Abstract
Objective
We tested the hypothesis that somatosensory system injury would more strongly affect movement than motor system injury in children with unilateral cerebral palsy (USCP). This hypothesis was based on how somatosensory and corticospinal circuits adapt to injury during development: while the motor system can maintain connections to the impaired hand from the uninjured hemisphere, this doesn't occur in the somatosensory system. As a corollary, cortical injury strongly impairs sensory function, so we hypothesized that cortical lesions would impair hand function more than subcortical lesions.
Methods
Twenty-four children with unilateral CP had physiological and anatomical measures of the motor and somatosensory systems and lesion classification. Motor physiology was performed with transcranial magnetic stimulation and somatosensory physiology with vibration-evoked EEG potentials. Tractography of the corticospinal tract and the medial lemniscus were performed with diffusion tensor imaging, and lesions were classified by MRI. Anatomical and physiological results were correlated with measures of hand function using two independent statistical methods.
Results
Children with disruptions in the somatosensory connectivity, and cortical lesions had the most severe upper extremity impairments, particularly somatosensory function. Motor system connectivity was significantly correlated with bimanual function, but not unimanual function or somatosensory function.
Interpretation
Both sensory and motor connectivity impact hand function in children with USCP. Somatosensory connectivity could be an important target for recovery of hand function in children with USCP.
Keywords: Somatosensory Evoked Potentials, Corticospinal Tract, Unilateral Spastic Cerebral Palsy
Introduction
Injury to the developing brain often results in impaired movement, known as cerebral palsy1. Injury to one hemisphere usually leads to unilateral spastic cerebral palsy (USCP), which primarily impairs hand function on the side opposite (contralateral) to the brain injury. Many studies of USCP have identified injury of the corticospinal tract (CST), the principal pathway for voluntary movement, as a determinant of upper extremity impairment2. Prior studies have shown that the CST integrity2-4 and organization5,6 correlate with hand function. In addition to movement deficits, an estimated 90% of children with USCP also have impaired somatosensory function7. Several studies have demonstrated correlations between the magnitude of somatosensory and movement impairments8,9. The close interaction of motor and sensory systems can make it challenging to determine their relative contribution to functional impairments. We interrogated anatomy and physiology of the CST and touch systems using homologous methods to determine their relative contribution to hand function.
A key determinant of the degree to which motor or somatosensory systems contribute to upper extremity impairment in USCP is the adaptation of each system to developmental brain injury. During normal development, the CST descends from motor cortex to establish bilateral connections to the spinal cord10. These bilateral projections are pruned through the third trimester and early childhood11, leading to a predominantly contralateral system (Fig 1A). If brain injury occurs early in development or affects a large portion of the CST, early bilateral CST projections from the uninjured hemisphere persist, and the impaired hand is controlled via connections from the ipsilateral (uninjured) hemisphere12 (Fig 1B and C). These ipsilateral connections, found in more than half of children with USCP, have the capacity to support substantial motor function10,13, 14.
Figure 1.
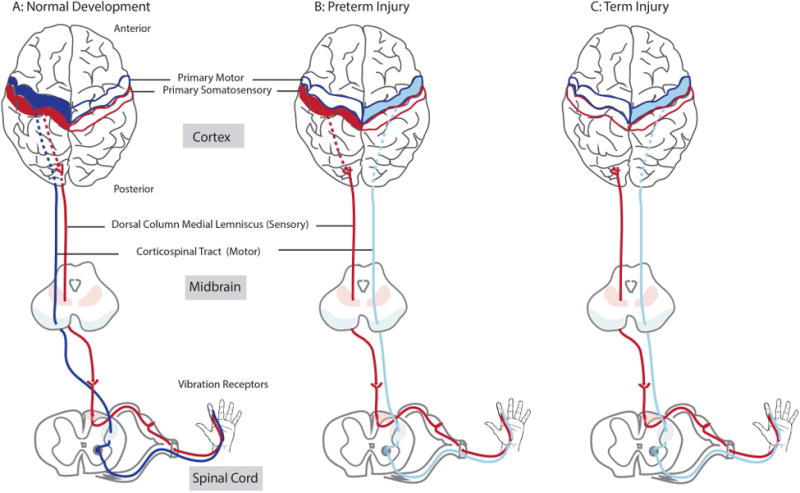
An illustration of the motor and somatosensory connections in normal development and early brain injury. These are models based on previous studies of the effects of injury timing on connectivity. (A) Typical development. After initial bilateral projections, the motor tract (corticospinal tract (CST), is largely pruned to the contralateral one. The somatosensory tract mediating touch, vibration, and proprioception (dorsal column-medial lemniscus (DC-ML)). (B) In case of preterm injury, the contralateral CST is often disrupted, and the ipsilateral CST persists from the uninjured hemisphere. Thalamocortical projections (interrupted line) that are part of the DC-ML develop later and may skirt periventricular lesions to reach primary somatosensory cortex in the contralateral cortex. (C) In case of a larger injury (such as MCA) that can often occur at full term, the thalamocortical projections and primary somatosensory cortex may be disrupted as well. (Colored version of Figure is available online)
Somatosensory adaptation to injury is less robust15-17 and differs from CST development in two important ways. First, axonal connections in the touch somatosensory system project almost entirely to one hemisphere, even after injury early in development13. Second, somatosensory axons can navigate around brain lesions that occur before the early third trimester to reach primary somatosensory cortex12. A common developmental brain injury affects periventricular (PV)15,18 white matter. Thalamocortical projections can skirt PV lesions and reach their cortical target, albeit with decreased integrity18-20. In contrast, middle cerebral artery (MCA) infarcts are often large, occur later, and usually do not spare thalamocortical connections18,19.
The relatively weak adaptation of the sensory system and the strong adaptation of the motor system to early injury leads us to the hypothesis that loss of somatosensory connections will more strongly correlate with upper extremity impairment than loss of contralateral motor connections. The impairments are predicted to be in both somatosensation and in movement. Further, we also hypothesized that hand function would be more impaired after MCA lesions than PV lesions, since MCA lesions are more likely to disrupt somatosensory circuits. Analyzing results by lesion will allow classification based on conventional and clinically indicated brain imaging. We tested these hypotheses by assessing anatomy and physiology of both the motor system and the somatosensory system in children with USCP.
Methods and Materials
2.1 Participants
Twenty-four children (13M/11F, average age 10.5±3.3, years.months) with congenital USCP participated (Table 1). The inclusion criteria were: congenital USCP, presence of hemiparesis that limits daily function, ability to lift arms 15 cm above table surface and grasp light objects, and enrolled in mainstream school. The exclusion criteria were: health problems that would interfere with participation (including visual impairment), seizures after 2 years of age, severe spasticity (Ashworth ≥3), hand surgery within one year, injection of botulinum toxin in upper extremity within six months, or metallic implants. Informed assent was obtained from participants and consent from parents/legal guardians. The research procedures were IRB approved by Burke Medical Research Institute, Teachers College (Columbia University), and Weill Cornell Medical College.
Table 1. Participant demographics, injury type and location, physiology and anatomical connectivity.
