Summary
Overuse, which is defined as the provision of medical services that are more likely to cause harm than good, is a global problem that afflicts rich and poor countries alike. This article reviews the definition of overuse, methods for measuring overuse, harms from overuse, and the evidence for worldwide overuse of many types of services.
Introduction
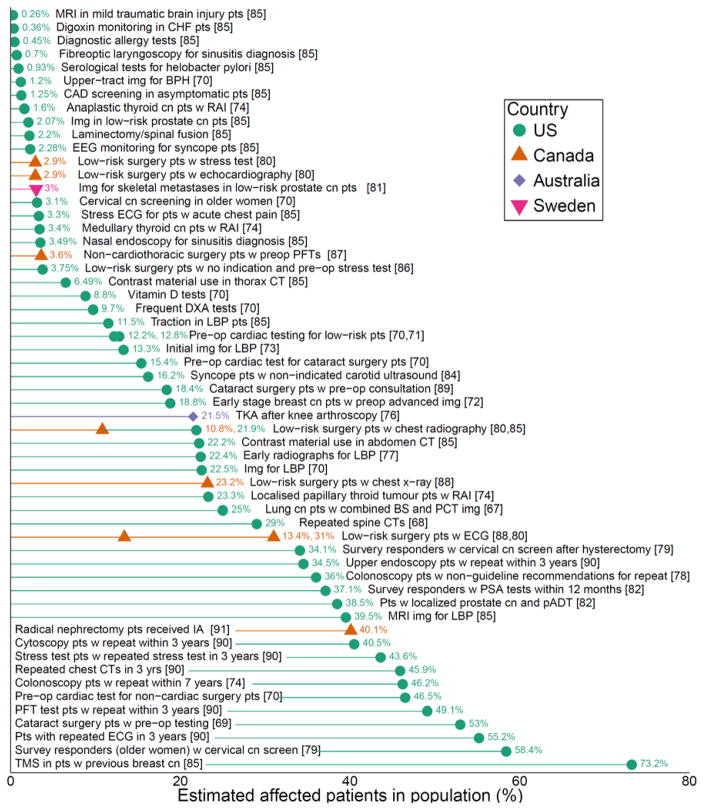
Overuse, which Chassin and Galvin defined as the provision of medical services for which the potential for harm exceeds the potential for benefit,1 is increasingly recognized around the world. Directly measuring overuse requires a definition of appropriate care, which is often challenging. In the United States, estimates of spending on overuse vary widely: conservative estimates based on direct measurement of individual services range from 6% to 8% of total health care spending,2 while studies of geographic variation (an indirect measure) put the proportion of Medicare spending on overuse closer to 29%.3 Around the world, overuse of some individual services may be as high as 80% of cases (see Figure 2: Overuse of Selected Services in Four Countries).4 While overuse has been best documented in high-income countries (HICs), low- and middle-income countries (LMICs) are not immune, and evidence suggestive of widespread overuse is accumulating from countries and health systems as diverse as Australia,5 Spain,6 Israel,7 Brazil,8 and Iran.9 Overuse can coexist with unmet health needs, particularly in LMICs.
Figure 2. Overuse of Selected Services in Four Countries.
Figure 2 explanatory note: Estimates from the literature of the proportion of patients that received various low-value services, out of the relevant patient population. The populations are based in four locations (US: circle/green, Canada: triangle/orange, Australia: diamond/purple, Sweden: upside-down triangle/pink). Abbreviations: Patients (pts); with (w); cancer (cn); imaging (img); preoperative (preop); total knee arthroplasty (TKA); lower back pain (LBP); computed tomography (CT); benign prostate hyperplasia (BPH); primary androgen deprivation therapy (pADT); bone scintigraphy (BS); positron emission tomography (PET); tumour marking studies (TMS); dual-energy x-ray absorptiometry (DXA); echocardiography (ECG); pulmonary function test (PFT); ipsilateral adrenalectomy (IA); radioactive iodine treatment (RAI); carotid artery disease (CAD); congestive heart failure (CHF); magnetic resonance imaging (MRI). Figure adapted and updated from Chalmers and Elshaug.4
The purpose of this paper is to highlight the significance of the problem of overuse and explore what is known about its scope and consequences around the world. We draw on five systematic reviews 4,10–13 on overuse to help inform this paper, supplemented with pearling of reference lists and additional structured searches of scientific and grey literature. Subsequent papers in this series examine underuse around the world, the causes of overuse and underuse, and potential remedies for both.
What is overuse?
“Though the doctors treated him, let his blood, and gave him medications to drink, he nevertheless recovered.”
- Leo Tolstoy, War and Peace
While Chassin and Galvin’s definition of overuse is succinct, and may have broad intuitive appeal, it is difficult to operationalize. To directly measure overuse requires a definition of appropriateness for a service, based on evidence for the balance of benefits and harms for a population or individuals. However, quantifying benefits and harms is often problematic, because evidence of benefits is often incomplete; for many services, harms have been poorly documented;14 and the threshold between appropriate and inappropriate care may vary among patients or groups of patients. In addition, the role of costs in defining low value services varies in different settings (see Box 1: The role of cost in defining overuse and low-value services).
BOX 1. The role of cost in defining overuse and low-value services.
Eliminating clearly ineffective services would reduce both potential harm to patients and excess costs, since ineffective treatments and tests cannot be cost effective. Unfortunately, clearly ineffective services are greatly outnumbered by grey-zone interventions. Many grey-zone interventions benefit very few patients or provide only small benefit relative to costs, and thus are not cost effective. Paying for such low-value services poses an opportunity cost, leaving less money available for addressing unmet health needs and reducing funds to improve the socio-economic determinants of health. While cost-effectiveness analysis, which can quantify these tradeoffs, is formally considered in coverage decisions in some HICs, such as Australia, Canada and the UK 21–23 and in an increasing number of LMICs, 24 it is not included in appropriateness determinations in the US. 25
Ultimately, overuse can be thought to occur along a continuum of services. At one end lie tests and treatments that are universally beneficial when used on the appropriate patient, such as blood cultures in a young otherwise healthy patient with sepsis, and insulin for patients with Type 1 diabetes. At the other end of the continuum are services that are entirely ineffective, futile, or pose such high risk of harm to all patients they should never be delivered, such as the drug combination fenfluramine-phentermine for obesity.15 Most tests and treatments fall into a more nebulous grey zone, 16,17 which includes: services that offer scant benefit to most patients (e.g. glucosamine for osteoarthritis of the knee); those for which the balance of benefits and harms varies substantially among patients (e.g. opioids for chronic pain, antidepressant medications for adolescents); and the many services that are backed by little evidence to help decide which patients, if any, might benefit and by how much (e.g. routine blood testing in patients with hypertension). 18 (See Figure 1: Grey Zone Services.) Even when robust consensus processes have led to criteria defining appropriateness of tests and treatments (such as those developed for cardiology services in the U.S.), appropriateness can remain uncertain in many individual cases.19
Figure 1. Grey Zone Services.
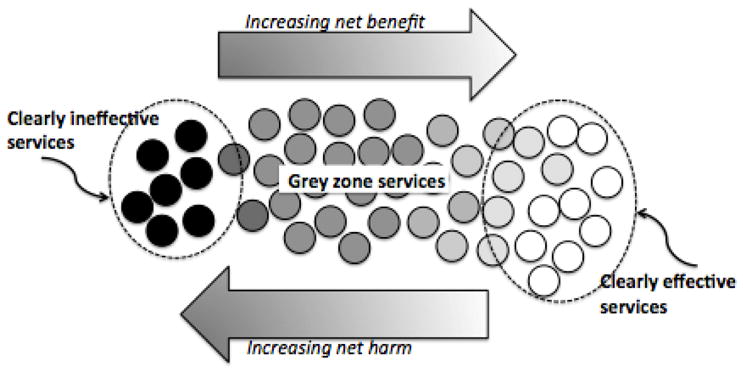
Some medical tests and treatments are of clear benefit, and some are clearly ineffective and therefore offer only net harm. There is clear underuse of effective services, and clear overuse of ineffective services. Many services fall into a more nebulous grey zone, where evidence is lacking, or the services is delivered to inappropriate patients, or to patients who are poorly informed.
Chassin and Galvin’s simple formulation is further muddied by the question of whose values and preferences should determine the balance between potential benefits and acceptable harms. Certainly different patients facing a choice among potentially beneficial treatments will vary in their views of the tradeoffs of each.20 Thus individual patients’ values and preferences may be critical for defining appropriate care for many conditions in the grey zone. Unfortunately clinicians often have poor understanding of patient values, incorrectly assuming in some cases that a patient prefers to avoid aggressive or invasive intervention, and in others that the patient wants more rather than less care. This “preference misdiagnosis” contributes to overuse (and underuse) when clinicians deliver a service that is wrong for that individual patient.
Measuring overuse
Overuse can be measured in a variety of ways. Overuse of a specific service can be measured directly in a population, using patient registries or medical records. This approach requires a reliable definition of appropriateness for a given service, generally using an evidence- or consensus-based guideline, or a multidisciplinary iterative panel process (e.g. the RAND Appropriateness Method) to define necessary and unnecessary use. Rates of overuse are then calculated as either the proportion of delivered services that are inappropriate or as the proportion of patients with a disorder who receive the service inappropriately. This direct measure is the most reliable indicator of overuse, and a growing body of literature, including several systematic reviews, 4,10–13 have employed it. There are, however, several challenges inherent in this approach when applied to many health care interventions.12 First, as discussed above, evidence for defining appropriate care is lacking in many clinical situations, precluding direct measurement of overuse of those services. Second, even if evidence is available, guidelines often lack necessary details for defining the appropriateness of care in individual patients, while iterative panel processes, which incorporate more nuance, are costly and time consuming. Electronic health records (EHR), and the development of large datasets informed by clinical information from EHRs has facilitated measurement of overuse in some contexts (e.g. the U.S. Veteran’s Affairs system26,27) and may have broader applicability in the future. However, EHRs alone are not likely to enable widespread direct measurement of overuse.
