Abstract
Background
Adsorption of albumin onto urine collection and analysis containers may cause falsely low concentrations.
Methods
We added 125I-labeled human serum albumin to urine and to phosphate buffered solutions, incubated them with 22 plastic container materials and measured adsorption by liquid scintillation counting.
Results
Adsorption of urine albumin (UA) at 5–6 mg/l was <0.9%; and at 90 mg/l was <0.4%. Adsorption was generally less at pH 8 than pH 5 but only 3 cases had p <0.05. Adsorption from 11 unaltered urine samples with albumin 5–333 mg/l was <0.8%. Albumin adsorption for the material with greatest binding was extrapolated to the surface areas of 100 ml and 2 l collection containers, and to instrument sample cups and showed <1% change in concentration at 5 mg/l and <0.5% change at 20 mg/l or higher concentrations. Adsorption of albumin from phosphate buffered solutions (2–28%) was larger than that from urine.
Conclusions
Albumin adsorption differed among urine samples and plastic materials, but the total influence of adsorption was <1% for all materials and urine samples tested. Adsorption of albumin from phosphate buffered solutions was larger than that from urine and could be a limitation for preparations used as calibrators.
Keywords: Urine albumin, Albumin adsorption, Urine containers
1. Introduction
Chronic kidney disease is a significant public health problem worldwide, and diagnosis is hampered by the lack of standardized early detection methods. Urinary albumin (UA) measurement is widely used for detection of chronic kidney disease, but albumin adsorption to collection and analysis containers may cause falsely low measurements. A joint working group of the National Kidney Disease Education Program and the International Federation of Clinical Chemistry and Laboratory Medicine identified surface adsorption onto containers as one of the pre-analytical variables that could influence interpretation of urine albumin results especially at lower concentrations important for early detection [1].
Investigators have used both chemical and physical methods including radioisotopes [2], atomic force microscopy [3], fluorescence and infrared spectroscopy [4], and ellipsometry [5] to study the interactions of proteins with surfaces. Many have studied albumin adsorption in search of promising materials for use in implantable medical devices, but the albumin concentrations used in these studies approached the concentrations in human serum or plasma, which are considerably greater than those in urine. Only a few studies have examined albumin adsorption to containers at the concentrations found in human urine. Holmberg and Hou [6] reported on the competitive adsorption of proteins in buffered mixtures onto polymer surfaces and the complex nature of this process. A few investigators have reported adsorption onto particular containers [7] and explored the use of surfactants such as Triton X-100 and Tween-20 to reduce albumin adsorption [8]. Bakker reported up to 90% reduction in albumin adsorption using surfactants [9]. Because the pH of urine varies widely, the influence of pH and surfactants are important considerations for measurement of albumin adsorption onto container surfaces.
2. Materials and methods
Fig. S1 (supplemental data) provides a flow chart showing the series of experimental components that are described in detail in the following sections.
2.1. Containers
We purchased urine collection containers, centrifuge tubes, storage vials, and sample analysis cups from companies indicated in Table S1 (supplemental data). The polyethylene terephthalate materials with a hydrophilic coating claiming to reduce bovine serum albumin adsorption to 1/10,000 of that for polystyrene were donated by Sumitomo-Bakelite, Japan through their U.S. subsidiary Wako.
2.2. Labeled and unlabeled human serum albumin
We used custom-labeled 125I-HSA from Perkin Elmer (88–93 mCi/mg, in 0.01 mol/l sodium phosphate buffer, pH 7.4, containing 0.0027 mol/l KCl and 0.137 mol/l NaCl) to prepare mixtures of labeled and unlabeled HSA. HSA (crystallized cat # A8763-1G) was from Sigma-Aldrich Chemical Co. We assumed that 125I-HSA and unlabeled HSA had the same adsorption properties.