Index: Contra: contralateral; ipsi: ipsilateral; *: No TMS Hotspot Found; T/P: Technical Problem; I/D: Incomplete Data; N/A: No significant SEP classification in the unaffected hemisphere.
| Demographics | Injury | Physiology | Anatomy | |||||
|---|---|---|---|---|---|---|---|---|
| P | Age | Sex | Lesion | Cortex | Motor | Sensory | Motor | Sensory |
| (y.m) | (TMS) | (EEG) | (DTI) | (DTI) | ||||
| 1 | 13.06 | m | PV | R | Ipsi | Present | Absent | Present |
| 2 | 8.10 | f | PV | R | Ipsi | Present | Absent | Present |
| 3 | 16.10 | f | PV | L | Ipsi | Present | Absent | Present |
| 4 | 18.12 | f | MCA | L | Ipsi | Present | Absent | Present |
| 5 | 8.01 | m | Other | R | Ipsi | Present | Absent | Present |
| 6 | 9.06 | m | MCA | L | Ipsi | Absent | Absent | Absent |
| 7 | 9.06 | f | MCA | L | Ipsi | Absent | Absent | Absent |
| 8 | 12.03 | m | MCA | L | Ipsi | Absent | Absent | Present |
| 9 | 10.07 | m | MCA | L | Ipsi | Absent | Present | Present |
| 10 | 13.08 | f | Other | L | Ipsi | Absent | no DTI | no DTI |
| 11 | 9.03 | m | no MRI | L | Ipsi | Absent | no DTI | no DTI |
| 12 | 10.01 | m | PV | L | Contra | Present | Present | Present |
| 13 | 10.03 | f | PV | L | Contra | Present | Present | Present |
| 14 | 11.01 | m | PV | L | Contra | Present | Present | Present |
| 15 | 7.08 | f | PV | R | Contra | Present | Absent | Present |
| 16 | 7.02 | f | MCA | L | * | Absent | Absent | Absent |
| 17 | 11.01 | m | no MRI | L | Contra | Absent | no DTI | no DTI |
| 18 | 7.05 | f | PV | R | Contra | N/A | Present | Present |
| 19 | 7.01 | m | Other | R | Contra | N/A | Present | Present |
| 20 | 7.12 | f | PV | L | Contra | I/D | no DTI | no DTI |
| 21 | 12.05 | m | PV | R | Contra | T/P | Present | Present |
| 22 | 13.08 | m | PV | L | Ipsi | T/P | Present | Present |
| 23 | 7.02 | f | PV | L | * | Absent | Absent | Absent |
| 24 | 8.03 | m | MCA | L | * | N/A | Absent | Present |
2.2 Experimental paradigms
Overview
In each participant, we measured the anatomy and physiology of the motor and the somatosensory system. Diffusion Tensor Imaging (DTI) was used to analyze the presence of motor and somatosensory tracts. Lesion type was analyzed using structural MRI. Somatosensory physiology was tested with somatosensory evoked potentials (SEPs)– stimulating the hand and measuring evoked potentials in the brain with electroencephalography (EEG). Motor physiology was tested with motor evoked potentials (MEPs) from single-pulse transcranial magnetic stimulation (TMS) of motor cortex and measuring hand muscle responses with electromyography (EMG).
EEG SEPs
31 channel EEG was recorded with NeuroPrax (NeuroConn, GmbH, Germany) data acquisition system at 4096Hz, referenced to the right mastoid. Vibrotactile stimulation was delivered to the pad of each index finger (Fig 2A) to preferentially stimulate the touch/proprioception system. The paradigm was designed based on previous studies21. Motors with 175Hz vibration frequency (1200 vibration trains of 100msec duration and 150-1300msec interstimulus-interval) were used to stimulate Pacinian corpuscles. 600 vibration trains were presented per hand, randomly distributed across the two hands. A transistor-transistor logic (TTL) pulse from the microcontroller was used to precisely mark the vibration onset in the EEG.
Figure 2.
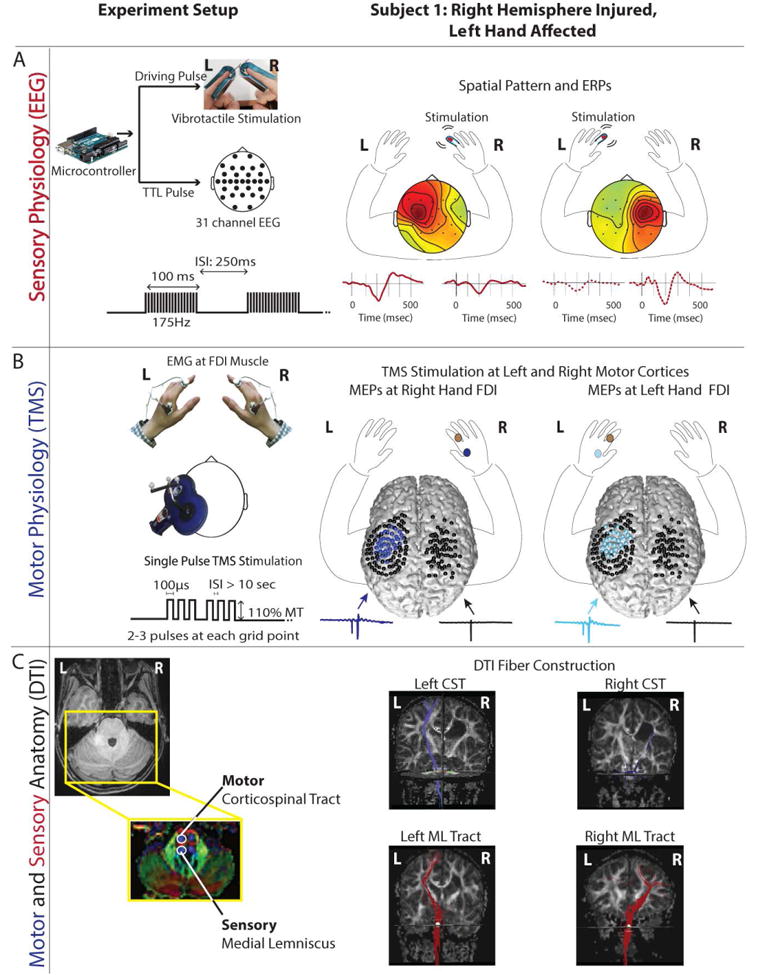
Experimental methods. The left panel shows the methods for data acquisition for assessment of physiology and anatomy. The right panel shows the results for one example participant with right hemisphere PV injury with preserved somatosensory connections and ipsilateral motor connections. (A) Sensory physiology. Left: EEG SEPs were evoked with vibrotactile stimulation applied to the tip of each index finger. Right panel shows the spatial topographies and SEPs for left and right hand stimulation. Comparable contralateral evoked response is observed for stimulation of both the left (more affected) and right (less affected) hand. (B) Motor Physiology. Left: Single pulse TMS stimulation applied using neuronavigation evoked MEPs in the first dorsal interosseus muscle. Right: black spheres indicate the sites of stimulation projected onto a 3D rendering of the patient's cortex. The locations that evoked an MEP are shown in dark blue for the contralateral (right) hand and light blue for the ipsilateral (left) hand (same scheme as Fig 1). (C) Anatomy: Left: DTI of the motor and somatosensory tracts displayed on a color-coded DTI FA map. In FA maps the red indicates fibers running along left-right direction, green inferior-superior and blue anterior-posterior. The CST (anterior pair of blue) and medial lemniscus (posterior pair of blue) were seeded on an axial slice at pons level. Right: CST (shown in blue) is found in the left (uninjured) hemisphere, but not in the right hemisphere with the PV lesion. In contrast, the somatosensory tracts (shown in red) are present in both the left and the right hemisphere.
EEG was pre-processed with a 1-20Hz bandpass filter, downsampled to 512Hz and epoched into -100ms to 600ms trials relative to vibration onset. Trials with artifacts were automatically eliminated using pre-specified statistical metrics. The analysis procedure was defined by the challenges of processing brain injury pediatric EEG. Specifically, there were larger and more frequent artifacts and noise as the participants were young and tests were performed in an awake resting state. Additionally, we expected a lower signal-to-noise ratio and unspecific alterations in the spatial and temporal responses, due to neuroplastic changes. To limit subjectivity in analysis, we followed an objective procedural pipeline similar to previously published methods22. First we extracted relevant spatial filters using 31 channel EEG data for each hemisphere based on the largest invariance across the stimulation trials. This spatial filter was applied to the epoched data to extract the evoked response per trial. Next, a Riemannian geometry based classifier23 was built to classify left vs. right hand evoked response in each hemisphere. Classification outcome was quantified as area-under-the-curve and controlled for over-fitting using Monte-Carlo cross-validation with repeated random sub-sampling. The significance was tested with a permutation method24.