A growing literature seeks to expand knowledge of overuse through an indirect measure: identifying unexpected variations in health care utilization. Variations in utilization which are not attributable to differences in patient or population characteristics have been documented both within and among countries and health systems.1–5,8,28,29 While these variations are often not related to overuse (or underuse) per se, but rather to different rates of discretionary care (or services for which the evidence does not point clearly to a right answer,16 such as revisit interval for diabetics), unexpectedly high rates of use of a particular service can reflect overuse.6,7 In more recent years, investigators have used large databases to explore variations in the use of specific services as a method for documenting probable overuse.30,31 Table 1 provides examples of both direct and indirect evidence documenting overuse of specific services around the world. Some investigators have moved beyond individual services to evaluate rates of general overuse in a system by evaluating variations in the use of groups of possibly overused services, 5,32–34 but these methods are not yet well established.
Table 1.
Direct and Indirect Evidence of Overuse Globally
| Clinical Category | Direct documentation of inappropriate care | Indirect evidence |
|---|---|---|
| Musculoskeletal procedures |
Spain: Rates of inappropriate total knee replacement of 26% and of total hip replacement 25% 49 US: Rate of inappropriate total knee replacement 34% 50 |
International: 4-fold variation across countries and 2–3 fold variation within countries in rates of knee replacement 51 England: 13-fold regional variation in rates of arthroscopic knee lavage52 US: 5-fold regional variation in adjusted rates of total hip and knee replacement 53 |
| Cardiovascular procedures |
Italy: Rate of inappropriate percutaneous coronary intervention (PCI) 22% and of inappropriate coronary angiography 30% 54 US: Rate of inappropriate PCI 1.1% for acute indications and 11.6% for non-acute indications with variation across hospitals (6.0%–16.7%) 55 Brazil: rate of inappropriate coronary angiography 20% 56 |
International 9-fold variation in use of PCI and 5-fold variation in use of CABG across OECD countries 28 US: Rates of elective percutaneous coronary interventions vary 10-fold within the state of California57 India: A second opinion centre reported recommending against cardiac interventions in 55% of patients in whom intervention initially recommended58 |
| Hysterectomy |
Taiwan59: 20% of hysterectomies inappropriate Switzerland60: 13% of hysterectomies inappropriate US61: rates of inappropriate hysterectomies between 16% and 70% across studies |
Canada62: 2.7-fold variation in rates of hysterectomy across regions within Ontario Netherlands63: 2.2-fold regional variation in rates of hysterectomy of bleeding; 2.3-fold regional variation in rates for pelvic organ prolapse India64: prevalence of up to 9.8% overall, with 1/3 of hysterectomies performed in women under age 35 (likely inappropriate in this age group) |
| Antibiotics for acute diarrhea |
Italy65: among children hospitalized for acute diarrhea, 9% received antibiotics inappropriately China66: 57% received antibiotics inappropriately; among those with an indication for antibiotics, 21% were NOT treated (adults) Thailand67: 55% of children with acute diarrhea received antibiotics inappropriately |
US68: 10.4% of patients with diarrhea received antibiotics (often likely inappropriate) India69: 71% of children with acute diarrhea received antibiotics (despite recommendation against routine use) India70: Rates of antibiotic use for acute diarrhea 43% in public facilities and 69% in private facilities (despite recommendation against routine use) |
Related concepts
We use the term “overuse” to refer to any services that are unnecessary in any way and for any reason. The related terms, “overtreatment” and “overtesting,” indicate the inappropriate delivery of particular types of services.
Another related term, overdiagnosis, is commonly described as the diagnostic labeling of abnormalities or symptoms that are indolent, non-progressive or regressive, and that if left untreated or treated later will not cause significant distress or shorten the person’s life.35 This definition can be complicated by the varying natural history of specific diseases, and does not entirely encompass the various settings in which overdiagnosis occurs or the role it plays in overuse. 36 Overdiagnosis can occur as a consequence of screening (including recommended screening). For some screening tests, such as cervical cancer screening,37 the small risk of overdiagnosis and subsequent overtreatment are outweighed by the reduction in risk of death. For other screening tests, however, the balance is less clear38 and overdiagnosis may be a significant driver of overuse in the form of aggressive overtreatment of clinically insignificant findings.5,39 (Paper 3 in this series, “Drivers of Poor Medical Care,” will discuss in greater detail overdiagnosis and other drivers of overuse, such as defensive medicine, which has been associated with aggressive diagnostic testing in the U.S.40 and has been identified by physicians in several countries41–43 as an important reason for overusing tests and treatments.)
Overdiagnosis can also result when the definition of disease or abnormality is widened, leading to populations previously considered “normal” or healthy being labeled as diseased. This phenomenon is referred to as overmedicalization (or in some cases disease mongering) and can result in treatment of essentially healthy patients in whom potential benefit is small and likely to be outweighed by harms. A review of recent US guidelines showed that for ten of the 16 guidelines studied, disease definition had been widened, potentially leading to overuse.44 Lowering risk thresholds for treating cholesterol has led to a growing proportion of populations in many countries being prescribed lipid-lowering drugs with unclear benefit.45,46 A broadened definition of chronic kidney disease that is used in many countries, while potentially beneficial for ensuring safe drug dosing, has led to large numbers of asymptomatic older people being labeled as ill; as many as 30% of older adults diagnosed with moderately advanced kidney disease (stage 3A) have no urine markers of kidney damage.47 In children, overdiagnosis can occur in such frequently diagnosed conditions as Attention Deficit Hyperactivity Disorder (ADHD), bacteremia, food allergy, hyperbilirubinemia, obstructive sleep apnea, and urinary tract infection.48
Worldwide prevalence of overuse
There is increasing recognition that overuse is a problem around the world, but how significant the problem is has not yet been defined. A 2012 systematic review of the prevalence of the overuse of services in the US noted that the majority of studies that directly measured overuse were focused on a relatively small number of services.12 However, indirect evidence, such as studies of geographic variation, suggests that overuse is not limited to these services in the U.S. 71 A more recent systematic review4 of global overuse categorized 83 overused or low-value services from studies including large sample sizes (more than 800 patients).27,72–95 These authors identified studies from four countries (with US studies predominating) and found that rates of overuse of various services ranged from about 1 to 80 percent (see Figure 2). For many HICs and for LMICs, the evidence of overuse is sparser and largely indirect, though it appears to be growing. (See for example a 2014 report on geographic variation in health care in 13 countries.51) Below, we describe rates of overuse around the world of a selection of clinical services. We focused our attention on services that have been most commonly described in systematic reviews and other literature, and services whose overuse has the potential for significant impact on patients or health systems.
Overuse of Medications
One of the best-documented examples of overuse of medications in both HICs and LMICs is the inappropriate use of antibiotics, a worldwide problem with significant consequences for antimicrobial resistance. Many studies have addressed inappropriate antibiotic use in patients with viral upper respiratory infections. A 2012 systematic review of overuse in the US system found 59 studies documenting widely variable rates of overuse of antibiotics for upper respiratory infections.12 In Europe, there are high rates of antibiotic prescribing for viral URIs in Poland, Sweden, and the UK with half of patients receiving unnecessary antibiotics,96–98 and across the continent studies have documented variable rates of antibiotic prescribing for patients with acute cough, with no associated differences in rates of recovery,99 suggesting overuse.
Evidence of antibiotic overuse in LMICs is largely indirect. Global consumption of antibiotic drugs increased by 36% between 2000 and 2010, with emerging economies such as Brazil, Russia, India, China, and South Africa accounting for 76% of this increase.100 The extent to which this increase represents overuse is not known, though a 2015 systematic review of medication use in China and Vietnam found evidence for antibiotic overuse in both countries13 and a 2005 systematic review 11 of patterns of antibiotic use, which included studies from around the globe, found high rates of inappropriate use including substantial patient use of “leftover” antibiotics. Similarly, a 2013 Cochrane review of the effect of interventions to improve antibiotic prescribing for hospitalised patients included studies from both HICs and LMICs, suggesting wide recognition of the problem of inappropriate antibiotic use, though the review did not directly quantify rates.101
In other clinical arenas, unexpectedly high rates of prescribing of specific drugs in individual health systems suggest overuse. Bevacizumab, an expensive and generally ineffective treatment for breast cancer, is not recommended by the National Institute for Health and Care Excellence (NICE) in the UK and its US Food and Drug Administration marketing authorisation for breast cancer was withdrawn. However, the drug is reimbursed by health insurers in Colombia for all (licensed and unlicensed) cancer indications at great expense to the country’s health care system.102 Similarly, erythropoiesis stimulating agents such as epoetin a and b and darbapoetin have been widely and inappropriately used in Romania to treat ribavirin-induced anaemia in patients with Hepatitis C and organ transplantations in the absence of supporting evidence.103
Overuse of screening tests
High rates of inappropriate use of screening tests have been documented, often in the context of concurrent underuse of the test in appropriate populations. In the US, where there is widespread public support for cancer screening,104 there has been documented overuse of screening for cervical cancer 105,106 in very low risk women and of mammography in women with limited life expectancy who are unlikely to benefit from diagnosis and treatment. 107 Inappropriate use of screening colonoscopy has been found in both the US and Canada. 108–110
Few studies have evaluated rates of inappropriate cancer screening outside of North America. A notable exception is South Korea’s aggressive use of ultrasound screening, which has led to a 15-fold increase in incidence of papillary thyroid cancers. The death rate from this cancer has remained unchanged over the period of increased screening, and it is estimated that 99.7–99.9% of screen-detected thyroid cancers in Korea represent overdiagnosis.111 Patients then subjected to unnecessary thyroidectomy face an 11% risk of hypoparathyroidism and 2% risk of vocal cord paralysis, demonstrating clear downstream harms of the inappropriate screening. Despite low levels of appropriate mammography screening and general doubts about the cost-effectiveness of mammograms,112 there are reports of touring mammography vans in India providing indiscriminate breast cancer screening in women as young as 18 years old,113 much of which represents clear overuse.