2.3. Urine and human serum albumin solutions
For adsorption studies using urine, we collected unidentified, fresh human urine samples or used leftover, unidentified urine samples from Grady Health System or Virginia Commonwealth University Hospital using a protocol approved by the CDC Human Subjects Review Committee. Urine samples were transported and stored at 4–8 °C and used within 30 days of collection. We measured both albumin concentration and pH and used some of the samples without adjustment. We used some of the samples with very low urine albumin concentrations as baseline urine to dilute samples with higher concentrations (pooled urine). We prepared stock unlabeled HSA solutions (1 g/l) in both baseline urine and 10 mmol/l phosphate-buffered saline (PBS). From these stock solutions, we prepared dilutions to contain 5 and 100 mg/l. We adjusted these solutions to pH 4.0, 5.0, or 8.0 using 2 mol/l HCl or 2 mol/l NaOH just prior to adjusting the final volume. To examine the effects of surfactants, we prepared buffered HSA solutions to contain 0.1 v/v% Triton X-100 and 0.05 v/v% Tween-20. We stored all solutions refrigerated in small aliquots in 20 ml glass liquid scintillation counting (LSC) vials and spiked with the 125I-HSA as needed to obtain specific activities of 107–1010 counts per minute/mg (cpm/mg). All chemicals were analytical grade.
2.4. Adsorption and retention measurements
We conducted adsorption studies by incubating container materials with urine samples or buffered HSA solutions at room temperature (23.0 ± 2.0 °C), for specific time periods (usually 300 min). Disks (1.032 cm diameter, 0.836 cm2) were punched from plastic materials when possible and 100 μl of solutions was placed on the surface. Instrument sample cups were filled with 200 μl of solutions which covered an area of approximately 1.43 cm2. After incubation we transferred the solution, five water rinses, and the pipet tip to a glass LSC vial and the material (disk, cone, sample cup, or sample vial) to a separate counting vial. To calculate “total counts” we added the counts for both vials and used that total to calculate specific activity. This approach allowed us to measure both the albumin retained in solution and the albumin adsorbed onto the material. For some experiments, we only counted the material (disk, cup or vial) and prepared two separate vials using an equal volume of the solution incubated on the material (either 100 or 200 μl) to determine specific activity. For containers that could not be cut or transferred to LSC vials, we used the latter approach. In all cases, we added 10 ml of liquid scintillation cocktail (Ultima Gold, Perkin Elmer) to each vial and counted for 1 min with a Perkin Elmer Tri-carb 3100TR Liquid Scintillation Counter using a protocol for 125I. The counter was calibrated weekly with 3H and 14C reference solutions.
We calculated specific activity (Sp.Ac.) according to the following equation:
Vol is the volume counted (100 μl for disks and 200 μl for cones, sample analysis cups or vials) and Cb is the total concentration of albumin (labeled + unlabeled). We calculated the albumin concentration in the labeled solution from data provided on the certificate of analysis. We measured the albumin concentration in both urine and buffered HSA solutions by immunoassay (IA) prior to spiking with 125I-labeled HSA, then calculated the total albumin concentration for use in the equation above. We measured albumin concentrations using IA with a Roche Hitachi 912 Clinical Analyzer using Roche Tina-Quant reagents, calibrators, and controls according to the manufacturer’s instructions. We also measured buffered HSA solutions with and without the surfactants to determine whether surfactants interfered with the albumin IA. We calculated the surface adsorption for each disk, cup, or other container according to the following equation:
To further support our 125I estimates of albumin retention in urine samples, we measured albumin concentrations in urine samples using IA before and after 10 serial transfers from one container to the next over 300 min (30 min intervals per container) in both polystyrene and hydrophilic-coated sample vials.
To measure the effects of pH on albumin concentration, we mixed 2 urine samples to obtain an albumin concentration of approximately 10 mg/l. We adjusted the pH of duplicate 3 ml aliquots to values from 4.0 to 9.5 in increments of 0.5 pH units with microliter quantities of 2 mol/l NaOH or 2 mol/l HCl. After overnight refrigeration, we measured the albumin concentrations by IA; then re-adjusted each aliquot to pH 5.7 (the pH of the original pooled urine sample) and re-measured the albumin concentration.