The goal was to categorize the presence or absence of an SEP from left versus right hand in the injured hemisphere, as determined by the classifier. SEPs in the unaffected hemisphere acted as a control; if a participant's SEP could not be significantly classified in the unaffected hemisphere, due to low signal-to-noise ratio, then the SEPs were categorized as ‘undetermined’ (n=3, Table 1). Participants' with significant classification of SEP in the unaffected hemisphere were classified as ‘Present’ if SEP in the affected hemisphere could be significantly classified, and ‘Absent’ otherwise.
TMS MEPs
Single-pulse TMS was used to analyze corticospinal pathways (Fig 2B). The participant's structural MRI was used to guide the TMS coil to the motor cortex, using neuronavigation (Brainsight) software. First, the region of the motor cortex that elicits the largest MEP was localized. Centered around this region, 3 concentric circular grids were created. Stimuli were delivered at each of these grid points in both hemispheres and the MEPs were recorded at the first dorsal interosseus and wrist flexor muscles, using bipolar surface EMG electrodes. An MEP was considered present if an EMG response (>50μV) was observed within 40msec. The pulses were delivered at 110% of the participant's resting motor threshold. The goal was to categorize the motor connectivity as contralateral vs. ipsilateral. This was accomplished with the Lateralization Index (LI)25, defined as the percentage of the responsive sites for the affected hand, in the injured hemisphere compared to the total number of sites in both hemispheres. If more than 50% of total map area was located in the injured hemisphere, LI>0.5, the participant was classified as having a contralateral CST, and ipsilateral otherwise.
DTI
DTI was used to construct motor and somatosensory tracts. The goal was to assess the presence or absence of motor and somatosensory fiber tracts from the injured and the uninjured hemisphere. 3T DTI scans were acquired with 75 slices (112x112 resolution) and 55 or 65 directions (b = 800s/mm2). Deterministic tractography was performed with Diffusion Toolkit26 using the 2nd-order Runge-Kutta fiber propagation method. TrackVis26 was used to visualize the fractional anisotropy (FA) color maps to seed regions-of-interest (ROI) (Fig 2C). Motor (targeting CST27, but would include the corticobulbar fibers as well) and somatosensory tracts (targeting the medial lemniscus)27,28 were seeded using an axial slice of the pons on a DTI FA image that most clearly distinguished the tracts for the uninjured hemisphere. Tracts were then visually inspected in 3-D to ensure that they intersected the motor or sensory cortex. For reliability, D.G. and A.B. performed tractography independently and blindly. In the four participants who did not have visible somatosensory tracts on the lesioned side, we performed noise correction by excluding noisy images in DTI Studio29; tracts could not be reconstructed even after noise correction.
Structural MRI
High-resolution 176 slice (256x256) T1-weighted brain images were acquired with 3T MRI to characterize lesion type. Lesions were classified as a periventricular (PV), middle cerebral artery (MCA) or other (Fig 3), by a pediatric neurologist in consultation with a neuroradiologist as needed.
Figure 3.
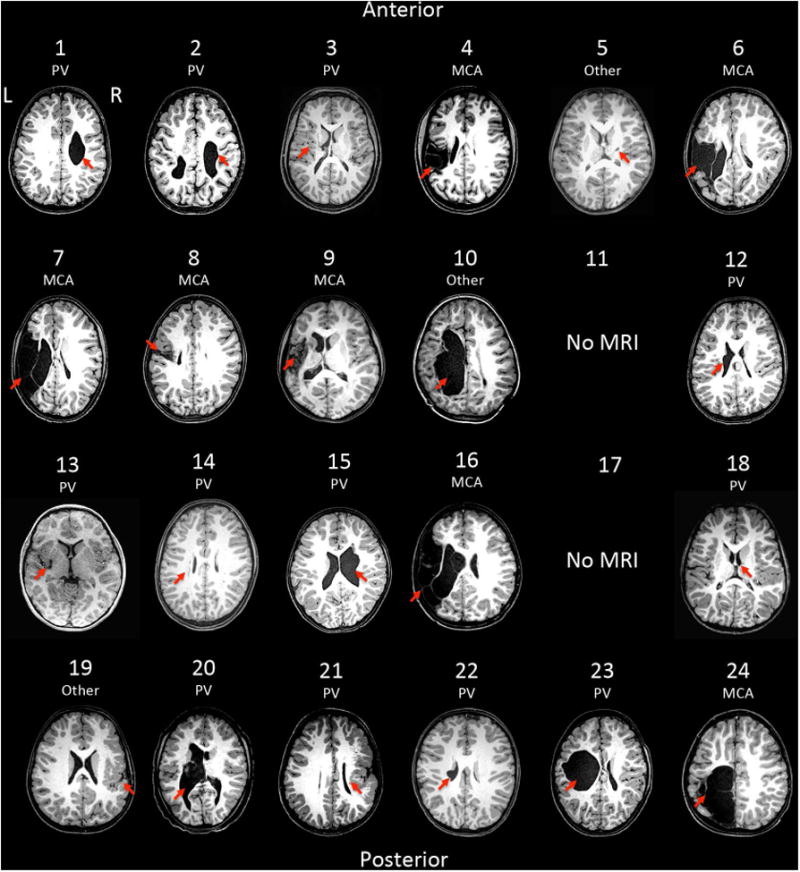
Structural MRI's for all participants, indicating (shown by arrow) the location and type of the lesion. PV: Periventricular, and MCA: Middle Cerebral Artery.
Tests of Hand Movement and Sensation
Five standardized tests were administered to analyze hand function. Unimanual dexterity was assessed with two tests: 1) Jebsen-Taylor Test of Hand Function (JTTHF)30: a timed test of 6 activities—flipping cards, object placement, simulated eating, stacking checkers, and transporting empty and full cans. 2) Box&Blocks31: measure the number of blocks moved between 2 boxes in 60 seconds. Bimanual dexterity was assessed with the Assisting Hand Assessment (AHA)32–structured play using both hands. Sensation was assessed with 1) Cooper Stereognosis33: a measure of the ability to recognize 10 objects placed in the hand with touch. and 2) Two-Point Discrimination34: which test the ability to discriminate 2 points placed 2-15mm apart. As these tests produce non-linear values we linearized and standardized all scores to a continuous scale between 0 (minimum) and 1 (maximum) to compare them. The transformation functions used were: AHA= score/100; Stereognosis= (score/10)2; JTTHF= - (log(score/1080))/4; Box&Blocks= score/60; 2-Point= 1-((score-2)/13). Raw and standardized scores are shown in Supplementary Table. For some participants (n=12), hand function measures were available from one timepoint. For others, scores and EEG from 2 timepoints were available and were averaged, to reduce measurement variability. AHA was our primary outcome because out of the available tests for children with hemiplegia, it best captures the use of impaired hand in daily activities, 2) it is robust, well-validated, and highly reliable.
2.3 Statistical Analysis
Standard univariate and multivariate analyses were performed to first investigate the relationship between the binary physiological and anatomical connectivity (present versus absent) and hand function. For all univariate analysis, significance was tested with a non-parametric permutation (5000 permutations) test24 based on Welch's t-statistic suitable for unequal variance and sample sizes. To compare our five dependent response variables (Cooper, 2-Point, JTTHF, Box & Blocks, AHA), by each of our five binary factors (EEG, DTI somatosensory, TMS, DTI motor, lesion), we used a non-parametric one-way MANOVA35 with permutation significance testing. The statistics were performed on the normalized data, but they were similar to outcomes from raw data (not shown). Sample sizes were: TMS (contra=9, ipsi=12), EEG (present=9, absent=9), DTI motor (present=8, absent=12), DTI sensory (present=16, absent=4), Lesion (PV=12, MCA=7). Effect size was computed with Cohen's d,(for univariate) and Ƞ2 (for MANOVA).
