010 NHANES cycle were much lower. These findings suggest that the effect of TFAs on blood lipids remains, even at low TFA concentrations. Further studies are needed to verify this observation. We observed a negative association between TFA concentrations and alcohol consumption. The reasons for this observation are not fully understood and may warrant further investigation.
The 4 TFAs measured in this study are considered major TFAs in foods (25). Reports on TFAs in blood are inconsistent with regard to the number of different TFAs measured. Overall, >10 different TFAs have been reported in blood, with trans vaccenic acid and elaidic acid being reported frequently as the most abundant among the different TFAs (26–29). Thus, the TFAs measured in this study provide information about the overall TFA exposure in this population.
This study assessed changes in TFA exposure or intake by measuring TFAs in plasma of fasting individuals instead of estimating intake, which is the main strength of this study. Furthermore, this is the only study, to our knowledge, that provides nationally representative data on TFAs in blood stratified by sex, race/ethnicity, and age. The TFA concentrations measured in this study reflect the overall TFA exposure including TFAs from all foods consumed by the study participants. Individual TFAs occur in partially hydrogenated vegetable oils as well as in fat from ruminant animals. Therefore, they are not suitable as highly specific biomarkers for either of these food sources and thus cannot be used to estimate the intake of TFAs from a particular source.
In summary, these nationally representative data for the adult US population showed a 54% reduction in plasma TFA concentrations between 1999–2000 and 2009–2010. With the exception of age, the TFA decreases were the same for all population subgroups and differences between population subgroups remained the same. The 2009–2010 data will provide a valuable baseline to evaluate the impact of the recent FDA regulation, which categorizes TFAs as food additives.
Supplementary Material
Acknowledgments
We thank Tunde Frame, Ashley Ribera, Christina Waters, Judith Brumlow, Antoinette Smith, Marcela Muresan, Ashley Tippins, Chui Tse, Monir Clark, Magaly Mendez, Samantha McGunigale, Na Wei, Amber Wallace, Melissa Missinne, Meghan Vidal, Neha Ahuja, Christopher Ghattas, and Kelsey Wiley (Division of Laboratory Sciences, CDC) for the laboratory measurements of the plasma TFAs. We also thank Kelley Scanlon (Division of Nutrition, Physical Activity, and Obesity, CDC) for helpful comments.
Footnotes
The authors reported no funding received for this study.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official views or positions of the CDC/Agency for Toxic Substances and Disease Registry.
Supplemental Tables 1–11 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://ajcn.nutrition.org.
Abbreviations used: CGM, covariate-adjusted geometric mean; FDA, Food and Drug Administration; GM, geometric mean; HbA1c, glycated hemoglobin; MA, Mexican American; NHB, non-Hispanic black; NHW, non-Hispanic white; sumTFAs, sum of the 4 trans fatty acids; TC, total cholesterol; TFA, trans fatty acid.
The authors’ responsibilities were as follows—HWV and JLP: designed the research and edited the manuscript; HWV and HCK: conducted the research; NA and DAL: provided essential materials; HWV, SPC, and QY: analyzed the data; HWV and SPC: prepared the manuscript; HWV: had primary responsibility for the final content; and all authors: read and approved the final version of the manuscript. None of the authors reported a conflict of interest related to the study.
References
- 1.Teegala SM, Willett WC, Mozaffarian D. Consumption and health effects of trans fatty acids: a review. J AOAC Int. 2009;92:1250–7. [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services [Internet] Healthy People 2020. Version current. 2011 Aug 13; [cited 2011 Aug 13]. Available from: http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=21.