Experiments to examine rinsing efficiency, effects of specific activity on adsorption measurements, and kinetics of adsorption are described in the Supplemental Data File. Because we were storing our samples in glass counting vials, we compared adsorption in glass and polystyrene counting vials. We also compared adsorption onto materials from the sides, bottoms, and tops of a single polypropylene container and compared adsorption onto surfaces of two types of 15 ml centrifuge tubes (hydrophilic-coated PET and polystyrene). We measured adsorption onto glass and polystyrene counting vials and conical bottoms of hydrophilic-coated PET and polystyrene centrifuge tubes. We compared surface adsorption onto disks cut from the bottom, sides, and top of one polypropylene urine collection cup. These data are also shown in the Supplemental Data File.
2.5. Experimental design
We used an approach outlined in Introduction to Design of Experiments with JMP, Third Edition published by the SAS Institute [10], to develop the experimental design. We selected albumin concentrations (5 and 100 mg/l), pHs (4.0, 5.0 and 8.0), and times (0.5 and 5.0 h), and used published surfactant concentrations for Triton X-100 (0.1%) and for Tween-20 (0.05%).
2.6. Statistical analysis
We performed measurements in quadruplicate (except where noted otherwise).
We used SAS to compute means or geometric means, standard deviations, and 95% confidence intervals. We used analysis of covariance, t-tests, and Duncan’s Multiple Comparison tests to determine statistical significance of differences in retention and adsorption across materials and conditions.
2.7. Extrapolation of results to clinical testing
To evaluate the influence of adsorption for typical urine collection conditions, we extrapolated the adsorption per cm2 results to the surface areas of a 100 ml container with dimensions 5.72 cm diameter and 7.32 cm height (surface area 182 cm2) as well as to a 3 l container with a 2 l fill with dimensions 11.43 cm square and 25.4 cm tall (surface area 905 cm2). Our results did not account for differences in the surface-to-volume ratios or differences in diffusion rates between the urine containers and the volumes added to the plastic disks or instrument cups.
3. Results
3.1. Validation of experimental design conditions
The supplemental data includes information on several aspects of the experimental design. The kinetics of adsorption of albumin to surfaces was found to reach a maximum at 5–6 h (Fig. S2). Consequently, we used 5 h as the adsorption time recognizing that longer intervals would not change the adsorption proportion. The conditions thus were close to those for a random urine collection although no additional adsorption would be expected for longer times such as 24 h. Three rinses were sufficient to remove all non-adsorbed albumin from surfaces (Fig. S3). A stable measurement of adsorption occurred when the specific activity of 125I labeled HSA was 107 to 109 cpm/mg (Fig. S4). We determined that glass counting vials adsorbed significantly less albumin than polystyrene (p < 0.01), except for the higher albumin concentration at pH 8 (p = 0.51). The hydrophilic coated vials adsorbed significantly less albumin than polystyrene, p < 0.01 (Fig. S5).
3.2. Albumin adsorption onto container materials
Fig. 1 shows the adsorption of albumin from urine and from PBS solutions onto a number of different container materials. Note that not all materials were investigated in all experiments; however, the same numbers were used to identify the materials throughout the manuscript figures and tables. The numeric data and p values are in Tables S2 and S3 (supplemental data). Fig. 1A shows adsorption from low (5.3 mg/l at pH 5 and 5.8 mg/l at pH 8) and high (88 mg/l at pH 5 and 91 mg/l at pH 8) albumin concentrations in urine samples onto 2 types of instrument sample cups and disks from 16 types of container materials. The amount adsorbed differed among types of materials but was <1% for all, and was lower at pH 8 than at pH 5. Only 1 of 16 materials had a significant difference (p = 0.033) between the pHs at low albumin concentrations and 2 of 16 had a significant difference (p = 0.007 and 0.029) between the pHs at higher albumin concentrations. The amount of albumin retained in the urine applied to the plastics is shown in Table S4 (supplemental data). A mass balance of the albumin adsorbed onto surfaces and retained in the bulk solutions accounted for 99–101% of the albumin present in each condition, indicating no unexplained loss in the experimental manipulations (Table S4, supplemental data).