Next, multiple linear regression and cluster analyses were used to test our hypothesis that somatosensory connectivity has a larger significant contribution to hand function than motor connectivity: 1) Relative contribution of motor and sensory physiology (independent variables) to each hand function test (dependent variable) was analyzed using multiple linear regression of sensory physiology (EEG) vs. motor physiology (TMS). Separately, we compared sensory anatomy (DTIs) vs. motor anatomy (DTIm) for each hand function test. 2) Correlation between the sensation and movement tests was analyzed with Spearman Rho. 3) We used a complementary and data-driven approach based on Cluster Analysis36 to analyze the relative contributions of all measurements of connectivity (sensory and motor physiology, sensory and motor anatomy and lesion type) on hand function. Hand function tests were used to group participants into distinct groups. This was performed with a nonparametric dimension reduction visualization called t-distributed Stochastic Neighbor Embedding (t-SNE)37, which compresses the 5 hand function tests into a 2-dimensional representation. This allows clustering of participants based on function. We then compared these clusters of hand function with the measures of anatomical and physiological connectivity and lesion type using k-means clustering. The goal was to determine whether measures of connectivity would segregate cluster of participants depending on their hand function. The fit between hand function clusters and connectivity measures was quantified with Cohen's kappa38 (>0.75: excellent; 0.4-0.75: fair to good, and <0.40: poor).
3 Results
Table 1 summarizes participant demographics and test results.
Both sensory and motor connectivity correlate with hand function Sensory connectivity was assessed using anatomical and physiological measures. For sensory anatomy, DTI was used to determine whether the sensory pathway was present (n=16) or absent (n=4; Fig 2C). For sensory physiology, EEG was used to detect the presence of SEPs after vibrotactile stimulation of the index finger from each hand. SEPs were categorized as present (n=9) or absent (n=9; Fig 2A). For six children, EEG data had poor quality due to participant cooperation or technical issues.
There was a strong and significant association between sensory connectivity and hand function (Fig 4), with large effect sizes for SEPs (d=1.02-3.21) and DTI sensory (d= 0.92-2.34). Compared to children whose sensory tract was absent in the lesioned hemisphere by DTI, children who had an intact sensory tract had better movement and sensory ability of the impaired hand. Similarly, children whose SEP was present in the injured hemisphere had better movement and sensory ability than children without SEPs in the injured hemisphere. Multivariate analysis (MANOVA) of sensory connectivity across all 5 hand function tests showed significant difference of hand function in children with intact somatosensory connectivity (physiologically with SEPs and anatomically with DTI) versus those who did not (Table 2).
Figure 4.
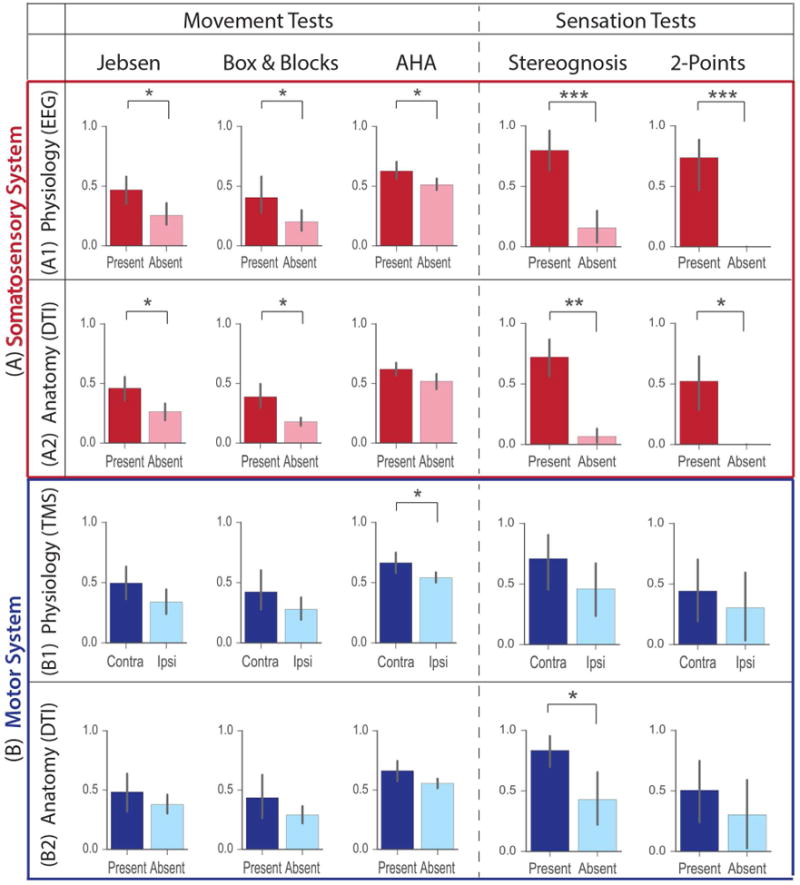
Correlation of somatosensory and motor anatomy and physiology with hand function. Movement tests (Jebsen Taylor, Box & Blocks and AHA) are shown in the left panel and sensation tests (Stereognosis and 2-point Discrimination) in the right. (A) Somatosensory Connectivity: The presence or absence of physiological connectivity in injured cortex, as measured with SEPs, was significantly correlated with all movement and sensation tests. The absence of a SEP was associated with poor hand function. The presence or absence of anatomical connections in injured cortex, as seen with DTI was significantly correlated with hand function for all test except AHA. Absence of tracts was associated with poor hand function.
(B) Motor Connectivity: The presence of predominant contralateral or ipsilateral physiological connections, as measured by TMS, was not significantly correlated with most movement and sensation test scores, except AHA. The presence or absence of anatomical connections in injured cortex, as seen in DTI, were not significantly correlated with hand function tests, except Stereognosis. The absence of the crossed CST was associated only with poor stereognosis. Significance was estimated by a non-parametric permutation test: *** = p < 0.001, ** = p < 0.01, * = p < 0.05. (Colored version of Figure is available online)
Table 2.
A) Effect sizes: Cohen's d was used for univariate analysis: small (0.2), medium (0.5), large (0.8), very large (1.3)50. Minimum effect size for representing a ‘practically’ significant effect (d=0.41)50. Eta2(η2) was used for MANOVA: small (0.02), medium (0.13), large (0.26)50. Partial eta2(ηp2)50 was used in multiple regression: small (0.01-0.06); medium (0.06-0.14); large (>= 0.14).
