Abstract
Despite its simplicity and efficacy, the promotion of hand washing for disease prevention remains a challenge particularly in resource-limited settings. Here we report on a quasi-experimental school-based study that aimed to improve habitual hand washing. Significant increases in hand washing occurred following improvements in hygiene and sanitation facilities (School A: t=13.86, p=0.0052). Smaller increases in hand washing occurred following education (School A: t=2.63; p=0.012; School B, no infrastructure improvements: t=1.66, p=0.239). Health policy and programming need to pay greater attention to the interplay of the structural, social, and individual dimensions of unique contextual environments that influence habitual behaviours.
Keywords: hand washing, water borne disease, hygiene and sanitation, quasi-experimental design, South Africa
Introduction
Diarrhoea is a serious global public health problem. The yearly global diarrheal disease burden is estimated at up to 98.9 million disability adjusted life years (DALYs) lost through incapacitation and premature deaths, with populations in low- and middle-income countries significantly affected (Murray & Lopez, 1996; Murray et al., 2012; Oria, Pinkerton, Lima, & Guerrant, 2010). Diarrhoea is to be responsible for 9% of deaths of children under the age of five in 2015, killing over 1,400 children a day, or 526,000 a year (UNICEF, 2017). Most diarrhea related deaths in children occur in those under the age of (UNICEF, 2017). Diarrhoea causing enteric pathogens are transmitted through improper or lack of hand washing after defecation (Curtis et al., 1995; Lanata, Huttly, & Yeager, 1998; LeBaron et al., 1990; Traore et al., 1994), improper handling of ready-to-eat foods (US Department of Health Services, 1999), contaminated drinking water (WHO/UNICEF, 2012), and person-to-person contact (Black et al., 1989).
In an effort to address this significant global disease burden, the United Nations established the Millennium Development Goal (MDG) 7C – by 2015, halve the proportion of people without sustainable access to safe drinking water and basic sanitation. This goal was updated to the Sustainable Development Goal 6 – to provide clean water and sanitation to everyone by 2030. Despite progress, worldwide one in nine people (783 million individuals) have no source of safe drinking water, and one in three (2.4 billion) lack improved sanitation – numbers that are projected to double by 2025 (Mara 2003, WHO/UNICEF 2015, The Water Project 2017). Furthermore, 946 million people practice open defecation (WHO/UNICEF 2017). Sub-Saharan Africa has frequently been cited as being slow to make progress to improve water, sanitation, and hygiene. According to the “25 years of progress on sanitation and drinking water” report, developed jointly by UNICEF and the WHO (2015), sub-Saharan Africa did not meet the drinking water target, with 68% of the population using improving drinking water, up 20 percentage points from 1990. In addition, only 30% of the population in sub-Saharan Africa use improved sanitation facilities, up only 6 percentage points from 1990 (UNICEF/WHO, 2015).
There are many possible ways to reduce diarrhoeal disease. Such initiatives have collectively been termed WASH (water, sanitation and hygiene). As evidenced by the failure to achieve the MDG 7C goal despite significant efforts, WASH programs are not always effective and some programmatic elements may prove more successful than others. A meta-analysis of non-randomized community-based studies, found a reduction in diarrhoea risk from WASH interventions in low- and middle-income countries ranged from 44% to 47% (Curtis & Cairncross, 2003; Fewtrell et al., 2005; Luby et al., 2005). Providing clean water alone offered a 27% reduction in diarrhoea (Clasen, Roberts, Rabie, Schmidt, & Cairncross, 2006; Clasen, Schmidt, Rabie, Roberts, & Cairncross, 2007). A benefit-cost analysis of WASH initiatives, identified basic sanitation as having a greater benefit to health and at a lower cost than improvements in potable water supply, particularly in rural areas (Hutton, 2015). The World Health Organization defines basic sanitation as the lowest-cost technology ensuring hygienic excreta and sullage disposal and a clean and healthful living environment both at home and in the neighbourhood of users. Access to basic sanitation includes safety and privacy in the use of these services. Improved sanitation facilities include public sewer connection, septic system connection, pour-flush latrine, simple pit latrine, ventilated improved pit latrine (WHO).
Improving water access and sanitation facilities are important components of the WASH agenda; however, hygiene, relating specifically to the behaviours of individuals is an equally important component. Aunger, Coombes, Curtis, Mosler, and Trevaskis (cited in Liu et al., 2012) argue:
Taps and toilets alone do not ensure that public health is protected. Key to preventing child deaths from diarrhoea is the safe use of such facilities. Having a toilet but then leaving it unused, or only partially used, allows pathogens into the domestic environment. Having water available, but then not using it for washing hands allows foods, drinks and surfaces to become contaminated.