Fig. 1.
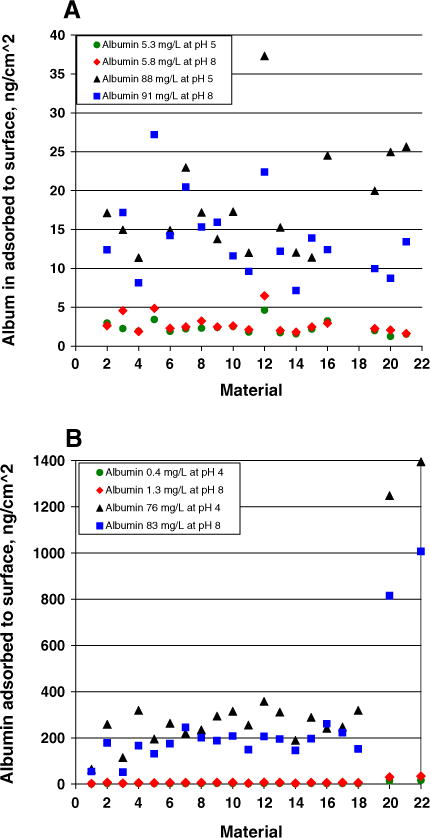
Adsorption of albumin onto plastic materials. Panel A shows adsorption from urine samples. Panel B shows adsorption from HSA in PBS. Materials 1–19 were disks cut from: hydrophilic-coated centrifuge tube (1), polystyrene centrifuge tube (2 & 12), polypropylene urine specimen containers (3, 4, 6, 11 & 13), polyethylene urine specimen containers (5 & 18), polyethylene terephthalate centrifuge tube (7), polypropylene centrifuge tubes (8, 9, 10, & 15), tef centrifuge tube (14), polyethylene 24-h urine collection jug (16), polysulfone centrifuge tube (17), and polypropylene screw cap for container #3 (19). Materials 20–22 were: polystyrene sample cups (20), hydrophilic coated vials (21), polyethylene sample cups (22).
Fig. 1B shows adsorption from solutions of albumin in PBS at low (0.4 mg/l at pH 4 and 1.3 mg/l at pH 8) and high (76 mg/l at pH 4 and 83 mg/l at pH 8) albumin concentrations, onto 2 types of instrument sample cups and 18 types of container materials. The adsorption from PBS solutions was somewhat larger (2–7%) than from the urine samples for most of the materials. However, larger amounts of albumin were adsorbed from PBS solutions (7–28%) for polystyrene and polyethylene sample cups. At the lower albumin concentrations, the influence of pH on adsorption could not be evaluated because the albumin concentrations differed by 3-fold which was sufficient to contribute to the different absolute amounts adsorbed. For the higher albumin concentrations, adsorption at pH 8 was less than at pH 4 for 18 of 20 materials but only 6 of 20 differences in adsorption at the 2 pHs were statistically significant (p = 0.029 to <0.001).
Fig. 2 shows adsorption from 11 urine samples with albumin concentrations of 5–333 mg/l and pH values of 4.8–7.5 onto 2 types of instrument sample cups and 2 types of container materials. The amount adsorbed differed among urine samples and material types. There was scatter in the data, but a general increase in the amount adsorbed occurred as the concentration in the bulk solution increased.
Fig. 2.