Significance and effect size for univariate and multivariate statistical analysis
| Significance and effect size for physiological and anatomical multiple regression analysis | ||||||
|---|---|---|---|---|---|---|
| A) Significance (p) | JTHHF | Box&Blocks | AHA | Stereo-gnosis | 2-Point | MANOVA |
| Sensory Physiology (EEG) | 0.0152 | 0.0462 | 0.0176 | 0.0008 | 0.0007 | 0.0003 |
| Sensory Anatomy (DTI) | 0.0417 | 0.0144 | 0.0753 | 0.001 | 0.028 | 0.0005 |
| Lesion | 0.0426 | 0.0948 | 0.0092 | 0.0026 | 0.014 | 0.0005 |
| Motor Anatomy (DTIm) | 0.3032 | 0.2042 | 0.0586 | 0.014 | 0.344 | 0.0952 |
| Motor Physiology (TMS) | 0.1076 | 0.1548 | 0.0186 | 0.0626 | 0.3508 | 0.2028 |
| Effect Size (d, η2) | JTHHF | Box&Blocks | AHA | Stereo-gnosis | 2-Point | MANOVA |
| Sensory Physiology (EEG) | 1.23 | 1.02 | 1.12 | 2.59 | 3.21 | 0.7 |
| Sensory Anatomy (DTIs) | 1.03 | 1.12 | 0.92 | 2.34 | 1.44 | 0.37 |
| Lesion | 1 | 0.76 | 1.3 | 2 | 1.74 | 0.42 |
| Motor Anatomy (DTI) | 0.53 | 0.71 | 1.02 | 1.21 | 0.48 | 0.15 |
| Motor Physiology (TMS) | 0.78 | 0.71 | 1.19 | 0.88 | 0.49 | 0.12 |
| Significance and effect size for univariate and multivariate statistical analysis | ||||||
| B1) Model p value | 0.033 | 0.124 | 0.032 | 0.001 | 0.001 | |
| Model r2 | 0.408 | 0.294 | 0.409 | 0.633 | 0.777 | |
| P val | 0.031 | 0.18 | 0.088 | 0.0009 | 0.0009 | |
| EEG | ηp2 | 0.308 | 0.144 | 0.207 | 0.5831 | 0.721 |
| CI | [−434.2 −23.5] | [ −5.0, 23.9] | [ −1.7, 20.9] | [2.8, 8.4] | [ −13.2, −4.8] | |
| Coeff | −228.9 | 9.4 | 9.6 | 5.6 | −9.0 | |
| P val | 0.38 | 0.22 | 0.123 | 0.579 | 0.379 | |
| TMS | ηp2 | 0.06 | 0.122 | 0.172 | 0.024 | 0.087 |
| CI | [ −312.2 127.3] | [ −6.6, 26.0] | [ −2.9, 21.3] | [ −2.2, 3.8] | [ −6.2, 2.6] | |
| Coeff | −92.4 | 9.7 | 9.2 | 0.8 | −1.8 | |
| B2) Model p value | 0.387 | 0.134 | 0.08 | 0.0002 | 0.08 | |
| Model r2 | 0.105 | 0.22 | 0.26 | 0.62 | 0.31 | |
| DTIs | p value | 0.184 | 0.174 | 0.381 | 0.001 | 0.047 |
| ηp2 | 0.101 | 0.112 | 0.045 | 0.479 | 0.269 | |
| CI | [ −436.3 90.6] | [ −4.9, 24.9] | [ −7.7, 19.2] | [2.4, 7.9] | [ −14.1, −0.09] | |
| Coeff | −172.9 | 10 | 5.7 | 5.2 | −7.1 | |
| DTIm | p value | 0.781 | 0.377 | 0.11 | 0.12 | 0.854 |
| ηp2 | 0.005 | 0.048 | 0.143 | 0.136 | 0.002 | |
| CI | [ −186.4 243.8] | [ −7.2, 18.0] | [ −2.2, 19.8] | [ −0.5, 4.0] | [ −5.6, 6.6] | |
| Coeff | 28.7 | 5.4 | 8.8 | 1.7 | 0.5 | |
Motor connectivity was assessed using anatomical (DTI) and physiological (TMS) measures and compared with tests of hand function. Using DTI (Fig 2C), we determined if the CST was present (n=8) or absent (n=12). During TMS, we stimulated the injured and uninjured hemispheres and recorded MEPs in the FDI and wrist flexor muscles of the affected hand (Fig 2B). In 9 children, bilateral responses were found; these children were categorized as either predominantly ipsilateral or contralateral depending on which motor cortex had the greater number of responsive sites. Predominantly contralateral responses were found in 10 children, and predominantly ipsilateral responses in 12 children. In two of the younger children (participants 23 and 24), no MEPs in the impaired hand were evoked with stimulation of either hemisphere, which is common in young children39.
Univariate analysis of TMS and DTI for each hand function test showed an association between motor connectivity and hand function, with smaller effect sizes (TMS d=0.49-1.19 and DTI motor d=0.48-1.21, Fig 4 and Table 2) than the corresponding somatosensory analysis. The presence of a contralateral TMS MEP was associated with significantly better performance on the AHA, but not with performance on any other test. Similarly, the presence of a contralateral CST, by DTI, was associated with significantly better performance in stereognosis, but not with performance on any other test. Multivariate analysis (MANOVA) of motor connectivity across all 5 hand function tests showed no significant difference of hand function for children who had intact anatomical connections by DTI or physiologically by TMS versus those who did not (Table 2).
To further test the influence of motor connectivity we performed additional analyses. We separated the children with the bilateral (where MEPs were evoked by TMS stimulation of both hemispheres (n=8) responses, from purely ipsilateral and purely contralateral, as performed in previous research studies 25. Using 3 categories did not change the general trend of relationships with the hand function tests. They were however underpowered to test for significance. We also assessed the correlation of the continuous LI values with the hand function tests and it was not found to be significantly correlated with JTTHF, Box & Blocks, 2-point at p<0.05, while it was significantly correlated with AHA and Stereognosis (both p<0.05).
Type of lesion correlates with hand function
Among 24 participants, 12 had clearly identifiable PV lesions, and 7 had lesions in the middle cerebral artery territory involving cortex. Three children had more complex lesions that could not be categorized as PV or MCA, while two children did not have an MRI. We hypothesized that impairments would be more severe in children with MCA lesion for two reasons. First these lesions often occur after the somatosensory tracts have been established, making them unable to skirt the lesion as has been observed for PV lesions. Second, these lesions often involve somatosensory cortex, which is critical for several sensory functions.
The impact of lesion type on hand function with univariate analysis is shown in Fig 5, and Table 2. Children with PV lesions had significantly better hand function scores than children with MCA lesions on all tests, except Box & Blocks. The effect size was moderate (d=0.76-2.00). Multivariate analysis (MANOVA) of lesion type (PV versus MCA) across the 5 hand function tests showed significant difference in hand function for children who had PV lesions versus those who had MCA (Table 2).
Figure 5.
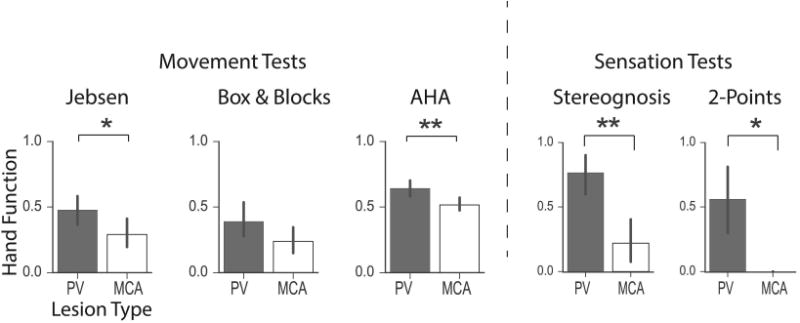
Correlation of lesion type and sensory and motor function. PV and MCA lesion types are significantly different in their impact on the motor (Jebsen Taylor and AHA) and sensory (Stereognosis and 2-Points) function. PV is generally associated with better hand movement and sensation scores than MCA, except for Box & Blocks. Significance was estimated by a non-parametric permutation test: *** = p < 0.001, ** = p < 0.01, * = p < 0.05.
Furthermore, we observe that lesion type was significantly associated with the state of functional somatosensory connectivity but not anatomical connectivity. 84% of participants with MCA lesions had absent SEPs, compared to 12% of with PV lesions (Fisher's Exact Test shows a statistically significant difference between these groups, p= 0.0163). Sensory DTI tracts were absent in 43% of participants with MCA lesions compared to 9% PV lesions (p= 0.137). Similarly, 100% of participants with MCA lesions had TMS ipsilateral connectivity compared to 37% with PV lesions (p= 0.0288). However, DTI motor tracts were absent in 85% of participants with MCA lesions and 83% of those with PV lesions (p= 0.112). This discrepancy between anatomy and physiology highlights the possibility that during recovery, structural tracts (anatomy) may be present, but not necessarily functional (physiology).
Relative contributions of motor and sensory connectivity and lesion type to hand function
The above univariate and multivariate analysis showed that the sensory physiology and anatomy and lesion type each significantly and independently correlated with hand function. In contrast, no significant effect was observed for motor physiology and anatomy on hand function. Next we compared the contribution of motor and sensory connectivity on hand function.
First, we used a model-based approach (multiple linear regression) to analyze the relative contribution of sensory and motor physiology and anatomy to hand function. This differs from the previous analyses where only a correlation between connectivity and function was tested. For physiology, comparing sensory physiology (EEG) vs motor physiology (TMS) (Table 2. B1), sensory physiology had a statistically significant contribution towards movement (JTHHF) and sensation tests (Stereognosis and 2-Point) (p< 0.05 for all) and had large effect sizes for all. In comparison, motor physiology did not have a statistically significant contribution for any of the 5 tests. For anatomy, comparing sensory anatomy (DTIs) vs. motor anatomy (DTIm) (Table 2. B2), sensory anatomy had a statistically significant contribution to sensory hand function tests (Stereognosis and 2-Point) with large effect sizes, while motor anatomy did not show contributions towards any of the 5 hand function tests.
