Capsule Summary
Higher maternal 25OHD levels at early pregnancy could reduce the associated risk of uncontrolled asthma status during pregnancy. Obesity might attenuate this effect and is a predictor of uncontrolled status during pregnancy.
Keywords: Asthma, Exacerbation, Pregnancy, BMI, Vitamin D
To the Editor
Asthma is one of the most common health problems during pregnancy. It has been suggested that asthma severity prior to pregnancy is associated with uncontrolled asthma status during pregnancy1, and this may result in adverse pregnancy outcomes, such as preterm birth and low birth weight2. The aims of our study were four-fold: To examine 1) whether the prevalence of uncontrolled asthma, assessed by the Asthma Control Test (ACT) during pregnancy is consistent with prior estimates; 2) whether baseline uncontrolled asthma status evaluated by ACT at subjects’ enrollment (10–18 weeks of gestation) would be associated with increased risk of uncontrolled asthma during the remainder of pregnancy; 3) whether serum vitamin D level (25OHD) is associated with asthma control status at enrollment; 4) whether body mass index (BMI) at enrollment affects the relationship of 25OHD and asthma control status at enrollment and during pregnancy. We performed these analyses on pregnant asthmatics from the Vitamin D Antenatal Asthma Reduction Trial (VDAART), where we randomized 881 pregnant women. After exclusion of 65 subjects (7.38%) due to post-randomization ineligibility, no return for the follow-up questionnaires or incomplete labor and delivery records, 816 subjects were included in the trial arms and 327 subjects (40.07%) who had physician-diagnosed asthma were included in this secondary analysis. The asthma control status was assessed at enrollment and monthly thereafter using the ACT. The study group characteristics, subject distributions by site and race, and additional methods have been provided in the article’s Online Repository at www.jacionline.org.
The prevalence of uncontrolled asthma status at enrollment was 12.8%. The asthmatic pregnant women with uncontrolled asthma status at enrollment experienced almost 4 times higher risk of having uncontrolled asthma months during the remainder of their pregnancy as compared with those with controlled asthma (aIRR: 3.8, 95% CI: 2.66–5.40). This finding concurs with prior evidence that suggests worsening of asthma activity during pregnancy is associated with baseline asthma control status1. Overall, 31.5% of the women experienced at least one episode of an uncontrolled asthma month during their pregnancy. Similarly, Schatz and colleagues in a prospective study of 330 asthmatic women demonstrated that asthma worsened in 35.0% of asthmatic women during pregnancy3.
Vitamin D levels may affect asthma activity, particularly in obese individuals4, 5. To our knowledge, no prior study has investigated the potential effect of 25OHD levels on asthma control status at early pregnancy. Therefore, we explored this relationship. We did not observe a significant difference in uncontrolled asthma status after enrollment between treatment and placebo groups (P=0.15), mostly due to higher frequency of uncontrolled asthma months in the treatment arm at enrollment (Supplement Table 1). However, asthmatic women with uncontrolled asthma at enrollment had lower serum 25OHD level compared with those whose asthma was controlled at enrollment (19.6 ± 8.8 ng/mL vs. 23.0 ± 10.4 ng/mL, P=0.04). After adjustment for potential confounders, the inverse association of asthma control status and 25OHD level persisted (Table 1A) such that a 10 ng/ml increase in 25OHD level resulted in up to 80.0% reduction in the risk of uncontrolled asthma status at early pregnancy (aOR10-unit: 0.19, 95%CI: 0.04–0.90). This effect showed dependency on the BMI measure at enrollment (P =0.03). Consequently, the positive effect of higher 25OHD level on asthma control was attenuated as the enrollment BMI progressively increased such that BMI >35 kg/m2 gradually increased the risk of uncontrolled asthma status at early pregnancy (Figure 1). It is noted that 25OHD level was inversely correlated with BMI at enrollment (rs=−0.33, P<0.001). BMI at enrollment was significantly higher in African Americans compared with non-African Americans (31.7 ± 9.0 vs. 28.6 ± 7.3 mg/kg2, P=0.001) and they also had more uncontrolled asthma (Supplemental Table 2). Prior reports have shown an inverse association between 25OHD levels and various parameters of asthma severity and control5, 6, but there is no prior report on this association in pregnant women.
Table 1.
| A Logistic regression results for variables of interests associated with uncontrolled asthma status at enrollment (10–18 weeks of gestation). Complete results are provided in Supplemental Table 3. | ||
|---|---|---|
| Terms | Univariable OR (95% CI) |
Multivariable aOR (95% CI) |
| 25OHD (ng/ml) at enrollment (10–18 weeks) | 0.96 (0.93–1.002) | 0.85 (0.73–0.99) |
| BMI at enrollment (kg/m2) | 1.07 (1.03–1.11) | 0.96 (0.87–1.05) |
| 25OHD*BMI at enrollment | 1.004 (0.999–1.008) | 1.005 (1.004–1.010) |
| B. Poisson regression results for variables of interest associated with uncontrolled asthma month during pregnancy (from enrollment to delivery). Complete results are provided in Supplemental Table 4. | ||
| Univariable IRR (95% CI) |
Multivariable aIRR (95% CI) |
|
| 25OHD (ng/ml) at enrollment (10–18 weeks) | 0.98 (0.96–0.99) | — |
| BMI at enrollment (kg/m2) | 1.04 (1.025–1.056) | 1.02 (1.006–1.038) |
Figure 1.