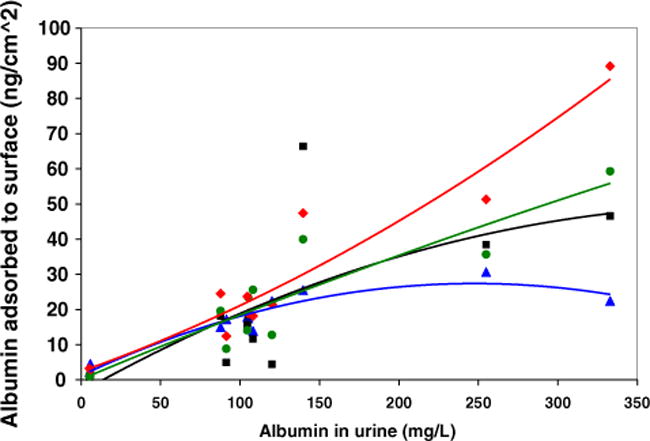
Adsorption of albumin from 11 urine samples. The lines are second order polynomial fits to the data; the equation for material 16 (worst case) is y = 2.68 × 10−4 (x2) + 0.160 (x) + 2.38. Plot symbols are: material 3 (triangle), material 16 (diamond), sample cup material 20 (square), hydrophilic vial material 21 (circle) for the same materials described in Fig. 1.
Fig. 3 shows that the surfactants Triton X-100 and Tween-20 significantly decreased the adsorption of albumin to PE and PS sample cups, and Fig. S6 (supplemental data) shows the same for PP disks; p < 0.001 for all three materials for both surfactants. However, Fig. S7 (supplemental data) shows that the surfactants caused an interference in the IA used here and consequently may not be generally useful for urine albumin sample collection or storage.
Fig. 3.
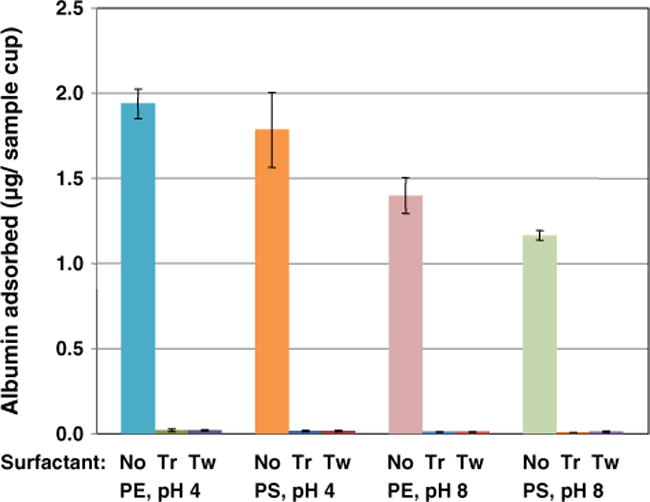
Albumin adsorbed to polyethylene (PE) and polystyrene (PS) instrument sample cups from 200 μl of 88 mg/l HSA in PBS plus 125I-labeled HSA after incubation for 5 h in the absence and presence of the surfactants Triton X-100 (Tr) and Tween 20 (Tw). Results are the mean of 4 measurements, and bars represent 95% confidence intervals.
Because the adsorption of albumin to surfaces was relatively small, we investigated the influence of 10 serial transfers of albumin solutions over 5 h to new polystyrene or hydrophilic-coated polyethylene terephthalate vials. Table 1 shows that the change in albumin concentrations was −3.5% to 2.4%, except for one urine sample which changed −8.9% for PS instrument sample cups.
Table 1.
Adsorption of albumin to sample cups after 10 transfers.
| Sample | Materiala | Albumin concentration, mg/l
|
Changec, % | |
|---|---|---|---|---|
| Initial | After 10 transfersb | |||
| HSA in PBS | PS | 12.7 | 13.1 | 3.1 |
| HSA in PBS | PS | 30.3 | 30.6 | 1.0 |
| Urine | PS | 25.9 | 23.6 | −8.9 |
| HSA in PBS | HPET | 13.0 | 2.4 | |
| HSA in PBS | HPET | 30.1 | −0.7 | |
| Urine | HPET | 25.0 | −3.5 | |
Materials were: PS, polystyrene instrument cup; HPET, hydrophilic coated vial.
Incubation was for 5 h with 10 sequential transfers to a new cup or vial at 30 min intervals.
Change in concentration vs. initial values.
3.3. Effects of pH on albumin measurements
Fig. S8 (supplemental data) shows that urine supplemented with HSA had a substantial decrease in albumin concentration measured by IA below pH 5. Readjusting the pH to its original values caused an increase in concentrations but did not fully restore the albumin concentrations to their original values.