Second, we assessed the correlation between the movement and sensation hand function tests. We found significant correlations between all pairs of movement and sensation tests (p<0.001 for all pairs) (Fig 6B).
Figure 6.
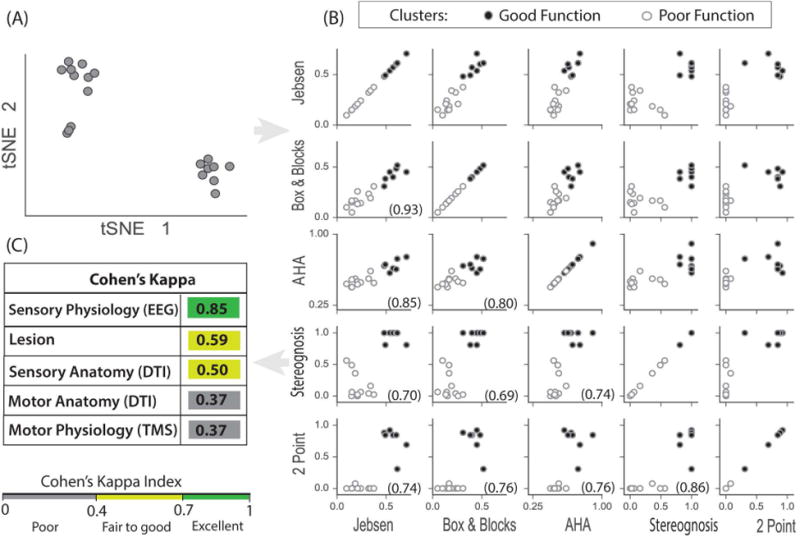
Clustering of behavioral data. (A) t-SNE allows projections of the 5 hand function tests into 2 dimensions (t-SNE 1 and t-SNE 2) which shows 2 distinct participant groups. (B) Clustering outcome from applying k-means clustering on 5 dimensional hand function data. The two clusters are marked for all pairs of hand movement and somatosensory test scores. Each circle represents a single participant, and circles are filled or open based on the 2 clusters. The clusters fall into the top right quadrant (good hand function: filled circles) and the lower left quadrant (poor hand function: open circles) for all pairs of hand function tests. All tests pairs were also significantly correlated, tested with non-parametric Spearman's rho (p < 0.001), rho is shown in each subplot in parentheses. (C) Cohen's kappa was used to estimate cluster agreement with anatomy, physiology and lesion type. Somatosensory physiology was the most sensitive indicator of good or poor hand function.
Third, we used a data-driven approach to find possible distinct groups based only on the hand function tests, rather than the sensory-motor anatomy or physiology. The t-SNE based visualization of the 5-dimension data (i.e., each movement and sensation test) in 2-dimensions revealed two highly distinct groups of comparable size (Fig 6A). To interpret the relationship of these groups with the hand function, we applied a k-means clustering, setting k= 2. To observe the relationship of these clusters and the hand function tests, we labeled the 2 clusters obtained from k-means clustering in all possible pairs of hand function test plots (shown as open and closed circles in Fig 6B). The clusters were found automatically in 2 quadrants: the upper right quadrant (good function) and the lower left quadrant (poor function). This segregation was stronger for the sensation tests than for the movement tests, as seen in the separation between the clustered participant groups in Fig 6B.
Then we determined how well each of the 5 measurement modalities (i.e. the motor and sensory anatomy and physiology plus lesion location) explained whether participants fall into these good versus poor function clusters. We measured agreement of each measurement category (absent/present for DTI and EEG, ipsi/contra for TMS, or PV/MCA for lesion) with the clusters using Cohen's kappa coefficient. As shown in Fig 6C, clusters strongly agree with the physiological somatosensory connectivity (EEG SEPs, kappa=0.85), fairly well with lesion and anatomical somatosensory categorization (kappa=0.59 and 0.50 respectively), and poorly with motor anatomy and physiology (kappa=0.37 and 0.37 respectively).
Thus, the above independent statistical procedures (univariate and multivariate analysis, multiple regression analyses and cluster analyses) all support the hypothesis that somatosensory connectivity (anatomy and physiology) is strongly correlated with hand function and has a larger relative contribution towards hand function compared to motor connectivity. In addition, lesion type strongly correlates with hand function as well; with MCA lesions associated with poor hand function and PV lesions with better hand function.
Discussion
Loss of somatosensory connectivity was strongly correlated with impairments of hand function in children with USCP. Motor connectivity was associated with impairments in bimanual function in children with USCP. Connectivity was determined using DTI for both the motor and somatosensory tracts. This outcome was achieved because we were able to assess the physiology and anatomy of these two systems and hand function in the same set of children. In addition, our procedures to extract the SEPs allowed detection of responses that can elude other detection methods. In particular, we presented a large number of trials in a short time and employed machine learning techniques to extract information from the EEG with relatively lower signal-to-noise ratio and spatio-temporal alterations in the neural responses due to developmental plasticity. Finally, the statistical and clustering methods employed supported the main hypothesis through two independent and objective measures.
Developmental response to injury
The main findings of our study can be understood in the context of the developmental response to injury. Participants with cortical damage tended to lack a classifiable SEP in the injured hemisphere, while participants with a subcortical lesion tended to have a classifiable SEP. This dichotomized relationship between lesion type and the somatosensory response supports our current working model. As illustrated in Fig 1B and C, the somatosensory connectivity is less likely to be affected in an early injury, such as a PV lesion, as these tracts are still developing and can skirt the lesion and reach the cortex. However, the established somatosensory connectivity is more likely to be disrupted in later injuries, such as the larger MCA lesions. The somatosensory connections do not have bilateral connections that might persist after unilateral injury, as the corticospinal system does.
These differences in the developmental alteration of the motor and somatosensory systems, often leads to motor control in the uninjured hemisphere and somatosensory function in the injured hemisphere (such as for participants 1-5). We thought that the two functions being segregated in opposite hemispheres would have a strong adverse effect on hand function, as these systems are highly interdependent. However, the effect of motor control for the affected hand being in the opposite hemisphere from somatosensory control was rather weak (i.e. no significant cross-effect). This suggests that ipsilateral motor control, while less optimal than typical contralateral control, may be a promising developmental alteration, even if sensory function is encoded in the opposite hemisphere. However, the integrity of the less adaptable somatosensory connections was significantly correlated with hand function. This implies that despite the motor adaptation, the integrity of the somatosensory connections had a relatively larger implication for the hand function. Developmental preservation of bilateral connections observed in children might make them less reliant on crossed CST connections than adults. This may help to explain why loss of CST connections may have a relatively weaker effect on hand function in children compared with adults40.
Limitations
The main limitation of the study is that it has relatively small number of participants, with heterogeneities in the time, size and location of injury. Nevertheless, we see significant contribution of the somatosensory system to hand function with large effect sizes (d=1.0 to 3.2). A larger cohort with more participants in each subgroup might make the outcomes more statistically significant, especially in case of the motor connectivity analysis which had relatively smaller effect sizes (d=0.5 to 1.2). Indeed, our group25 and others41 have shown that CST connectivity is significantly associated with hand function. In this study, many children had both contralateral and ipsilateral CSTs. In other studies, these children with a bilateral CST have been analyzed separately than children with purely contralateral or ipsilateral CSTs. In the current study, there was insufficient power to analyze children with bilateral CSTs separately. Thus, we categorized children as having an ipsilateral CST if a majority of the map was located in the hemisphere ipsilateral to the affected hand. Four of thirteen children categorized as ipsilateral had a contralateral CST. Analysis of larger groups of children with different patterns of connectivity can help better elucidate the relationship to hand control.