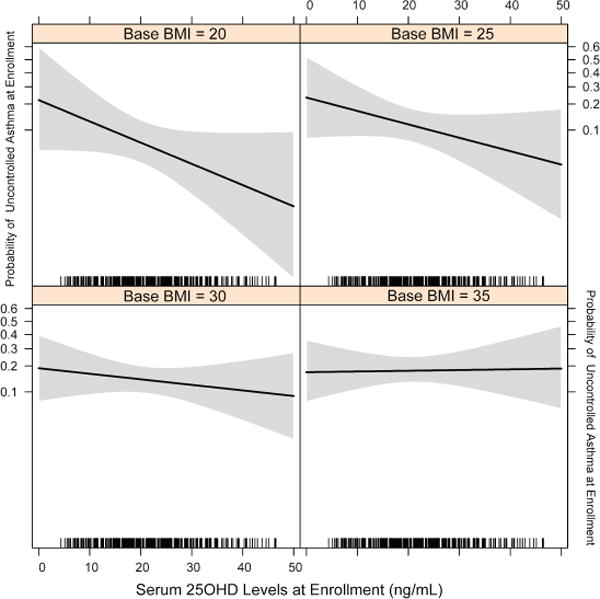
The dose-response relationship of serum 25OHD levels at enrollment (10–18 weeks of gestation) and the risk of uncontrolled asthma status at enrollment. Body Mass Index (BMI) attenuates the efficacy of maternal serum vitamin D level in reducing the risk of uncontrolled asthma. The grey shaded areas depict the 95% confidence bands of uncontrolled asthma risk point estimates.
Finally, we used Poisson regression modeling to further investigate the predictors of uncontrolled asthma during pregnancy. In the adjusted model, 25OHD level at entry was no longer a significant predictor of uncontrolled asthma, however, initial BMI remained as a main predictor of uncontrolled asthma during pregnancy (Table 1B). The incidence rate of uncontrolled asthma was increased by 25% for a 10-unit increase of BMI (aIRR10-unit: 1.25, 95%CI: 1.10–1.46). There are two points that could potentially explain the observed BMI effect on 25OHD risk modification of asthma control. First, it has been shown that vitamin D bioavailability (both 25OHD and 1,25OH2D3) is BMI-dependent and considerably decreases in obesity7. Second, there is evidence suggesting that high BMI, a recognized risk factor for asthma severity and exacerbation, might be linked to asthma through a chronic inflammatory response, physiologic changes to lung function and steroid resistance8. Our findings are in line with other studies recommending the importance of healthy BMI at the beginning of pregnancy, weight gain based on BMI categories during pregnancy and weight specific vitamin D supplementation during pregnancy9.
In summary, our data suggest that baseline asthma activity at early pregnancy is a predictor for asthma control status during pregnancy. In as much as 25OHD levels are associated with asthma control status at baseline, the correction of low vitamin D status at earliest opportunity during pregnancy might be beneficial to reduce the risk of uncontrolled asthma. BMI is a strong predictor of asthma control status during pregnancy, thus, closer monitoring of obese pregnant asthmatics may be warranted. Finally, the modification of the effect of 25OHD levels by BMI will need to be explored in future studies.
Supplementary Material
Acknowledgments
Funding: This study has received research support for Vitamin D Antenatal Asthma Reduction Trial (VDAART) by U01 HL091528 (to S.T.W. and A.A.L.) from the National Heart, Lung, and Blood Institute (NHLBI); and National Research Service Award T32-HL007427 (to H.M.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: VDAART was registered at ClinicalTrials.gov by ID# NCT00920621
References
- 1.Schatz M, Dombrowski MP, Wise R, Thom EA, Landon M, Mabie W, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol. 2003;112:283–8. doi: 10.1067/mai.2003.1516. [DOI] [PubMed] [Google Scholar]
- 2.Kallen B, Rydhstroem H, Aberg A. Asthma during pregnancy–a population based study. Eur J Epidemiol. 2000;16:167–71. doi: 10.1023/a:1007678404911. [DOI] [PubMed] [Google Scholar]
- 3.Schatz M, Harden K, Forsythe A, Chilingar L, Hoffman C, Sperling W, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol. 1988;81:509–17. [PubMed] [Google Scholar]
- 4.Mirzakhani H, Al-Garawi A, Weiss ST, Litonjua AA. Vitamin D and the development of allergic disease: how important is it? Clin Exp Allergy. 2015;45:114–25. doi: 10.1111/cea.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korn S, Hubner M, Jung M, Blettner M, Buhl R. Severe and uncontrolled adult asthma is associated with vitamin D insufficiency and deficiency. Respir Res. 2013;14:25. doi: 10.1186/1465-9921-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanlint S. Vitamin D and obesity. Nutrients. 2013;5:949–56. doi: 10.3390/nu5030949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baffi CW, Winnica DE, Holguin F. Asthma and obesity: mechanisms and clinical implications. Asthma Research and Practice. 2015;1:1–7. doi: 10.1186/s40733-015-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekwaru JP, Zwicker JD, Holick MF, Giovannucci E, Veugelers PJ. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS One. 2014;9:e111265. doi: 10.1371/journal.pone.0111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