3.4. Extrapolation of results to clinical testing
The influence of albumin adsorption on the clinical interpretation of results is shown in Table 2 based on the data in Fig. 2 for measurements of albumin adsorption from urine samples to 2 types of instrument sample cups and 2 types of plastic. Table S5 shows a similar clinical impact assessment for urine samples adsorbed to 18 plastic surfaces at 2 urine albumin concentrations and 2 pHs. Using either type of data, the influence of adsorption was proportionally greater at lower concentrations and caused <1% change in albumin concentration at 5 mg/l and <0.5% change at the clinical decision threshold of 20 mg/l.
Table 2.
Assessment of the influence of albumin adsorption from urine onto containers.a
| Urine albumin concentration, mg/1 | Albumin adsorbed to 24 h collection container (assume 2 l fill), μg | Albumin adsorbed to 100 ml collection container, μg | Albumin adsorbed to analyzer cup (assume 0.2 ml fill), μg | Change in concentration due to adsorption to 24 h collection container, % | Change in concentration due to adsorption to 100 ml container, % | Change in concentration due to adsorption to analyzer cup, % | Total change in albumin concentration if all 3 containers were used, % |
|---|---|---|---|---|---|---|---|
| 5 | 2.887 | 0.584 | 0.00574 | 0.029 | 0.117 | 0.574 | 0.720 |
| 10 | 3.630 | 0.734 | 0.00722 | 0.018 | 0.073 | 0.361 | 0.453 |
| 20 | 5.154 | 1.042 | 0.0103 | 0.013 | 0.052 | 0.256 | 0.321 |
| 50 | 10.02 | 2.026 | 0.0199 | 0.010 | 0.041 | 0.199 | 0.250 |
| 100 | 19.09 | 3.860 | 0.0380 | 0.010 | 0.039 | 0.190 | 0.238 |
| 200 | 40.87 | 8.265 | 0.0813 | 0.010 | 0.041 | 0.203 | 0.255 |
| 300 | 67.50 | 13.65 | 0.1343 | 0.011 | 0.045 | 0.224 | 0.281 |
The amount adsorbed was calculated from the polynomial regression for the worst case for 11 urine samples over the albumin concentration interval 5–333 mg/1 to the 4 plastic materials in Fig. 2.
4. Discussion
The focus of this study was to measure the influence of albumin adsorption onto a variety of material surfaces that may be used for collection, storage, and measurement of albumin in urine. Our primary goal was to determine whether adsorption of urine albumin onto collection and analysis containers would compromise the ability to measure urine albumin accurately at concentrations used to detect early kidney disease.
The total amount of albumin adsorbed increased as the concentration in urine increased. However, the influence of adsorption on concentration was greater at lower urine concentrations because the amount adsorbed was a larger fraction of the total concentration. Albumin adsorption from urine samples with approximately the same concentrations was seen to vary considerably, and this variability could be influenced by differences in the urine matrix [11]. Based on worst case adsorption conditions, we concluded that albumin adsorption onto urine collection containers and instrument analysis cups was <1% (>0.05 mg/l) at 5 mg/l, <0.5% (<0.1 mg/l) at the frequently used decision threshold of 20 mg/l and proportionally less at higher concentrations. Consequently, adsorption of albumin is not likely to influence interpretation of results in clinical situations.
The trivial amount of albumin adsorption could be minimized, if desired, by alkalinization or buffering of samples, by limiting the number of transfers of urine to different containers, and by selection of container materials for which albumin adsorption has been found to be minimal. Overall, the order of least adsorption of albumin onto materials was hydrophilic-coated polyethylene terephthalate < polypropylene < glass < polystyrene < polyethylene. There were very few statistically or clinically significant differences in adsorption of albumin between pHs of 5 and 8 for urine samples. However, for HSA in PBS at a concentration of approximately 80 mg/l, there were several conditions with significantly more adsorption at pH 4 than at pH 8.