Hand washing is considered an accessible, low cost behaviour for preventing diarrhoea (Jamison et al., 2006) and other diseases. In a Cochrane Review of 14 randomized institution- and community-based control trials, hand washing reduced childhood diarrhoea morbidity by 32 to 39% (Ejemot-Nwadiaro, Ehiri, Meremikwu, & Citchley, 2008). In a study among slum dwellers in Bangladesh, the use of soap for hand washing resulted in a sharp reduction in incidence of Shigella infections without any improvement in environmental conditions or drinking water quality (Shahid, Greenough, Samadi, Huq, & Rahaman, 1996).
However, the low cost and relative effectiveness of hand washing is highly dependent on availability of clean water and soap. As such, we conducted a quasi-experimental study among young learners in resource poor communities in Limpopo, South Africa. The study addressed the structural environment (access to water and sanitation resources), the social environment (norms of hand washing), and the individual (knowledge for reasoned action). By paying attention to the interplay of environmental factors that influence individual health behaviours, health policy and practice is better able to understand, predict, and alter patterns of healthy behaviour.
Background
Despite its simplicity, the promotion of routine hand washing remains a challenge. Significant efforts and repeated interventions have been unsuccessful in establishing hand washing as a habitual behaviour, particularly in resource-limited settings (Ejemot-Nwadiaro et al., 2008). The practice of hand washing and the factors that influence hand-washing behaviour among individuals and communities are complex, and in sub-Saharan Africa confronted with socio-economic and cultural challenges that undermine efforts to improve hygiene in the general population (Akpabio & Takara, 2014). Hand washing, as with other health behaviours, is influenced by knowledge of best practice, social customs, and availability of resources (Hoque, Mahalanabis, Alam, & Islam, 1995; Hoque, Mahalanabis, Pelto, & Alam, 1995).
Psychological factors influencing hand washing include knowledge of risk factors, attitude, norms, perceived ability, and self-regulation (Mosler, 2012). Knowledge of the causes of diarrhoea encourages behaviours that reduce or prevent disease particularly when behaviours are further motivated by social pressure to redefine behavioural norms (Graf, Meierhofer, Wegelin, & Mosler, 2008). Hans-Joachim Mosler (2012) argues that perceived ability and self-efficacy further contribute to sustaining hand washing behaviour change. A study conducted in two primary schools in Malawi concluded that both teachers and students had reasonable appreciation for hygiene. However, there was a limited understanding of disease transmission and a failure to put knowledge into practice, with continued open defecation despite the presence of sanitation facilities (Grimason et al., 2014). In a review of the literature on water, sanitation and hygiene among indigenous populations, Jimenez and colleagues (2014) identified that low-resource populations have lower rates of disease despite having limited access to improved infrastructure. For example, traditional hygiene habits by locals were more effective at preventing disease than those practised by outsiders coming to live in an indigenous village (Kroeger, Schulz, Witte, Skewes-Ramm, & Etzler, 1992). Recent research indicates that soil-transmitted helminths are significantly higher among settlers than among indigenous groups (Briones-Chavez et al., 2013). Consequently, researchers argue that these ‘software’ components of rationalized behaviour change or hygiene promotion, are more important than the ‘hardware’ of infrastructure and resources for sustained hygiene and sanitation practices (Bailie, Stevens, & McDonald, 2012; McDonald & Bailie, 2010; Mosler, 2012).
Even so, the structural environment has the ability to either promote or prevent safe hygiene and sanitation practices. Sanitation facilities may not be accessible, be in such poor condition that they are no longer user friendly, or not physically paired with hand washing facilities. Grimason’s (2014) study of the Malawi primary schools found that the sanitation facilities were not child friendly, which likely contributed to their lack of use and preference for the bush. Furthermore, hygiene behaviours are generally habitual and grounded in social norms learnt and inherited from family and community members. Common customary practices, or social norms, such as the dipping of hands into a shared bowl of water (often without soap) may contribute to, rather than prevent, pathogen transmission (Ehiri et al., 2001; Kaltenthaler, Waterman, & Cross, 1991; Schmitt et al., 1997). However, as a commonly practiced custom in many low-resource communities, including the communities in this study, the practice is difficult to alter. Low resource communities are unlikely to overcome diarrhoea burdens until infrastructure is significantly improved.