Overuse of diagnostic tests
Overuse of testing appears to be common, driven by availability, apparent objectiveness, and the increasing sensitivity of tests to detect disease. Despite few systematic analyses of inappropriate use of diagnostic tests in general, some specific diagnostic services have been evaluated around the world. Overuse of endoscopy, for instance, appears to be common globally. In primary care practices in Switzerland, 14% of colonoscopy referrals and 49% of referrals for upper endoscopy represented overuse.114,115 Elsewhere in Europe, appropriateness rates for endoscopy have been reported in Portugal, Spain, Italy, and Norway, with overuse accounting for between 13% and 33% of tests, 116–119 and at an Israeli center 16% of endoscopies were unnecessary.120 Studies in the US have reported overuse rates as high as 60%121. In Saudi Arabia, which has open access to endoscopy, nearly half of procedures were inappropriate.122 Interestingly, a Dutch study found that only about a quarter of patients received appropriate colonoscopy after removal of colorectal adenomas, with both overuse and underuse of needed surveillance observed.123
Overuse of therapeutic procedures
Surgery and other invasive procedures are likely to be commonly overused in high-income countries. Though rates of directly-measured overuse were not reported, Elshaug and colleagues identified more than 150 “low-value” services in use in Australia,5 and in the US, up to 42% of Medicare beneficiaries were found to have received at least one of 26 low-value treatments, with these low-value interventions accounting for 2.7% of overall Medicare spending. 32 Such findings are suggestive of widespread overuse of these services.
There is ample data from around the world on the overuse of several cardiovascular procedures, despite clear and broadly accepted appropriateness criteria.124 Inappropriate percutaneous coronary intervention has been documented in many countries, with prevalence of 4–12% in the US; 55,125 10%–14% in Germany, 126,127 16% in Italy; 128 22% in Israel;7 20% in Spain; 6 and 3.7% in Korea. 129 In one second-opinion centre in India, 55% of recommended cardiac stents or surgery were deemed inappropriate. 58
Site of care delivery
The site of care delivery and the intensity of care provided are relevant to overuse since more intense care poses greater risk of complications (and is more costly). If more intense care does not improve outcomes for a condition compared with less invasive or intensive care, it represents overuse. Hospital care has been found to be overused in both HICs and LMICs. A 2000 systematic review 10 of the appropriateness of hospital admissions found widely varying rates of inappropriate hospitalizations around the world, ranging from 1% to 54% of hospitalizations. Rates of overuse of hospital care in specific countries (using established criteria to determine appropriateness) were 18–25% in France,130 33% in Germany,131 19% among internal medicine admissions in Portugal,132 7.4% at a referral center in Spain,133 27% in rural hospitals in China,134 and widely variability across three Egyptian hospitals, with rates between 0% and 79%.135 In addition, studies have shown wide variations in rates of hospital use both within and among countries,136,137 suggesting possible overuse as well as underuse of hospital care in different locations. Many of these variations are particularly striking with regard to “ambulatory care-sensitive” conditions, or conditions for which high-quality primary care is likely to prevent the need for hospitalization.138 Overuse of hospitalization for ambulatory care-sensitive conditions demonstrates that overuse of one (usually more aggressive) service can result from underuse of another, often less aggressive, service.
End of life care
In many countries, evidence exists for the overuse of aggressive care for dying patients and simultaneous underuse of appropriate palliative care. Despite evidence that the majority of people around the world prefer to die at home, 139–144 about half die in hospital worldwide, with considerable variation among countries. 145 Inappropriately aggressive cancer care near the end of life has been identified as a common problem in Canada,146 the US,147 and the UK 148 with regional variations observed.147 Overuse of aggressive end-of-life care in the UK, for example, has included futile insertion of PEG tubes 149 and administration of chemotherapy that hastens death, 150 while futile ICU care at the end of life has been reported in Canada,151 the US, 152 and Brazil. 153 A study from Korea found that the majority of terminal cancer patients received futile intravenous nutrition in the last week of life, with discussions of palliation in only 7% of cases. 154
While few systematic assessments of end-of-life care have been performed in LMICs, futile care at the end of life is likely not limited to high-income countries. In one study in India, nearly half of cancer patients were diagnosed late and received futile radiotherapy.155 In Brazil, one in five cancer patients was taking a useless medication, most often a statin.156 Overall it is likely that overuse of aggressive care and underuse of palliative care at the end of life is commonplace in both HICs and LMICs.
Harms to patients and health systems
Overuse is likely to harm patients physically, psychologically and financially, and could threaten the viability of health systems by driving up costs and diverting resources. However, our ability to collect strong evidence describing the direct consequences of overuse on patients and health systems has been impeded by the same factors that challenge our ability to document overuse itself, including an incomplete evidence base for effectiveness and little reporting of harms from treatments.157 Much of what we know about harms of overuse derives from estimates and extrapolations.
Harms to patients
There are few studies directly documenting patient harms from overuse, though some estimates of the rate of physical harm to patients from overuse can be inferred from data on adverse events and studies of overuse of specific treatments. For example, Cushner et. al used outcomes from a global orthopaedic registry for total knee and hip arthroplasty to estimate a rate of 7–8% for serious adverse events (including severe infection, revision, cardiovascular events and death).158 Other researchers estimate that more than 20% of total knee replacements in Spain and 30% in the US are inappropriate. 50,159 Thus we can estimate that 2–3% of patients undergoing arthroplastic surgery in those two countries are unnecessarily harmed by an inappropriate procedure, with approximately 14,000 patients suffering harm from unnecessary knee and hip arthroplasty per year in the US alone. Other examples of documented harm from overuse include high rates of overuse of implantable vena cava filters and low rates of appropriate removal,160 with known excess venous thrombotic complications in 10% of patients who receive them;161 and continued overuse of tight glycemic control in the intensive care unit despite evidence of higher rates of hypoglycemic complications without reductions in mortality.162
Psychological harms from overuse have been documented for few clinical situations but may be common. Several authors have noted that hospitalized patients may be physically isolated unnecessarily,163 with negative consequences including loneliness, feelings of stigmatization, and depression.164 Screening for breast cancer is known to lead to diagnosis of precancerous lesions such as ductal carcinoma in situ,165 which has been associated with anxiety for several years after the diagnosis and patient overestimation of future cancer risk.166–168
Patients can also suffer from being inappropriately labeled as “ill” as a result of unnecessary testing. As early as 1967, Bergman and Stamm found that among adolescents with heart murmurs which had been previously (and possibly unnecessarily) evaluated and deemed “innocent,” 40% continued to be subjected to restricted activity and 63% had parents who continued to believe their child to be unhealthy. 169 Harm from labeling can also occur in the context of mental illness. For example, ADHD is widely acknowledged to be overdiagnosed and overtreated in the US and other HICs, and is also overtreated in some LMICs 170 (even as some children with ADHD fail to receive appropriate treatment). There is scant research on the impact of an ADHD diagnosis on childrens’ sense of self-esteem and ability to modulate their own behavior, but the label has been shown to impact teacher expectations and peer interactions, which can substantially influence children’s self-perceptions. 171–173
Financial costs represent a potentially important but poorly documented source of harm from overuse to patients. In the US, cost has been framed as a known consequence of all medical care 174 and of cancer treatment in particular,175 with medical bills contributing to over half of personal bankruptcies,176 although the contribution of overuse is not known. Similarly, in Australia, parents of children with cancer reported high out of pocket expenses,177 and the World Health Organization has decried “medical indebtedness” across the globe. Health care is a major source of impoverishment and indebtedness among the poor of India 178,179 and 15% of rural Vietnamese families with one member with a chronic illness experience financial catastrophe.180 Determining the financial burden of overuse on patients requires active investigation in the future.
Harms to health care systems
While there are few direct measurements of the proportion of health care spending attributable to overuse, evidence is emerging to suggest the cost may be considerable. A study of inappropriate use of bone scans for US Medicare beneficiaries with prostate cancer found that 21% and 48% of patients at low and moderate risk of bony metastases underwent at least one scan, despite recommendations against scanning in these groups, at a cost of $11,300,000 annually.181 High rates of overuse are estimated by experts to contribute substantially to health care spending in the US (and to its mediocre quality);182 based on a conservative estimate, the US spent at least $270 billion on overuse in 2013 2 (even as millions of Americans lack adequate access to basic health care). Overuse may also strains health care budgets in other countries.183 In Australia, where many common services are believed to be overused,5 the growth in health care expenditure from the rising volume of medical services has been identified as the greatest threat to the financial position of the government, and a bigger cause of health cost increases than population growth or ageing.184
Of particular concern is the potential financial impact of overuse on LMICs. The use of expensive advanced technology in HICs, such as new cancer biologics, imaging devices, and multi-focal cataract replacement lenses, spreads through globalized markets to LMICs, potentially crowding out less technological (and potentially higher value) means of promoting population health.185 In India, for example, private health insurance and formal sector employees’ insurance programs cover expensive cancer drugs for a tenth of the country’s population, even as the general population does not have access to many basic health interventions.178 While the extent to which the use of expensive services represents true overuse as opposed to lower-value care from a public health perspective is not clear, overuse is a potential threat both to the viability of public budgets and to population health in LMICs.
Worldwide trends in overuse
Is overuse getting better or worse? It is a difficult question to answer for a number of reasons. First, we are only beginning to conceptualize overuse as a general system problem and to develop system-level metrics.186 There are no measures in general use. Second, health care systems are complex and dynamic;187 reducing or eliminating overuse of one service or in one site of care can encourage overuse in another.
We do know that there has been increased attention among health ministers, clinicians, policy makers and the public to overuse during the last 5 to 10 years, particularly in HICs but also in some LMICs. However, awareness of the problem has not automatically led clinicians to deliver the right care. In the US, for example, concerns about caesarean delivery rates have existed for decades but rates continued to rise (from 21% in 1996 to 31% in 2006 188). Despite longstanding concerns about overuse of imaging with CT and MRI, their use has increased by 8% to 10% annually between 1996 and 2010.189
In LMICs, overuse appears to be on the rise, at least for certain services. For example, rates of caesarean delivery rose from 19% to 49% among low-risk deliveries in Tanzania between 2000 and 2011,190 with rates also rising over time in India, Nepal, and Bangladesh. 191 Financial incentives and government policies can contribute to dramatic overuse. In China, government cuts in subsidies led hospitals to charge patients for care, 192,193 potentially contributing to notably high rates of cesarean delivery (46% in one study in a rural area).194 Amid allegations of frank physician corruption and kickbacks from the pharmaceutical industry and diagnostic centres, there are reports from India of inappropriate use of drugs, diagnostic tests and procedures, 195 including strikingly high rates of hysterectomies.196 These trends appear to be recent and likely reflect increases in overuse over the last decade, but there are few data documenting longitudinal changes.