Moreover, studies about the relationship between hand function and time/type or site of injury, attribute the early anatomical injury to the individual's current state of hand function as measured by hand function tests. This does not take into account interventions, compensatory strategies, or other neurological impairments, which can have an impact on hand function42. However, despite this expected variation we observed a significant overall impact of lesion type on hand function in a small participant group. Nevertheless, it should be noted that this study has been performed with a small group and should be replicated in a larger group. In addition, this study has used dichotomous variables, which allowed direct comparison of all anatomy and physiology measures. However, this can limit our understanding of how the strength of measure can influence hand function. Future studies could potentially use continuous variables not just for TMS laterality, but for other variables as well.
Conclusions
The somatosensory findings reinforce the importance of considering the anatomical and functional state of this system in USCP at an early age. Considering previous studies where somatosensory training43 or peripheral nerve stimulation44 has indicated promise in improving motor deficits, specifically demonstrated in diplegic CP45, it might be useful to evaluate the inclusion of such training early on in the rehabilitation of the motor function in children with USCP. This might be especially useful for the sub-group of children who had a larger cortical-subcortical lesion like MCA , that occurs close to birth , or who have a disrupted somatosensory physiology or anatomy. Conversely, intensive motor training has shown concurrent improvements in the somatosensory function46 , although , these improvements in movement and sensation were not found to be correlated and somatosensory improvements did not last.
Another promising intervention for the motor rehabilitation in CP , has been constraint induced movement therapy47. In this approach the less-affected hand is constrained to force the use of the more-affected hand. It remains to be tested whether a similar somatosensory constraint may be beneficial depending on how viable the somatosensory connections are from the injured hemisphere. Further, brain stimulation has also been used in CP to alter motor cortex excitability48,49. Our results suggest that connectivity, particularly of the somatosensory system, is critical for hand use in these children. Insofar as non-invasive brain stimulation can alter activity of these cortical networks, it could be a useful tool to improve hand function.
In conclusion, this study demonstrates the importance of both sensory and motor connectivity on hand movement and sensation. These associations were compared directly through 2 independent methods: multivariate and clustering analyses, and both demonstrated strong effects of sensory connectivity and more moderate effects of motor connectivity. Lesion type was found to be correlated with hand function as well. These analyses support our main hypothesis and point to somatosensation as a possible critical driver of hand function in children with USCP. A better understanding of these two circuits integral to hand function could offer new approaches to therapy.
Supplementary Material
Supplementary Table 1: Three movement tests (JTHHF, Box & Blocks, AHA) and two sensation tests (Stereognosis, 2-Points) were measured for each subject. Index: <>: Unable to make conclusion or lack of attention. Outcome measures for movement and sensation hand function tests.
Acknowledgments
This work was supported by Horace Goldsmith Foundation (D.G), NIH-R01-HD076436 (K.M.F), NIH-K08-NS073796-05 (J.B.C), Blythedale Children's Hospital, Carvel Foundation and the Mai Family Foundation. We thank Lindsey Soles for helping with DTI analysis. We thank faculty, students, and volunteers at Teachers College, Columbia University for integrating neurophysiology experiments with ongoing CP study. We thank Jacqueline A. Bello, MD, for help with interpretation of lesions on MRI. We thank Dr. Linda Gerber (CTSC- Weill Cornell) for help with statistical analysis. Research reported in this publication was supported by the National Center For Advancing Translational Science of the National Institute of Health Under Award Number UL1TR000457
Footnotes
Author Contributions: Study concept and design: D.G, A.B, J.B.C, K.M.F, Data acquisition and analysis: D.G, A.B, J.B.C, K.M.F, H-C.K, C.F, A.M.G, Drafting manuscript and figures: D.G, J.B.C, K.M.F
Conflicts of Interest: Authors have no conflicts to report.
Contributor Information
Disha Gupta, Burke Medical Research Institute, White Plains, NY, USA, Weill Cornell Medicine, New York, NY, USA, Computational Science & Engineering, Cornell University, Ithaca, USA, Helen Hayes Hospital, West Haverstraw, NY, USA.
Alexandre Barachant, Burke Medical Research Institute, White Plains, NY, USA.
Andrew M. Gordon, Teachers College, Columbia University, NY, USA.
Claudio Ferre, Burke Medical Research Institute, White Plains, NY, USA.
Hsing-Ching Kuo, Alberta Children's Hospital Research Institute, University of Calgary, Calgary, AB, Canada.
Jason B. Carmel, Burke Medical Research Institute, White Plains, NY, USA, Weill Cornell Medicine, New York, NY, USA, Blythedale Children's Hospital, Valhalla, NY, USA.
Kathleen M. Friel, Burke Medical Research Institute, White Plains, NY, USA, Weill Cornell Medicine, New York, NY, USA, Blythedale Children's Hospital, Valhalla, NY, USA.
References
- 1.Ostensjø S, Carlberg EB, Vøllestad NK. Motor impairments in young children with cerebral palsy: relationship to gross motor function and everyday activities. Dev Med Child Neurol. 2004;46(9):580–9. doi: 10.1017/s0012162204000994. [DOI] [PubMed] [Google Scholar]
- 2.Friel KM, Kuo HC, Carmel JB, et al. Improvements in hand function after intensive bimanual training are not associated with corticospinal tract dysgenesis in children with unilateral cerebral palsy. Exp Brain Res. 2014;232(6):2001–9. doi: 10.1007/s00221-014-3889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleyenheuft Y, Grandin CB, Cosnard G, et al. Corticospinal dysgenesis and upper-limb deficits in congenital hemiplegia: a diffusion tensor imaging study. Pediatrics. 2007;120(6):1502–11. doi: 10.1542/peds.2007-0394. [DOI] [PubMed] [Google Scholar]
- 4.Duque J, Thonnard JL, Vandermeeren Y, et al. Correlation between impaired dexterity and corticospinal tract dysgenesis in congenital hemiplegia. Brain. 2003;126:732–47. doi: 10.1093/brain/awg069. [DOI] [PubMed] [Google Scholar]
- 5.Staudt M, Gerloff C, Grodd W, et al. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann Neurol. 2004;56:854–863. doi: 10.1002/ana.20297. [DOI] [PubMed] [Google Scholar]
- 6.Holmstrom L, Vollmer B, Tedroff K, et al. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Dev Med Child Neurol. 2010;52(2):145–52. doi: 10.1111/j.1469-8749.2009.03496.x. [DOI] [PubMed] [Google Scholar]
- 7.Bleyenheuft Y, Gordon AM. Precision grip control, sensory impairments and their interactions in children with hemiplegic cerebral palsy: a systematic review. Research in Developmental Disabilities. 2013;34(9):3014–28. doi: 10.1016/j.ridd.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 8.Wingert JR, Burton H, Sinclair RJ, et al. Tactile sensory abilities in cerebral palsy: Deficits in roughness and object discrimination. Dev Medicine and Child Neurology. 2008;50(11):832–838. doi: 10.1111/j.1469-8749.2008.03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon AM, Duff SV. Relation between clinical measures and fine manipulative control in children with hemiplegic cerebral palsy. Dev. Med Child Neurology. 1999;41(9):586–591. doi: 10.1017/s0012162299001231. [DOI] [PubMed] [Google Scholar]