Albumin adsorption was larger from PBS solutions than from urine samples at comparable albumin concentrations. This observation is not surprising since urine contains a variety of other components that may modify the adsorption of albumin to the surface. However, adsorption should be considered and minimized for preparation, storage and use of buffered albumin solutions as reference materials for calibration of measurement procedures.
The most significant apparent losses of albumin from urine and buffered HSA solutions occurred at pH values <5. The albumin concentrations of some of the HSA solutions prepared by weight to be 5 mg/l, were close to zero at pH 4.0 using the Roche Tina-Quant IA. We conclude that urine albumin concentration measured using this IA may be falsely low when the urine pH is below 5.0. The molecular conformation of albumin changes between pH 5.0 and 3.5. The molecule changes from the native, N conformation to an electrophoretically faster-moving F conformation known as the N–F transition. This transition is thought to involve structural changes in the helix that may cause irreversible denaturation [12] affecting albumin’s stability, its adsorption characteristics and its immunoreactivity in some measurement procedures. Heerspink et al. [13] reported that urine alkalinization to pH N >8.0 prevented the decline in UA concentration otherwise observed during prolonged frozen storage at −20 °C for urine samples from diabetic patients. These observations lead us to postulate that alkalinization or buffering of fresh urine samples may be a useful component of a standardized protocol for UA measurement using IA.
Adding surfactants to the urine appeared to reduce the adsorption of albumin. However, there was an artifactual influence of the surfactants on the IA measurement procedure used which limited any general conclusions. However, given the small influence of adsorption to containers, using surfactants to reduce adsorption would not improve the clinical interpretation of results.
4.1. Strengths
Strengths of this investigation were that we were careful to limit the number of transfers of solutions to 2 and we did not centrifuge any of the samples. The primary conclusions were based on adsorption from native urine samples and reinforced by data from solutions of buffered HSA. Radioactively labeled albumin was used to quantitate the adsorption, making conclusions independent of a typical IA or other chemical measurement procedure. The mass balance of albumin adsorbed from or retained in solutions indicated that there were no unexplained losses of albumin.
4.2. Limitations
Limitations of this study include the small numbers and concentration ranges of native urine samples that we tested. Our extrapolation of adsorption losses to urine containers based on data collected by incubation on 1.032 cm diameter disks of plastic materials or on fixed small volumes in sample cups assumed similar rates of diffusion and did not correct for differences in surface-to-volume ratios. Uettwiller et al. [14] showed that adsorption decreases along with the surface-to-volume ratios (R). For our disks, urine collection cups, and 24-h collection containers, the values of R were 8.36, 1.82 and 0.45 cm2/ml which means that adsorption onto the urine collection cups and 24-h collection container would be even less than that shown by our data. Mixing of urine in a container would increase the rate of diffusion from the bulk solution to the surface which would increase the potential amount adsorbed. Consequently, the adsorption extrapolation to larger containers represented a worse case situation. The surface areas of the plastic disks were all the same (0.836 cm2), however, the actual contact areas varied because the 100 μl of liquid did not completely cover the disk. The differences in contact area were not corrected for, but would have a minimal influence on conclusions because the disks were nearly completely covered with a thin film of liquid.
4.3. Conclusions
Albumin adsorption differed among urine samples and plastic materials, but the total influence of adsorption was <1% for all materials and urine samples tested and unlikely to cause erroneous assessment of an individual’s albumin excretion. Adsorption of albumin from phosphate buffered solutions was larger than from urine and could be a limitation for preparations used as calibrators.
Supplementary Material
Acknowledgments
We thank the NKDEP-IFCC Joint Working Group for Standardization of Urinary Albumin for developing the framework, the National Center for Chronic Disease Prevention and Health Promotion at the CDC for providing funding, and the Newborn Screening and Molecular Biology Branch at the CDC for use of their radiation laboratory. We thank Elizabeth Monsell for assisting with the experimental design, Theodosia Prater for technical assistance and Pamela Olive for assisting with operation of the Hitachi 912.