Wealthy countries are experimenting with specific initiatives to address overuse, such as NICE’s “do not do” list, 197 attention to low-value practices in Australia, 5 and the Choosing Wisely® campaign, 198 but there are few studies in either HICs or LMICs addressing the impact of such initiatives. Additionally, EHRs have been used as tools to reduce overuse locally199 and could be employed more broadly in the future. The last paper in this series, “Levers for Addressing Medical Underuse and Overuse: Achieving High-Value Health Care,” reviews efforts around the world to reduce overuse.
Conclusion
There is strong evidence of widespread overuse of several specific services in multiple countries, suggesting that overuse is common around the world and may be growing. However, this paper highlights a key challenge: measuring overuse and developing robust evidence for its prevalence in health services and patient populations. There is a clear need for a research agenda to develop that evidence.14 Given that overuse likely causes harm to both patients and health systems, physicians, politicians and policy-makers in both HICs and LMICs must understand overuse and act to reduce it.
Key Messages.
Overuse is difficult to measure and has not been well characterized.
Most studies of overuse come from high-income countries, but there is growing evidence that overuse is a global problem.
Overuse likely causes patients physical, psychological and financial harm.
Overuse deflects resources from public health and other social spending in both poor and wealthy countries.
Overuse occurs across a wide range of medical specialties.
Acknowledgments
Work for this paper was supported by The Commonwealth Fund, a national, private foundation based in New York City that supports independent research on health care issues and makes grants to improve health care practice and policy. The views presented here are those of the authors and not necessarily those of The Commonwealth Fund, its directors, officers, or staff. The authors are indebted to Sarah Quddusi and Yi Wang for assistance with references; Prakash Shakya for researching the international literature on cardiology and overuse at the end of life; and Joseph Colucci and Carissa Fu for technical assistance.
Footnotes
Contributors
All authors participated in the development of the report, including conception, provision of data and references, writing of the manuscript, revision of the draft, and approval of the final version. SB and DK wrote drafts, which were improved and revised by all other authors. KChalmers developed Figure 2.
Declaration of Interests
AGE receives salary support as the HCF Research Foundation Principal Research Fellow, and holds research grants from The Commonwealth Fund and Australia’s National Health and Medical Research Council (ID 1109626 and 1104136). AGE receives consulting/sitting fees from Cancer Australia, the Capital Markets Cooperative Research Centre-Health Quality Program, NPS MedicineWise (facilitator of Choosing Wisely Australia), The Royal Australasian College of Physicians (facilitator of the EVOLVE program) and the Australian Commission on Safety and Quality in Health Care. JD reports grants from NHMRC. VS and SB receive support from a grant from the Robert Wood Johnson Foundation. DK was supported by a Cancer Center Support Grant from the National Cancer Institute to Memorial Sloan Kettering Cancer Center (award number P30 CA008748). KC, KChalmers, SN, DS, PG, and IH report nothing to disclose.
Views expressed by the authors are their own and do not necessarily represent the views of their employing, affiliated or associated organisations.
References
- 1.Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA : the journal of the American Medical Association. 1998;280(11):1000–5. doi: 10.1001/jama.280.11.1000. [DOI] [PubMed] [Google Scholar]
- 2.Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA : the journal of the American Medical Association. 2012;307(14):1513–6. doi: 10.1001/jama.2012.362. [DOI] [PubMed] [Google Scholar]
- 3.Wennberg JE, Fisher ES, Skinner JS. Geography and the debate over Medicare reform. Health affairs. 2002;(Suppl Web Exclusives):W96–114. doi: 10.1377/hlthaff.w2.96. [DOI] [PubMed] [Google Scholar]
- 4.Chalmers K, Elshaug AG. An inventory of low-value care measured from two perspectives: Inappropriate care versus wasted services. 2016 Unpulbished Manuscript Under Review. [Google Scholar]
- 5.Elshaug AG, Watt AM, Mundy L, Willis CD. Over 150 potentially low-value health care practices: an Australian study. Med J Aust. 2012;197(10):556–60. doi: 10.5694/mja12.11083. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar MD, Fitch K, Lazaro P, Bernstein SJ. The appropriateness of use of percutaneous transluminal coronary angioplasty in Spain. International journal of cardiology. 2001;78(3):213–21. doi: 10.1016/s0167-5273(01)00385-0. discussion 21–3. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg A, Yalonetsky S, Kopeliovich M, Azzam Z, Markiewicz W. Appropriateness of diagnosis of unstable angina pectoris in patients referred for coronary arteriography. Experimental and clinical cardiology. 2008;13(3):133–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Barros AJ, Santos IS, Matijasevich A, et al. Patterns of deliveries in a Brazilian birth cohort: almost universal cesarean sections for the better-off. Revista de saude publica. 2011;45(4):635–43. doi: 10.1590/s0034-89102011005000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahadori F, Hakimi S, Heidarzade M. The trend of caesarean delivery in the Islamic Republic of Iran. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2014;19(Suppl 3):S67–70. [PubMed] [Google Scholar]
- 10.McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds. A systematic review of methods and results. Health Policy. 2000;53(3):157–84. doi: 10.1016/s0168-8510(00)00092-0. [DOI] [PubMed] [Google Scholar]
- 11.Kardas P, Devine S, Golembesky A, Roberts C. A systematic review and meta-analysis of misuse of antibiotic therapies in the community. Int J Antimicrob Agents. 2005;26(2):106–13. doi: 10.1016/j.ijantimicag.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Korenstein D, Falk R, Howell EA, Bishop T, Keyhani S. Overuse of health care services in the United States: an understudied problem. Arch Intern Med. 2012;172(2):171–8. doi: 10.1001/archinternmed.2011.772. [DOI] [PubMed] [Google Scholar]
- 13.Mao W, Vu H, Xie Z, Chen W, Tang S. Systematic review on irrational use of medicines in China and Vietnam. PloS one. 2015;10(3):e0117710. doi: 10.1371/journal.pone.0117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan DJ, Brownlee S, Leppin AL, et al. Setting a research agenda for medical overuse. Bmj. 2015;351:h4534. doi: 10.1136/bmj.h4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachdev M, Miller WC, Ryan T, Jollis JG. Effect of fenfluramine-derivative diet pills on cardiac valves: a meta-analysis of observational studies. Am Heart J. 2002;144(6):1065–73. doi: 10.1067/mhj.2002.126733. [DOI] [PubMed] [Google Scholar]
- 16.Sirovich B, Gallagher PM, Wennberg DE, Fisher ES. Discretionary decision making by primary care physicians and the cost of U.S. Health care. Health affairs (Project Hope) 2008;27(3):813–23. doi: 10.1377/hlthaff.27.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra AS, JS Technology Growth and Expenditure Growth in Health Care. Journal of Economic Literature. 2012;50(3):645–80. [Google Scholar]
- 18. [accessed February 8, 2016];BMJ Clinical Evidence. http://clinicalevidence.bmj.com/x/index.html.
- 19.Huang X, Rosenthal MB. Overuse of Cardiovascular Services: Evidence, Causes, and Opportunities for Reform. Circulation. 2015;132(3):205–14. doi: 10.1161/CIRCULATIONAHA.114.012668. [DOI] [PubMed] [Google Scholar]
- 20.Blank T, Graves K, Sepucha K, Llewellyn-Thomas H. Understanding treatment decision making: contexts, commonalities, complexities, and challenges. Ann Behav Med. 2006;32(3):211–7. doi: 10.1207/s15324796abm3203_6. [DOI] [PubMed] [Google Scholar]
- 21.Lopert R. Evidence-based decision-making within Australia’s pharmaceutical benefits scheme. Issue Brief (Commonw Fund) 2009;60:1–13. [PubMed] [Google Scholar]
- 22.Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7(5):518–28. doi: 10.1111/j.1524-4733.2004.75003.x. [DOI] [PubMed] [Google Scholar]
- 23.Rocchi A, Menon D, Verma S, Miller E. The role of economic evidence in Canadian oncology reimbursement decision-making: to lambda and beyond. Value Health. 2008;11(4):771–83. doi: 10.1111/j.1524-4733.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- 24.Shillcutt SD, Walker DG, Goodman CA, Mills AJ. Cost effectiveness in low- and middle-income countries: a review of the debates surrounding decision rules. Pharmacoeconomics. 2009;27(11):903–17. doi: 10.2165/10899580-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold MR, Sofaer S, Siegelberg T. Medicare and cost-effectiveness analysis: time to ask the taxpayers. Health affairs (Project Hope) 2007;26(5):1399–406. doi: 10.1377/hlthaff.26.5.1399. [DOI] [PubMed] [Google Scholar]