- 3.Institute of Medicine. Dietary Reference Intakes: energy, carbohydrates, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): National Academies Press; 2002. [Google Scholar]
- 4.US Department of Health and Human Services; USDA. Dietary Guidelines for Americans, 2005. 6. Washington (DC): US Government Printing Office; 2005. [Google Scholar]
- 5.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, et al. Diet and lifestyle recommendations revision 2006. A scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 6.USDA; US Department of Health and Human Services. Dietary Guidelines for Americans 2010. 7. Washington (DC): US Government Printing Office; 2010. [Google Scholar]
- 7.Department of Health and Human Services, Food and Drug Administration. Food labeling: trans fatty acids in nutrition labeling; consumer research to consider nutrient content and health claims and possible footnote or disclosure statements; proposed rule. Federal Register. 2003 Jul 11;68(133) [cited 2015 Nov 24]. Available from: https://www.federalregister.gov/articles/2003/07/11/03-17526/food-labeling-trans. [PubMed] [Google Scholar]
- 8.Department of Health and Human Services, Food and Drug Administration. Final determination regarding partially hydrogenated oils; notice. Federal Register. 2015 Jun 17;80(116) [cited 2015 Nov 24]. Available from: https://www.federalregister.gov/articles/2015/06/17/2015-14883/final-determination-regarding-partially-hydrogenated-oils. [Google Scholar]
- 9.Doell D, Folmer D, Lee H, Honigfort M, Carberry S. Updated estimate of trans fat intake by the US population. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29:861–74. doi: 10.1080/19440049.2012.664570. [DOI] [PubMed] [Google Scholar]
- 10.Vesper HW, Kuiper HC, Mirel LB, Johnson CL, Pirkle JL. Levels of plasma trans-fatty acids in non-Hispanic white adults in the United States in 2000 and 2009. JAMA. 2012;307:562–3. doi: 10.1001/jama.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National Health and Nutrition Examination Survey: sample design, 2011–2014. Vital Health Stat 2. 2014;162:1–33. [PubMed] [Google Scholar]
- 12.Lagerstedt SA, Hinrichs DR, Batt SM, Magera MJ, Rinaldo P, McConnel JP. Quantitative determination of plasma c8-c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol Genet Metab. 2001;73:38–45. doi: 10.1006/mgme.2001.3170. [DOI] [PubMed] [Google Scholar]
- 13.Taylor JK. Quality assurance of chemical measurements. Chelsea (MI): Lewis Publishers; 1987. [Google Scholar]
- 14.National Center for Health Statistics. National Health and Nutrition Examination Survey [Internet]. Version current. 2016 Feb 24; [cited 2016 Mar 8]. Available from: http://www.cdc.gov/nchs/nhanes.htm.
- 15.Bernert JT, Jr, McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and non-smokers. J Anal Toxicol. 2000;24:333–9. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Technical Report Series 894. WHO; 2000. Obesity: preventing and managing the global epidemic. [cited Jan 2014]. Available from: http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ [PubMed] [Google Scholar]
- 17.Shah BV, Barnwell BG, Bieler GS. SUDAAN user’s manual, release 7. Research Triangle Park (NC): Research Triangle Institute; 1996. [Google Scholar]
- 18.Korn EL, Graubard BI. Confidence intervals for proportions with small expected number of positive counts estimated from survey data. Surv Methodol. 1998;24:193–201. [Google Scholar]
- 19.Woodruff RS. Confidence intervals for medians and other position measures. J Am Stat Assoc. 1952;47:635–47. [Google Scholar]
- 20.Sternberg MR, Schleicher RL, Pfeiffer CM. Regression modeling plan for 29 biochemical indicators of diet and nutrition measured in NHANES 2003–2006. J Nutr. 2013;143:948S–56S. doi: 10.3945/jn.112.172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoav B, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 22.Mozaffarian D, Jacobson MF, Greenstein JS. Food reformulations to reduce trans fatty acids. N Engl J Med. 2010;362:2037–9. doi: 10.1056/NEJMc1001841. [DOI] [PubMed] [Google Scholar]
- 23.Department of Health and Human Services, Food and Drug Administration. Tentative determination regarding partially hydrogenated oils; request for comments and for scientific data and information; notice. Federal Register. 2013 Nov 8;78(217) [cited 2015 Nov 24]. Available from: https://www.federalregister.gov/articles/2013/11/08/2013-26854/tentative-determination-regarding-partially-hydrogenated-oils-request-for-comments-and-for. [Google Scholar]
- 24.Wang DD, Li Y, Chiuve SE, Hu FB, Willett WC. Improvements in US diet helped reduce disease burden and lower premature deaths, 1999–2012: overall diet remains poor. Health Aff (Millwood) 2015;34:1916–22. doi: 10.1377/hlthaff.2015.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gebauer SK, Psota TL, Kris-Etherton PM. The diveristy of health effects of individual trans fatty acid isomers. Lipids. 2007;42:787–99. doi: 10.1007/s11745-007-3095-8. [DOI] [PubMed] [Google Scholar]
- 26.Baylin A, Kim MK, Donovan-Palmer A, Siles X, Dougherty L, Tocco P, Campos H. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol. 2005;162:373–81. doi: 10.1093/aje/kwi213. [DOI] [PubMed] [Google Scholar]
- 27.Kuhnt K, Kraft J, Moeckel P, Jahreis G. Trans-11-18:1 is effectively Δ9-desaturated compared with trans-12-18:1 in humans. Br J Nutr. 2006;95:752–61. doi: 10.1079/bjn20051680. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Avila N, Mata-Granados JM, Ruiz-Jiménez J, Luque de Castro MD. Fast, sensitive and highly discriminant gas chromatography-mass spectrometry method for profiling analysis of fatty acids in serum. J Chromatogr A. 2009;1216:6864–72. doi: 10.1016/j.chroma.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 29.Enke U, Jaudszus A, Schleussner E, Seyfarth L, Jahreis G, Kuhnt K. Fatty acid distribution of cord and maternal blood in human pregnancy: special focus on individual trans fatty acids and conjugated linoleic acids. Lipids Health Dis. 2011;10:247–57. doi: 10.1186/1476-511X-10-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.