Abbreviations
- UA
urinary albumin
- HSA
human serum albumin
- IA
immuno-turbidimetric assay
- LSC
liquid scintillation counting
- Sp.Ac
specific activity
- HPET
hydrophilic-coated polyethylene terephthalate
- PE
polyethylene
- PP
polypropylene
- PS
polystyrene
- PET
polyethylene terephthalate
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cca.2014.01.035.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Miller WG, Bruns DE, Hortin GL, Sandberg S, AAkre KM, McQueen MJ, et al. National Kidney Disease Education Program-International Federation of Clinical Chemistry Working Group on Standardization of Albumin in Urine. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009;55(1):24–38. doi: 10.1373/clinchem.2008.106567. [DOI] [PubMed] [Google Scholar]
- 2.Liu F, Zhou M, Zhang F. 125I Labelling of human serum albumin and fibrinogen and a study of protein adsorption properties on the surface of titanium oxide film. Appl Radiat Isot. 1998;49:67–72. doi: 10.1016/s0969-8043(97)00262-5. [DOI] [PubMed] [Google Scholar]
- 3.Sheller NB, Petrash S, Foster MD, Tsukruk VV. Atomic force microscopy and X-ray reflectivity studies of albumin Adsorbed onto self-assembled monolayers of hexadecyltrichlorosilane. Langmuir. 1998;14(16):4535–44. [Google Scholar]
- 4.Sevastianov VI, Pokidysheva EN, Maklakova IA, Bagrov SN. Use of a fluorescence method for probing protein adsorption to the surface of intraocular lenses. Biomed Eng. 1998;32(5):247–50. [Google Scholar]
- 5.Ying P, Viana AS, Abtantes LM, Jin G. Adsorption of human serum albumin onto gold: a combined electrochemical and ellipsometric study. J Colloid Interface Sci. 2004;279(1):95–9. doi: 10.1016/j.jcis.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 6.Holmberg M, Hou X. Competitive protein adsorption–multilayer adsorption and surface induced protein aggregation. Langmuir. 2009;25:2081–9. doi: 10.1021/la8031978. [DOI] [PubMed] [Google Scholar]
- 7.Hara F, Shiba K. Nonspecific binding of urinary albumin on preservation tube. Jpn J Clin Chem. 2003;32(Suppl. 1):28–9. [Google Scholar]
- 8.Zhang M, Ferrari M. Reduction of albumin adsorption onto silicon surfaces by Tween 20. Biotechnol Bioeng. 1997;56:618–25. doi: 10.1002/(SICI)1097-0290(19971220)56:6<618::AID-BIT4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 9.Bakker AJ. Immunoturbidimetry of urinary albumin: prevention of adsorption of albumin; influence of other urinary constituents. Clin Chem. 1986;34(1):82–6. [PubMed] [Google Scholar]
- 10.Goupy J, Creighton L. Introduction to design of experiments with JMP examples. Third. SAS Institute; 2008. [Google Scholar]
- 11.Sviridov D, Hortin GL. Urine albumin measurement: effects of urine matrix constituents. Clin Chim Acta. 2009;404:140–3. doi: 10.1016/j.cca.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Dockal M, Carter DC, Ruker F. Conformational transitions of the three recombinant domains of human serum albumin depending on pH. J Biol Chem. 2000;275(5):3042–50. doi: 10.1074/jbc.275.5.3042. [DOI] [PubMed] [Google Scholar]
- 13.Heerspink HJL, Nauta FL, van der Zee CP, Brinkman JW, Gansevoort RT, de Zeeuw D, et al. Alkalinization of urine samples preserves albumin concentrations during prolonged frozen storage in patients with diabetes mellitus. Diabet Med. 2009;26:556–9. doi: 10.1111/j.1464-5491.2009.02721.x. [DOI] [PubMed] [Google Scholar]
- 14.Uettwiller I, Dubut D, Voute N. Quantification of protein adsorption onto the surface of single-use flexible containers. Bioprocess International. 2006 Jun; (Supplement) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