- 26.Partin MR, Powell AA, Bangerter A, et al. Levels and variation in overuse of fecal occult blood testing in the Veterans Health Administration. J Gen Intern Med. 2012;27(12):1618–25. doi: 10.1007/s11606-012-2163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson MR, Grubber J, Grambow SC, et al. Physician Non-adherence to Colonoscopy Interval Guidelines in the Veterans Affairs Healthcare System. Gastroenterology. 2015;149(4):938–51. doi: 10.1053/j.gastro.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Corallo AN, Croxford R, Goodman DC, Bryan EL, Srivastava D, Stukel TA. A systematic review of medical practice variation in OECD countries. Health Policy. 2014;114(1):5–14. doi: 10.1016/j.healthpol.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Sirovich BE, Woloshin S, Schwartz LM. Too Little? Too Much? Primary care physicians’ views on US health care: a brief report. Archives of internal medicine. 2011;171(17):1582–5. doi: 10.1001/archinternmed.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makarov DV, Soulos PR, Gold HT, et al. Regional-Level Correlations in Inappropriate Imaging Rates for Prostate and Breast Cancers: Potential Implications for the Choosing Wisely Campaign. JAMA Oncol. 2015;1(2):185–94. doi: 10.1001/jamaoncol.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handelsman DJ. Pharmacoepidemiology of testosterone prescribing in Australia, 1992–2010. Med J Aust. 2012;196(10):642–5. doi: 10.5694/mja11.11277. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz AL, Landon BE, Elshaug AG, Chernew ME, McWilliams JM. Measuring low-value care in Medicare. JAMA Intern Med. 2014;174(7):1067–76. doi: 10.1001/jamainternmed.2014.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nassery N, Segal JB, Chang E, Bridges JF. Systematic overuse of healthcare services: a conceptual model. Appl Health Econ Health Policy. 2015;13(1):1–6. doi: 10.1007/s40258-014-0126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatia RS, Levinson W, Shortt S, et al. Measuring the effect of Choosing Wisely: an integrated framework to assess campaign impact on low-value care. BMJ Qual Saf. 2015;24(8):523–31. doi: 10.1136/bmjqs-2015-004070. [DOI] [PubMed] [Google Scholar]
- 35.Moynihan R, Henry D, Moons KG. Using evidence to combat overdiagnosis and overtreatment: evaluating treatments, tests, and disease definitions in the time of too much. PLoS medicine. 2014;11(7):e1001655. doi: 10.1371/journal.pmed.1001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carter SM, Rogers W, Heath I, Degeling C, Doust J, Barratt A. The challenge of overdiagnosis begins with its definition. BMJ. 2015;350:h869. doi: 10.1136/bmj.h869. [DOI] [PubMed] [Google Scholar]
- 37.Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Systematic reviews. 2013;2:35. doi: 10.1186/2046-4053-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Etzioni R, Gulati R, Mallinger L, Mandelblatt J. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Intern Med. 2013;158(11):831–8. doi: 10.7326/0003-4819-158-11-201306040-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Independent UKPoBCS. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–86. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 40.Carrier ER, Reschovsky JD, Katz DA, Mello MM. High physician concern about malpractice risk predicts more aggressive diagnostic testing in office-based practice. Health affairs (Project Hope) 2013;32(8):1383–91. doi: 10.1377/hlthaff.2013.0233. [DOI] [PubMed] [Google Scholar]
- 41.Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081–3. doi: 10.1001/archinternmed.2010.155. [DOI] [PubMed] [Google Scholar]
- 42.Hiyama T, Yoshihara M, Tanaka S, et al. Defensive medicine practices among gastroenterologists in Japan. World J Gastroenterol. 2006;12(47):7671–5. doi: 10.3748/wjg.v12.i47.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elli L, Tenca A, Soncini M, Spinzi G, Buscarini E, Conte D. Defensive medicine practices among gastroenterologists in Lombardy: between lawsuits and the economic crisis. Dig Liver Dis. 2013;45(6):469–73. doi: 10.1016/j.dld.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Moynihan RN, Cooke GP, Doust JA, Bero L, Hill S, Glasziou PP. Expanding disease definitions in guidelines and expert panel ties to industry: a cross-sectional study of common conditions in the United States. PLoS medicine. 2013;10(8):e1001500. doi: 10.1371/journal.pmed.1001500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Staa TP, Smeeth L, Ng ES, Goldacre B, Gulliford M. The efficiency of cardiovascular risk assessment: do the right patients get statin treatment? Heart. 2013;99(21):1597–602. doi: 10.1136/heartjnl-2013-303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polinski JM, Donohue JM, Kilabuk E, Shrank WH. Medicare Part D’s effect on the under- and overuse of medications: a systematic review. Journal of the American Geriatrics Society. 2011;59(10):1922–33. doi: 10.1111/j.1532-5415.2011.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moynihan R, Glassock R, Doust J. Chronic kidney disease controversy: how expanding definitions are unnecessarily labelling many people as diseased. BMJ. 2013;347:f4298. doi: 10.1136/bmj.f4298. [DOI] [PubMed] [Google Scholar]
- 48.Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013–23. doi: 10.1542/peds.2014-1778. [DOI] [PubMed] [Google Scholar]
- 49.Cobos R, Latorre A, Aizpuru F, et al. Variability of indication criteria in knee and hip replacement: an observational study. BMC Musculoskelet Disord. 2010;11:249. doi: 10.1186/1471-2474-11-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134–43. doi: 10.1002/art.38685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geographic Variations in Health Care: What Do We Know and What Can Be Done to Improve Health System Performance? 2014. Paris: OECD Publishing; 2014. [Google Scholar]
- 52.The NHS Atlas of Variation in Healthcare. Reducing unwarranted variation to increase value and improve quality. National Health Service (NHS); 2011. [Google Scholar]
- 53.Tomek IM, Goodman DC, Esty AR, Bell J-E, Fisher ES. Trends and Regional Variation in Hip, Knee and Shoulder Replacement. Dartmouth Atlas Surgery Report. 2010 [PubMed] [Google Scholar]
- 54.Carpeggiani C, Marraccini P, Morales MA, Prediletto R, Landi P, Picano E. Inappropriateness of cardiovascular radiological imaging testing; a tertiary care referral center study. PloS one. 2013;8(11):e81161. doi: 10.1371/journal.pone.0081161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan PS, Patel MR, Klein LW, et al. Appropriateness of percutaneous coronary intervention. JAMA : the journal of the American Medical Association. 2011;306(1):53–61. doi: 10.1001/jama.2011.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gontijo RV, Proietti FA, Amaral CF, de Rezende NA. Appropriateness use of coronary angiography in patients with suspected ischemic heart disease in Brazil. International journal of cardiology. 2005;104(3):348–9. doi: 10.1016/j.ijcard.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 57.Brownlee S, Hurley V. Elective Heart Procedures in California: A Close-Up of Geographic Variation. California Healthcare Foundation; 2014. [Google Scholar]
- 58.Iyer M. The Times of India. Jan 4, 2015. 44% advised unnecessary surgery: 2nd opinion-givers. [Google Scholar]
- 59.Chao YM, Tseng TC, Su CH, Chien LY. Appropriateness of hysterectomy in Taiwan. J Formos Med Assoc. 2005;104(2):107–12. [PubMed] [Google Scholar]
- 60.Schilling J, Abou Hadeed M, Fink D, et al. Evaluation of Swiss guidelines for the indication for hysterectomy in relation to patient outcome. Gynakol Geburtshilfliche Rundsch. 2009;49(4):315–9. doi: 10.1159/000301104. [DOI] [PubMed] [Google Scholar]
- 61.Lawson EH, Gibbons MM, Ingraham AM, Shekelle PG, Ko CY. Appropriateness criteria to assess variations in surgical procedure use in the United States. Arch Surg. 2011;146(12):1433–40. doi: 10.1001/archsurg.2011.581. [DOI] [PubMed] [Google Scholar]
- 62.Hall RE, Cohen MM. Variations in hysterectomy rates in Ontario: does the indication matter? Cmaj. 1994;151(12):1713–9. [PMC free article] [PubMed] [Google Scholar]
- 63.Hanstede MM, Burger MJ, Timmermans A, Burger MP. Regional and temporal variation in hysterectomy rates and surgical routes for benign diseases in the Netherlands. Acta Obstet Gynecol Scand. 2012;91(2):220–5. doi: 10.1111/j.1600-0412.2011.01309.x. [DOI] [PubMed] [Google Scholar]
- 64.Desai S, Sinha T, Mahal A. Prevalence of hysterectomy among rural and urban women with and without health insurance in Gujarat, India. Reprod Health Matters. 2011;19(37):42–51. doi: 10.1016/S0968-8080(11)37553-2. [DOI] [PubMed] [Google Scholar]
- 65.Lo Vecchio A, Liguoro I, Bruzzese D, et al. Adherence to guidelines for management of children hospitalized for acute diarrhea. Pediatr Infect Dis J. 2014;33(11):1103–8. doi: 10.1097/INF.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 66.Hou FQ, Wang Y, Li J, Wang GQ, Liu Y. Management of acute diarrhea in adults in China: a cross-sectional survey. BMC Public Health. 2013;13:41. doi: 10.1186/1471-2458-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Osatakul S, Puetpaiboon A. Appropriate use of empirical antibiotics in acute diarrhoea: a cross-sectional survey in southern Thailand. Ann Trop Paediatr. 2007;27(2):115–22. doi: 10.1179/146532807X192480. [DOI] [PubMed] [Google Scholar]
- 68.Carpenter LR, Pont SJ, Cooper WO, et al. Stool cultures and antimicrobial prescriptions related to infectious diarrhea. J Infect Dis. 2008;197(12):1709–12. doi: 10.1086/588142. [DOI] [PubMed] [Google Scholar]
- 69.Pathak D, Pathak A, Marrone G, Diwan V, Lundborg CS. Adherence to treatment guidelines for acute diarrhoea in children up to 12 years in Ujjain, India--a cross-sectional prescription analysis. BMC infectious diseases. 2011;11:32. doi: 10.1186/1471-2334-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kotwani A, Chaudhury RR, Holloway K. Antibiotic-prescribing practices of primary care prescribers for acute diarrhea in New Delhi, India. Value Health. 2012;15(1 Suppl):S116–9. doi: 10.1016/j.jval.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 71.Wennberg JE, Fisher ES, Goodman DC, Skinner JS. Tracking the Care of Patients with Severe Chronic Illness-The Dartmouth Atlas of Health Care. 2008 [PubMed] [Google Scholar]