- 10.Friel K, Martin JH. Rebalancing corticospinal activity promotes recovery of motor skill and anatomical integrity after inactivation during a critical period. J Neurosci. 2007;27:11083–90. doi: 10.1523/JNEUROSCI.2814-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eyre JA. Corticospinal tract development and its plasticity after perinatal injury. Neurosci Biobehav Rev. 2007;31(8):1136–49. doi: 10.1016/j.neubiorev.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Staudt M. Brain plasticity following early life brain injury: insights from neuroimaging. Semin Perinatol. 2010 Feb;34(1):87–92. doi: 10.1053/j.semperi.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Holloway V, Gadian DG, Vargha-Khadem F, et al. The reorganization of sensorimotor function in children after hemispherectomy. A functional MRI and somatosensory evoked potential study. Brain. 2000;123(12):2432–2444. doi: 10.1093/brain/123.12.2432. [DOI] [PubMed] [Google Scholar]
- 14.Mackey A, Stinear C, Sott S, et al. Upper limb function and cortical organization in youth with unilateral cerebral palsy. Frontiers Neurology. 2014;5(117):1–9. doi: 10.3389/fneur.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staudt M. (Re-)organization of the developing human brain following periventricular white matter lesions. Neurosci Biobehav Rev. 2007;31(8):1150–6. doi: 10.1016/j.neubiorev.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Wilke M, Staudt M, Juenger H, et al. Somatosensory system in two types of motor reorganization in congenital hemiparesis: topography and function. Hum Brain Mapp. 2009;30(3):776–88. doi: 10.1002/hbm.20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzzetta A, Bonanni P, Biagi L, et al. Reorganisation of the somatosensory system after early brain damage. Clin Neurophysiol. 2007;118(5):1110–21. doi: 10.1016/j.clinph.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Sannia A, Natalizia AR, Parodi A, et al. Different gestational ages and changing vulnerability of the premature brain. J Matern Fetal Neonatal Med. 2015;8S, 1:2268–72. doi: 10.3109/14767058.2013.796166. [DOI] [PubMed] [Google Scholar]
- 19.Hoon AH, Jr, Stashinko EE, Nagae LM, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 2009;51(9):697–704. doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadelis C, Ahtam B, Nazarova M, et al. Cortical somatosensory reorganization in children with spastic cerebral palsy: a multimodal neuroimaging study. Front Hum Neuroscience. 2014;8:725. doi: 10.3389/fnhum.2014.00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riquelme I, Montoya P. Developmental changes in somatosensory processing in cerebral palsy and healthy individuals. Clinical Neurophysiology. 2010;121:1314–20. doi: 10.1016/j.clinph.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Müller-Gerking J, Pfurtscheller G, Flyvbjerg H. Designing optimal spatial filters for single-trial EEG classification in a movement task. Clinical Neurophysiology. 1999;110((5)1):787–798. doi: 10.1016/s1388-2457(98)00038-8. [DOI] [PubMed] [Google Scholar]
- 23.Barachant A, Bonnet S, Congedo M, Jutten C. Classification of covariance matrices using a Riemannian-based kernel for BCI applications. Neurocomputing. 2013;112:172–178. [Google Scholar]
- 24.Maris E, Oostenvald R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–902007. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Smorenburg AR, Gordon AM, Kuo HC, et al. Does Corticospinal Tract Connectivity Influence the Response to Intensive Bimanual Therapy in Children With Unilateral Cerebral Palsy? Neurorehabil Neural Repair. 2016 doi: 10.1177/1545968316675427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang R, Benner T, Sorensen AG, Wedeen VJ. Diffusion toolkit: a software package for diffusion imaging data processing and tractography. Proc Intl Soc Magn Reson Med. 2007;15:3720. [Google Scholar]
- 27.Yang DS, Hong JH, Byun WM, et al. Identification of the medial lemniscus in the human brain: Combined study of functional MRI and diffusion tensor tractography. Neuroscience Letters. 2009;459:19–24. doi: 10.1016/j.neulet.2009.04.058. [DOI] [PubMed] [Google Scholar]
- 28.Purves D, Augustine GJ, Fitzpatrick D, et al. The Major Afferent Pathway for Mechanosensory Information: The Dorsal Column-Medial Lemniscus System Neuroscience. 2nd. Sunderland (MA): Sinauer Associates; 2001. pp. 189–207. [Google Scholar]
- 29.DTI Studio: software. available at: dsi-studio.labsolver.org.
- 30.Jebsen RH, Taylor N, Trieschmann RB, et al. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–319. [PubMed] [Google Scholar]
- 31.Volland G, Mathiowetz V. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39:386–91. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 32.Krumlinde-Sundholm L, Holmefur M, Kottorp A, Eliasson AC. The Assisting Hand Assessment: Current evidence of validity, reliability and responsiveness to change. Dev Med Child Neur. 2007;49:259–264. doi: 10.1111/j.1469-8749.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 33.Majnemer A, Cooper J. The determination of sensory deficits in children with hemiplegic cerebral palsy. J Child Neurology. 1995;10:300–9. doi: 10.1177/088307389501000412. [DOI] [PubMed] [Google Scholar]
- 34.Moberg E. 2 point discrimination test a valuable part of hand surgical rehabilitation eg in tetraplegia. Scandinavian Journal of Rehabilitation Medicine. 1990;22:127–134. [PubMed] [Google Scholar]
- 35.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46. [Google Scholar]
- 36.Lloyd SP. Least squares quantization in PCM, IEEE Transactions on Information Theory. 1982;28(2):129–137. [Google Scholar]
- 37.van der Maaten LJP, Hinton GE. Visualizing High-Dimensional Data Using t-SNE. Journal of Machine Learning Research. 2008;9:2579–2605. [Google Scholar]
- 38.Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20(1):37–46. [Google Scholar]
- 39.Koh TH, Eyre JA. Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex. Arch Dis Child. 1988;63(11):1347–52. doi: 10.1136/adc.63.11.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng W, Wang J, Chhatbar PY, et al. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann Neurol. 2015;78(6):860–70. doi: 10.1002/ana.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zewdie E, Damji O, Ciechanski P, et al. Contralesional Corticomotor Neurophysiology in Hemiparetic Children With Perinatal Stroke: Developmental Plasticity and Clinical Function. Neurorehabil Neural Repair. 2016;31(3):261–271. doi: 10.1177/1545968316680485. [DOI] [PubMed] [Google Scholar]
- 42.Priego Q, Lucas-Cuevas JI, Llana-Belloch AG. Effects of Exercise in People with Cerebral Palsy. A Review. Journal of Physical Education and Sport. 2014;14(1):36–41. [Google Scholar]
- 43.Kaelin-Lang A. Enhancing rehabilitation of motor deficits with peripheral nerve stimulation. NeuroRehabilitation. 2008;23(1):89–93. [PubMed] [Google Scholar]
- 44.Conforto AB, Ferreiro KN, Tomasi C, et al. Effects of somatosensory stimulation on motor function after subacute stroke. Neurorehabil Neural Repair. 2010;24:263–272. doi: 10.1177/1545968309349946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bumin G, Kayihan H. Effectiveness of two different sensory-integration programmes for children with spastic diplegic cerebral palsy. Disabil Rehabil. 2001;23(9):394–9. doi: 10.1080/09638280010008843. [DOI] [PubMed] [Google Scholar]
- 46.Kuo HC, Gordon AM, Henrionnet A, et al. The effects of intensive bimanual training with and without tactile training on tactile function in children with unilateral spastic cerebral palsy: A pilot study. Res Dev Disabil. 2016;49-50:129–39. doi: 10.1016/j.ridd.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon AM, Hung YC, Brandao M, et al. Bimanual Training and Constraint-Induced Movement Therapy in Children With Hemiplegic Cerebral Palsy- A Randomized Trial. Neurorehabil Neural Repair. 2011;25(8):692–702. doi: 10.1177/1545968311402508. [DOI] [PubMed] [Google Scholar]
- 48.Grecco LA, de Almeida Carvalho Duarte N, Mendonça ME, et al. Transcranial direct current stimulation during treadmill training in children with cerebral palsy: a randomized controlled double-blind clinical trial. Res Dev Disabil. 2014;35(11):2840–8. doi: 10.1016/j.ridd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 49.Kumru H, Murillo N, Samso JV, et al. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair. 2010;24(5):435–41. doi: 10.1177/1545968309356095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Three movement tests (JTHHF, Box & Blocks, AHA) and two sensation tests (Stereognosis, 2-Points) were measured for each subject. Index: <>: Unable to make conclusion or lack of attention. Outcome measures for movement and sensation hand function tests.