- 72.Backhus LM, Farjah F, Varghese TK, et al. Appropriateness of Imaging for Lung Cancer Staging in a National Cohort. Journal of Clinical Oncology. 2014;32(30):3428–35. doi: 10.1200/JCO.2014.55.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bible JE, Kadakia RJ, Kay HF, Zhang CE, Casimir GE, Devin CJ. Repeat spine imaging in transferred emergency department patients. Spine. 2014;39(4):291–6. doi: 10.1097/BRS.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 74.Chen CL, Lin GA, Bardach NS, Clay TH. Preoperative Medical Testing in Medicare Patients Undergoing Cataract Surgery. The New England Journal of Medicine. 2015;372(16):1530–8. doi: 10.1056/NEJMsa1410846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colla CH, Morden NE, Sequist TD, Schpero WL, Rosenthal MB. Choosing Wisely: Prevalence and Correlates of Low-Value Health Care Services in the United States. Journal of General Internal Medicine. 2014;30(2):221–8. doi: 10.1007/s11606-014-3070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Colla CH, Sequist TD, Rosenthal MB, Schpero WL, Gottlieb DJ, Morden NE. Use of non-indicated cardiac testing in low-risk patients: Choosing Wisely. BMJ Quality & Safety. 2014;24:149–53. doi: 10.1136/bmjqs-2014-003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crivello ML, Ruth K, Sigurdson ER, et al. Advanced imaging modalities in early stage breast cancer: preoperative use in the United States Medicare population. Annals of Surgical Oncology. 2012;20(1):102–10. doi: 10.1245/s10434-012-2571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fritz JM, Brennan GP, Hunter SJ. Physical Therapy or Advanced Imaging as First Management Strategy Following a New Consultation for Low Back Pain in Primary Care: Associations with Future Health Care Utilization and Charges. Health Services Research. 2015 doi: 10.1111/1475-6773.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goffredo P, Thomas S, Dinan M, Perkins J, Roman S, Sosa J. Patterns of use and cost for inappropriate radioactive iodine treatment for thyroid cancer in the United States: use and misuse. JAMA Internal Medicine. 2015;175(4):638–40. doi: 10.1001/jamainternmed.2014.8020. [DOI] [PubMed] [Google Scholar]
- 80.Goodwin JS, Singh A, Reddy N, Riall TS, Kuo Y-FF. Overuse of screening colonoscopy in the Medicare population. Archives of Internal Medicine. 2011;171(15):1335–43. doi: 10.1001/archinternmed.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harris IA, Madan NS, Naylor JM, Chong S, Mittal R, Jalaludin BB. Trends in knee arthroscopy and subsequent arthroplasty in an Australian population: a retrospective cohort study. BMC musculoskeletal disorders. 2013;14:143. doi: 10.1186/1471-2474-14-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jarvik JG, Gold LS, Comstock BA, et al. Association of Early Imaging for Back Pain With Clinical Outcomes in Older Adults. The Journal of the American Medical Association. 2015;313(11):1143–53. doi: 10.1001/jama.2015.1871. [DOI] [PubMed] [Google Scholar]
- 83.Kepka D, Breen N, King JB, Benard VB, Saraiya M. Overuse of papanicolaou testing among older women and among women without a cervix. JAMA internal medicine. 2014;174(2):293–6. doi: 10.1001/jamainternmed.2013.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirkham KR, Wijeysundera DN, Pendrith C, et al. Preoperative testing before low-risk surgical procedures. Canadian Medical Association Journal. 2015 doi: 10.1503/cmaj.150174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Makarov DV, Loeb S, Ulmert D, Drevin L, Lambe M, Stattin P. Prostate Cancer Imaging Trends After a Nationwide Effort to Discourage Inappropriate Prostate Cancer Imaging. Journal of the National Cancer Institute. 2013;105(17):1306–13. doi: 10.1093/jnci/djt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sammon JD, Abdollah F, Reznor G, et al. Patterns of Declining Use and the Adverse Effect of Primary Androgen Deprivation on All-cause Mortality in Elderly Men with Prostate Cancer. European Urology. 2015;68(1):32–9. doi: 10.1016/j.eururo.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 87.Sammon JD, Pucheril D, Diaz M, et al. Contemporary nationwide patterns of self-reported prostate-specific antigen screening. JAMA Internal Medicine. 2014;174(11):1839–41. doi: 10.1001/jamainternmed.2014.4117. [DOI] [PubMed] [Google Scholar]
- 88.Scott JW, Schwartz AL, Gates JD, Gerhard-Herman M, Havens JM. Choosing Wisely for Syncope: Low-Value Carotid Ultrasound Use. Journal of the American Heart Association. 2014;3:e001063. doi: 10.1161/JAHA.114.001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Segal JB, Bridges JFP, Chang H-Y, et al. Identifying Possible Indicators of Systematic Overuse of Health Care Procedures With Claims Data. Medical Care. 2014;52(2):157–63. doi: 10.1097/MLR.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 90.Sheffield KM, McAdams PS, Benarroch-Gampel J, et al. Overuse of Preoperative Cardiac Stress Testing in Medicare Patients Undergoing Elective Noncardiac Surgery. Annals of Surgery. 2013;257(1):73–80. doi: 10.1097/SLA.0b013e31826bc2f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun LY, Gershon AS, Ko DT, et al. Trends in Pulmonary Function Testing Before Noncardiothoracic Surgery. JAMA Internal Medicine. 2015;175(8):1410–2. doi: 10.1001/jamainternmed.2015.2087. [DOI] [PubMed] [Google Scholar]
- 92.Thanh NX, Rashiq S, Jonsson E. Routine preoperative electrocardiogram and chest x-ray prior to elective surgery in Alberta, Canada. Canadian Journal of Anesthesia/Journal canadien d’anesthésie. 2010;57(2):127–33. doi: 10.1007/s12630-009-9233-4. [DOI] [PubMed] [Google Scholar]
- 93.Thilen SR, Treggiari MM, Lange JM, Lowy E, Weaver EM, Wijeysundera DN. Preoperative Consultations for Medicare Patients Undergoing Cataract Surgery. JAMA Internal Medicine. 2014;174(3):380–8. doi: 10.1001/jamainternmed.2013.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Welch HG, Hayes KJ, Frost C. Repeat testing among Medicare beneficiaries. Archives of Internal Medicine. 2012;172(22):1745–51. doi: 10.1001/2013.jamainternmed.727. [DOI] [PubMed] [Google Scholar]
- 95.Yap SA, Alibhai SM, Abouassaly R, Timilshina N, Finelli A. Do we continue to unnecessarily perform ipsilateral adrenalectomy at the time of radical nephrectomy? A population based study. The Journal of Urology. 2012;187(2):398–404. doi: 10.1016/j.juro.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 96.Andre M, Odenholt I, Schwan A, et al. Upper respiratory tract infections in general practice: diagnosis, antibiotic prescribing, duration of symptoms and use of diagnostic tests. Scand J Infect Dis. 2002;34(12):880–6. doi: 10.1080/0036554021000026952. [DOI] [PubMed] [Google Scholar]
- 97.Gulliford MC, Dregan A, Moore MV, et al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open. 2014;4(10):e006245. doi: 10.1136/bmjopen-2014-006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Panasiuk L, Lukas W, Paprzycki P, Verheij T, Godycki-Cwirko M, Chlabicz S. Antibiotics in the treatment of upper respiratory tract infections in Poland. Is there any improvement? J Clin Pharm Ther. 2010;35(6):665–9. doi: 10.1111/j.1365-2710.2009.01136.x. [DOI] [PubMed] [Google Scholar]
- 99.Butler CC, Hood K, Verheij T, et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: prospective study in 13 countries. Bmj. 2009;338:b2242. doi: 10.1136/bmj.b2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14(8):742–50. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 101.Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;4:Cd003543. doi: 10.1002/14651858.CD003543.pub3. [DOI] [PubMed] [Google Scholar]
- 102.Guerrero R, Amaris A. Financing cancer care and control: Lessons from Colombia. Boston, MA: Harvard Global Equity Initiative; 2011. [Google Scholar]
- 103.Lopert R, Ruiz F, Chalkidou K. Applying rapid ‘de-facto’ HTA in resource-limited settings: experience from Romania. Health Policy. 2013;112(3):202–8. doi: 10.1016/j.healthpol.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 104.Schwartz LM, Woloshin S, Fowler FJ, Jr, Welch HG. Enthusiasm for cancer screening in the United States. Jama. 2004;291(1):71–8. doi: 10.1001/jama.291.1.71. [DOI] [PubMed] [Google Scholar]
- 105.Mathias JS, Gossett D, Baker DW. Use of electronic health record data to evaluate overuse of cervical cancer screening. J Am Med Inform Assoc. 2012;19(e1):e96–e101. doi: 10.1136/amiajnl-2011-000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sirovich BE, Welch HG. Cervical cancer screening among women without a cervix. Jama. 2004;291(24):2990–3. doi: 10.1001/jama.291.24.2990. [DOI] [PubMed] [Google Scholar]
- 107.Tan A, Kuo YF, Goodwin JS. Potential overuse of screening mammography and its association with access to primary care. Med Care. 2014;52(6):490–5. doi: 10.1097/MLR.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goodwin JS, Singh A, Reddy N, Riall TS, Kuo YF. Overuse of screening colonoscopy in the Medicare population. Arch Intern Med. 2011;171(15):1335–43. doi: 10.1001/archinternmed.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Murphy CC, Sandler RS, Grubber JM, Johnson MR, Fisher DA. Underuse and Overuse of Colonoscopy for Repeat Screening and Surveillance in the Veterans Health Administration. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hol L, Sutradhar R, Gu S, et al. Repeat colonoscopy after a colonoscopy with a negative result in Ontario: a population-based cohort study. CMAJ Open. 2015;3(2):E244–50. doi: 10.9778/cmajo.20140063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”--screening and overdiagnosis. The New England journal of medicine. 2014;371(19):1765–7. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 112.Khokhar A. Breast cancer in India: where do we stand and where do we go? Asian Pac J Cancer Prev. 2012;13(10):4861–6. doi: 10.7314/apjcp.2012.13.10.4861. [DOI] [PubMed] [Google Scholar]
- 113.Sirohi B. Cancer care delivery in India at the grassroot level: Improve outcomes. Indian J Med Paediatr Oncol. 2014;35(3):187–91. doi: 10.4103/0971-5851.142030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vader JP, Pache I, Froehlich F, et al. Overuse and underuse of colonoscopy in a European primary care setting. Gastrointest Endosc. 2000;52(5):593–99. doi: 10.1067/mge.2000.108716. [DOI] [PubMed] [Google Scholar]
- 115.Froehlich F, Burnand B, Pache I, et al. Overuse of upper gastrointestinal endoscopy in a country with open-access endoscopy: a prospective study in primary care. Gastrointest Endosc. 1997;45(1):13–9. [PubMed] [Google Scholar]
- 116.Eskeland SL, Dalen E, Sponheim J, Lind E, Brunborg C, de Lange T. European panel on the appropriateness of gastrointestinal endoscopy II guidelines help in selecting and prioritizing patients referred to colonoscopy--a quality control study. Scand J Gastroenterol. 2014;49(4):492–500. doi: 10.3109/00365521.2014.886715. [DOI] [PubMed] [Google Scholar]
- 117.Mangualde J, Cremers MI, Vieira AM, et al. Appropriateness of outpatient gastrointestinal endoscopy in a non-academic hospital. World J Gastrointest Endosc. 2011;3(10):195–200. doi: 10.4253/wjge.v3.i10.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arguello L, Pertejo V, Ponce M, Peiro S, Garrigues V, Ponce J. The appropriateness of colonoscopies at a teaching hospital: magnitude, associated factors, and comparison of EPAGE and EPAGE-II criteria. Gastrointest Endosc. 2012;75(1):138–45. doi: 10.1016/j.gie.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 119.Hassan C, Bersani G, Buri L, et al. Appropriateness of upper-GI endoscopy: an Italian survey on behalf of the Italian Society of Digestive Endoscopy. Gastrointest Endosc. 2007;65(6):767–74. doi: 10.1016/j.gie.2006.12.058. [DOI] [PubMed] [Google Scholar]
- 120.Keren D, Rainis T, Stermer E, Lavy A. A nine-year audit of open-access upper gastrointestinal endoscopic procedures: results and experience of a single centre. Can J Gastroenterol. 2011;25(2):83–8. doi: 10.1155/2011/379014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Keyhani S, Falk R, Howell EA, Bishop T, Korenstein D. Overuse and systems of care: a systematic review. Medical care. 2013;51(6):503–8. doi: 10.1097/MLR.0b013e31828dbafe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aljebreen AM, Alswat K, Almadi MA. Appropriateness and diagnostic yield of upper gastrointestinal endoscopy in an open-access endoscopy system. Saudi J Gastroenterol. 2013;19(5):219–22. doi: 10.4103/1319-3767.118128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van Heijningen EM, Lansdorp-Vogelaar I, Steyerberg EW, et al. Adherence to surveillance guidelines after removal of colorectal adenomas: a large, community-based study. Gut. 2015;64(10):1584–92. doi: 10.1136/gutjnl-2013-306453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hemingway H, Chen R, Junghans C, et al. Appropriateness criteria for coronary angiography in angina: reliability and validity. Ann Intern Med. 2008;149(4):221–31. doi: 10.7326/0003-4819-149-4-200808190-00003. [DOI] [PubMed] [Google Scholar]
- 125.Thomas MP, Parzynski CS, Curtis JP, et al. Percutaneous Coronary Intervention Utilization and Appropriateness across the United States. PloS one. 2015;10(9):e0138251. doi: 10.1371/journal.pone.0138251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brause M, Grande G, Mannebach H, Badura B. The impact of social and institutional characteristics on the appropriateness of invasive cardiologic procedures. Med Klin (Munich) 2006;101(3):226–34. doi: 10.1007/s00063-006-1028-6. [DOI] [PubMed] [Google Scholar]
- 127.Gandjour A, Neumann I, Lauterbach KW. Appropriateness of invasive cardiovascular interventions in German hospitals (2000–2001): an evaluation using the RAND appropriateness criteria. Eur J Cardiothorac Surg. 2003;24(4):571–7. doi: 10.1016/s1010-7940(03)00461-5. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 128.Medolago G, Marcassa C, Alkraisheh A, Campini R, Ghilardi A, Giubbini R. Applicability of the appropriate use criteria for SPECT myocardial perfusion imaging in Italy: preliminary results. Eur J Nucl Med Mol Imaging. 2014;41(9):1695–700. doi: 10.1007/s00259-014-2743-5. [DOI] [PubMed] [Google Scholar]
- 129.Choi JW, Cho J, Lee Y, et al. Microwave detection of metastasized breast cancer cells in the lymph node; potential application for sentinel lymphadenectomy. Breast Cancer Res Treat. 2004;86(2):107–15. doi: 10.1023/b:brea.0000032979.52773.fb. [DOI] [PubMed] [Google Scholar]
- 130.Lang T, Davido A, Logerot H, Meyer L. Appropriateness of admissions: the French experience. Int J Qual Health Care. 1995;7(3):233–8. doi: 10.1093/intqhc/7.3.233. [DOI] [PubMed] [Google Scholar]
- 131.Sangha O, Schneeweiss S, Wildner M, et al. Metric properties of the appropriateness evaluation protocol and predictors of inappropriate hospital use in Germany: an approach using longitudinal patient data. Int J Qual Health Care. 2002;14(6):483–92. doi: 10.1093/intqhc/14.6.483. [DOI] [PubMed] [Google Scholar]
- 132.Cordero A, Aguila J, Massalana A, Escoto V, Lopes L, Susano R. Appropriateness admissions to the Department of Internal Medicine of the Hospital de Santa Luzia (Elvas) evaluated by the AEP (Appropriateness Evaluation Protocol) Acta Med Port. 2004;17(2):113–8. [PubMed] [Google Scholar]
- 133.Soria-Aledo V, Carrillo-Alcaraz A, Campillo-Soto A, et al. Associated factors and cost of inappropriate hospital admissions and stays in a second-level hospital. Am J Med Qual. 2009;24(4):321–32. doi: 10.1177/1062860609337252. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Y, Chen Y, Zhang X, Zhang L. Current level and determinants of inappropriate admissions to township hospitals under the new rural cooperative medical system in China: a cross-sectional study. BMC health services research. 2014;14:649. doi: 10.1186/s12913-014-0649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Al-Tehewy M, Shehad E, Al Gaafary M, Al-Houssiny M, Nabih D, Salem B. Appropriateness of hospital admissions in general hospitals in Egypt. East Mediterr Health J. 2009;15(5):1126–34. [PubMed] [Google Scholar]
- 136.Busby J, Purdy S, Hollingworth W. A systematic review of the magnitude and cause of geographic variation in unplanned hospital admission rates and length of stay for ambulatory care sensitive conditions. BMC health services research. 2015;15:324. doi: 10.1186/s12913-015-0964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van den Berg MJ, van Loenen T, Westert GP. Accessible and continuous primary care may help reduce rates of emergency department use. An international survey in 34 countries. Family practice. 2015 doi: 10.1093/fampra/cmv082. [DOI] [PubMed] [Google Scholar]
- 138.Purdy S, Griffin T. Reducing hospital admissions. Bmj. 2008;336(7634):4–5. doi: 10.1136/bmj.39394.402465.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kulkarni P, Kulkarni P, Anavkar V, Ghooi R. Preference of the place of death among people of pune. Indian J Palliat Care. 2014;20(2):101–6. doi: 10.4103/0973-1075.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fukui S, Kawagoe H, Masako S, Noriko N, Hiroko N, Toshie M. Determinants of the place of death among terminally ill cancer patients under home hospice care in Japan. Palliat Med. 2003;17(5):445–53. doi: 10.1191/0269216303pm782oa. [DOI] [PubMed] [Google Scholar]
- 141.Barnato AE, Herndon MB, Anthony DL, et al. Are regional variations in end-of-life care intensity explained by patient preferences?: A Study of the US Medicare Population. Med Care. 2007;45(5):386–93. doi: 10.1097/01.mlr.0000255248.79308.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.De Roo ML, Miccinesi G, Onwuteaka-Philipsen BD, et al. Actual and preferred place of death of home-dwelling patients in four European countries: making sense of quality indicators. PloS one. 2014;9(4):e93762. doi: 10.1371/journal.pone.0093762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gomes B, Higginson IJ, Calanzani N, et al. Preferences for place of death if faced with advanced cancer: a population survey in England, Flanders, Germany, Italy, the Netherlands, Portugal and Spain. Ann Oncol. 2012;23(8):2006–15. doi: 10.1093/annonc/mdr602. [DOI] [PubMed] [Google Scholar]
- 144.Chen CH, Lin YC, Liu LN, Tang ST. Determinants of preference for home death among terminally ill patients with cancer in Taiwan: a cross-sectional survey study. J Nurs Res. 2014;22(1):37–44. doi: 10.1097/jnr.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 145.Bekelman JE, Halpern SD, Blankart C, et al. Comparison of site of death, health care utilization, and hospital expenditures for patients dying with cancer in 7 developed countries. Jama. 2016;315(3):272–83. doi: 10.1001/jama.2015.18603. [DOI] [PubMed] [Google Scholar]
- 146.Ho TH, Barbera L, Saskin R, Lu H, Neville BA, Earle CC. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol. 2011;29(12):1587–91. doi: 10.1200/JCO.2010.31.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Morden NE, Chang CH, Jacobson JO, et al. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health affairs (Project Hope) 2012;31(4):786–96. doi: 10.1377/hlthaff.2011.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Henson LA, Gomes B, Koffman J, Daveson BA, Higginson IJ, Gao W. Factors associated with aggressive end of life cancer care. Support Care Cancer. 2015 doi: 10.1007/s00520-015-2885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Johnston SD, Tham TC, Mason M. Death after PEG: results of the National Confidential Enquiry into Patient Outcome and Death. Gastrointest Endosc. 2008;68(2):223–7. doi: 10.1016/j.gie.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 150.Mort D, Lansdown M, Smith N, Protopapa K. Systemic Anti-Cancer Therapy: For better, for worse? London: 2008. [Google Scholar]
- 151.Palda VA, Bowman KW, McLean RF, Chapman MG. “Futile” care: do we provide it?” Why? A semistructured, Canada-wide survey of intensive care unit doctors and nurses. J Crit Care. 2005;20(3):207–13. doi: 10.1016/j.jcrc.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 152.Anstey MH, Adams JL, McGlynn EA. Perceptions of the appropriateness of care in California adult intensive care units. Crit Care. 2015;19(1):51. doi: 10.1186/s13054-015-0777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cruz VM, Camalionte L, Caruso P. Factors associated with futile end-of-life intensive care in a cancer hospital. Am J Hosp Palliat Care. 2015;32(3):329–34. doi: 10.1177/1049909113518269. [DOI] [PubMed] [Google Scholar]
- 154.Kim do Y, Lee SM, Lee KE, et al. An evaluation of nutrition support for terminal cancer patients at teaching hospitals in Korea. Cancer research and treatment : official journal of Korean Cancer Association. 2006;38(4):214–7. doi: 10.4143/crt.2006.38.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bansal M, Patel FD, Mohanti BK, Sharma SC. Setting up a palliative care clinic within a radiotherapy department: a model for developing countries. Support Care Cancer. 2003;11(6):343–7. doi: 10.1007/s00520-002-0418-4. [DOI] [PubMed] [Google Scholar]
- 156.Riechelmann RP, Krzyzanowska MK, Zimmermann C. Futile medication use in terminally ill cancer patients. Support Care Cancer. 2009;17(6):745–8. doi: 10.1007/s00520-008-0541-y. [DOI] [PubMed] [Google Scholar]
- 157.Saini P, Loke YK, Gamble C, Altman DG, Williamson PR, Kirkham JJ. Selective reporting bias of harm outcomes within studies: findings from a cohort of systematic reviews. BMJ. 2014;349:g6501. doi: 10.1136/bmj.g6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cushner F, Agnelli G, FitzGerald G, Warwick D. Complications and functional outcomes after total hip arthroplasty and total knee arthroplasty: results from the Global Orthopaedic Registry (GLORY) American journal of orthopedics. 2010;39(9 Suppl):22–8. [PubMed] [Google Scholar]
- 159.Quintana JM, Arostegui I, Escobar A, Azkarate J, Goenaga JI, Lafuente I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168(14):1576–84. doi: 10.1001/archinte.168.14.1576. [DOI] [PubMed] [Google Scholar]
- 160.Sarosiek S, Crowther M, Sloan JM. Indications, complications, and management of inferior vena cava filters: the experience in 952 patients at an academic hospital with a level I trauma center. JAMA Intern Med. 2013;173(7):513–7. doi: 10.1001/jamainternmed.2013.343. [DOI] [PubMed] [Google Scholar]
- 161.Group PS. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112(3):416–22. doi: 10.1161/CIRCULATIONAHA.104.512834. [DOI] [PubMed] [Google Scholar]
- 162.Niven DJ, Rubenfeld GD, Kramer AA, Stelfox HT. Effect of Published Scientific Evidence on Glycemic Control in Adult Intensive Care Units. JAMA Intern Med. 2015 doi: 10.1001/jamainternmed.2015.0157. [DOI] [PubMed] [Google Scholar]
- 163.Verlee K, Berriel-Cass D, Buck K, Nguyen C. Cost of isolation: daily cost of isolation determined and cost avoidance demonstrated from the overuse of personal protective equipment in an acute care facility. Am J Infect Control. 2014;42(4):448–9. doi: 10.1016/j.ajic.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 164.Kirkland KB. Taking off the gloves: toward a less dogmatic approach to the use of contact isolation. Clin Infect Dis. 2009;48(6):766–71. doi: 10.1086/597090. [DOI] [PubMed] [Google Scholar]
- 165.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer. 2013;108(11):2205–40. doi: 10.1038/bjc.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lauzier S, Maunsell E, Levesque P, et al. Psychological distress and physical health in the year after diagnosis of DCIS or invasive breast cancer. Breast Cancer Res Treat. 2010;120(3):685–91. doi: 10.1007/s10549-009-0477-z. [DOI] [PubMed] [Google Scholar]
- 167.Partridge A, Adloff K, Blood E, et al. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. J Natl Cancer Inst. 2008;100(4):243–51. doi: 10.1093/jnci/djn010. [DOI] [PubMed] [Google Scholar]
- 168.van Gestel YR, Voogd AC, Vingerhoets AJ, et al. A comparison of quality of life, disease impact and risk perception in women with invasive breast cancer and ductal carcinoma in situ. Eur J Cancer. 2007;43(3):549–56. doi: 10.1016/j.ejca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 169.Bergman AB, Stamm SJ. The morbidity of cardiac nondisease in schoolchildren. N Engl J Med. 1967;276(18):1008–13. doi: 10.1056/NEJM196705042761804. [DOI] [PubMed] [Google Scholar]
- 170.Conrad P, Bergey MR. The impending globalization of ADHD: Notes on the expansion and growth of a medicalized disorder. Social science & medicine. 2014;122:31–43. doi: 10.1016/j.socscimed.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 171.Batzle CS, Weyandt LL, Janusis GM, DeVietti TL. Potential impact of ADHD with stimulant medication label on teacher expectations. J Atten Disord. 2010;14(2):157–66. doi: 10.1177/1087054709347178. [DOI] [PubMed] [Google Scholar]
- 172.O’Driscoll C, Heary C, Hennessy E, McKeague L. Explicit and implicit stigma towards peers with mental health problems in childhood and adolescence. Journal of child psychology and psychiatry, and allied disciplines. 2012;53(10):1054–62. doi: 10.1111/j.1469-7610.2012.02580.x. [DOI] [PubMed] [Google Scholar]
- 173.Sherman J, Rasmussen C, Baydala L. The impact of teacher factors on achievement and behavioural outcomes of children with Attention Deficit/Hyperactivity Disorder (ADHD): a review of the literature. Educational Research. 2008;50(4):347–60. [Google Scholar]
- 174.Ubel PA, Abernethy AP, Zafar SY. Full disclosure--out-of-pocket costs as side effects. N Engl J Med. 2013;369(16):1484–6. doi: 10.1056/NEJMp1306826. [DOI] [PubMed] [Google Scholar]
- 175.Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health affairs. 2013;32(6):1143–52. doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Himmelstein DU, Thorne D, Warren E, Woolhandler S. Medical bankruptcy in the United States, 2007: results of a national study. The American journal of medicine. 2009;122(8):741–6. doi: 10.1016/j.amjmed.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 177.Cohn RJ, Goodenough B, Foreman T, Suneson J. Hidden financial costs in treatment for childhood cancer: an Australian study of lifestyle implications for families absorbing out-of-pocket expenses. J Pediatr Hematol Oncol. 2003;25(11):854–63. doi: 10.1097/00043426-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 178.La Forgia G, Nagpal S. Government-sponsored health insurance in India: Are you covered? World Bank Publications; 2012. [Google Scholar]
- 179.India tries to break cycle of health-care debt. Bull World Health Organ. 2010;88(7):486–7. doi: 10.2471/BLT.10.020710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Van Minh H, Xuan Tran B. Assessing the household financial burden associated with the chronic non-communicable diseases in a rural district of Vietnam. Glob Health Action. 2012;5:1–7. doi: 10.3402/gha.v5i0.18892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Falchook AD, Salloum RG, Hendrix LH, Chen RC. Use of bone scan during initial prostate cancer workup, downstream procedures, and associated Medicare costs. Int J Radiat Oncol Biol Phys. 2014;89(2):243–8. doi: 10.1016/j.ijrobp.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Emanuel EJ, Fuchs VR. The perfect storm of overutilization. JAMA : the journal of the American Medical Association. 2008;299(23):2789–91. doi: 10.1001/jama.299.23.2789. [DOI] [PubMed] [Google Scholar]
- 183.Tooke J. The Future of Healthcare in Europe: Meeting Future Challenges; Key Issues in Context. London: UCL; [Google Scholar]
- 184.Daley JMC, Savage J. Budget Pressures on Australian Governments 2014. Grattan Institute; 2014. [Google Scholar]
- 185.Schmidt H, Gostin LO, Emanuel EJ. Public health, universal health coverage, and Sustainable Development Goals: can they coexist? Lancet. 2015;386(9996):928–30. doi: 10.1016/S0140-6736(15)60244-6. [DOI] [PubMed] [Google Scholar]
- 186.Segal JB, Bridges JF, Chang HY, et al. Identifying possible indicators of systematic overuse of health care procedures with claims data. Med Care. 2014;52(2):157–63. doi: 10.1097/MLR.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 187.Unnecessary Tests and Procedures in the Health Care System. What Physicians Say About The Problem, the Causes, and the Solutions. PerryUndem Research/Communication; 2014. [Google Scholar]
- 188.MacDorman MF, Menacker F, Declercq E. Cesarean birth in the United States: epidemiology, trends, and outcomes. Clin Perinatol. 2008;35(2):293–307. v. doi: 10.1016/j.clp.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 189.Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. Jama. 2012;307(22):2400–9. doi: 10.1001/jama.2012.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Litorp H, Kidanto HL, Nystrom L, Darj E, Essen B. Increasing caesarean section rates among low-risk groups: a panel study classifying deliveries according to Robson at a university hospital in Tanzania. BMC Pregnancy Childbirth. 2013;13:107. doi: 10.1186/1471-2393-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Neuman M, Alcock G, Azad K, et al. Prevalence and determinants of caesarean section in private and public health facilities in underserved South Asian communities: cross-sectional analysis of data from Bangladesh, India and Nepal. 2014;4(12):e005982. doi: 10.1136/bmjopen-2014-005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kahler C. China’s Healthcare Reform: How Far Has It Come? China Business Review. 2011 [Google Scholar]
- 193.Jack Freifelder LJ. China Daily. 2014. More measures expected in China’s healthcare reform. [Google Scholar]
- 194.Long Q, Klemetti R, Wang Y, Tao F, Yan H, Hemminki E. High Caesarean section rate in rural China: is it related to health insurance (New Co-operative Medical Scheme)? Soc Sci Med. 2012;75(4):733–7. doi: 10.1016/j.socscimed.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 195.Berger D. Corruption ruins the doctor-patient relationship in India. Bmj. 2014;348:g3169. doi: 10.1136/bmj.g3169. [DOI] [PubMed] [Google Scholar]
- 196.Bhaumik S. Oxfam calls for new regulations to reduce unnecessary hysterectomies in private hospitals. Bmj. 2013;346:f852. doi: 10.1136/bmj.f852. [DOI] [PubMed] [Google Scholar]
- 197.NICE Savings and productivity collection. ‘do not do’ recommendations. National Health Service (NHS); [Google Scholar]
- 198.Levinson W, Kallewaard M, Bhatia RS, Wolfson D, Shortt S, Kerr EA. ‘Choosing Wisely’: a growing international campaign. BMJ Qual Saf. 2015;24(2):167–74. doi: 10.1136/bmjqs-2014-003821. [DOI] [PubMed] [Google Scholar]
- 199.Goldzweig CL, Orshansky G, Paige NM, et al. Electronic Health Record-Based Interventions for Reducing Inappropriate Imaging in the Clinical Setting: A Systematic Review of the Evidence. Washington (DC): Department of Veterans Affairs; 2015. [PubMed] [Google Scholar]