In this light, the promotion of hand washing behaviours requires structural (hardware), social (community norms) and individual (psychological/software) changes. Among children aged 7–12 years in the Maradi region of Niger, improvements in health infrastructure in schools including clean water, latrines, and hand washing stations, combined with health education, significantly improved hygiene-related habits in the beneficiary schools and communities, with a significant decrease in reported cases of diarrhoea and abdominal pains (Mainassara & Tohon, 2014). Public health policy and practice should recognize the relationship of all human behaviours to an interplay of complex components that make up our unique environmental contexts. As confirmed by Akpabio and Takara (2014) in their review of WASH literature from sub-Saharan Africa, individuals and groups respond to actual or potential WASH problems through contextual filters mostly related to structural, social, and individual factors.
Methods
Setting
We conducted this quasi-experimental study at neighbouring primary schools in a community in the Vhembe district of Limpopo, situated approximately 35km from the district capital, Thohoyandou. Age standardized death rates from diarrhoea in Limpopo Province are 1.5 times higher than the national average, and 3 times higher than the neighbouring Gauteng Province (Bradshaw, 2006). Approximately 10.5 million people in South Africa do not have access to proper sanitation facilities. In the Limpopo Province and the Vhembe District, 2.15 and 0.6 million households do not have access to improved sanitation facilities, respectively (DWAF, 2010). This northern area of South Africa, bordering Zimbabwe, receives high annual rainfalls that result in lush vegetation and plentiful food productivity. Projections from studies conducted locally suggest that households could collect sufficient rainwater runoff during the wet season to provide for household and small scale agricultural use throughout the dry season (Ndiritu, Odiyo, Makungo, Ntuli, & Mwaka, 2011). Despite the abundance of water as a natural resource in the area, access to safe drinking water, free from human and animal faecal matter and pathogens, remains low and few households have the economic resources to purchase water storage tanks for drinking, cooking or hygiene purposes.
Data collection
The University of Virginia, in partnership with the University of Venda, has conducted collaborative community programs and research projects on the environment, water quality and enteric disease within the study communities for almost a decade (Demarest, Pagsuyoin, Learmonth, Mellor, & Dillingham, 2013; Mellor, Abebe, Ehdaie, Dillingham, & Smith, 2014; Mellor, Smith, Samie, & Dillingham, 2013). Consequently, researchers, community members, traditional leaders, and school staff had established long-standing relationships. Interventions directed at the structural environment (infrastructure improvements) and the social environment (education and addressing risky normative practices) were conducted and corresponding observational data of hand washing behaviours were collected between the months of June and August 2014. The schools are referred to as ‘School A’ and ‘School B’ in an effort to maintain confidentiality. All interventions were developed and implemented in collaboration with local stakeholders, primarily school faculty and staff.
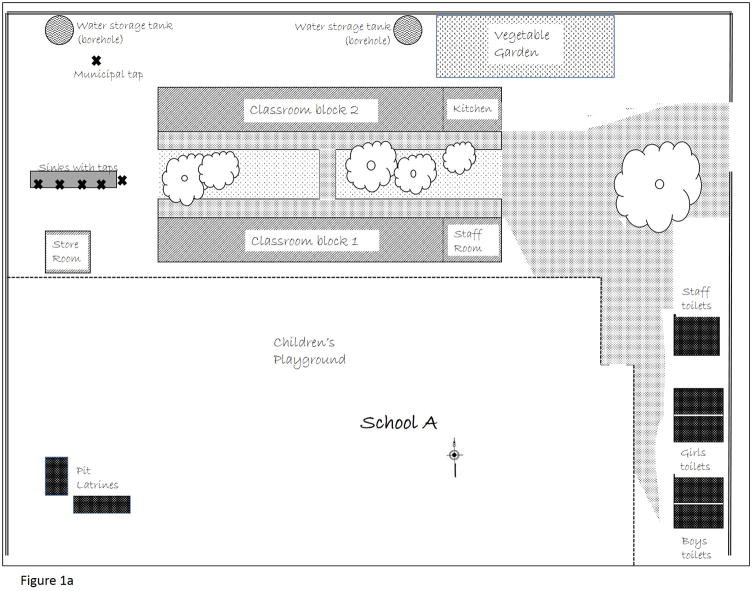
At the time of this study, School A hosted 400 children between the ages 6 and 13 years. The school had two water sources: municipality treated water servicing a single standpipe on school grounds and a privately installed borehole supplying untreated water to a series of outdoor taps with sinks and privately constructed toilet facilities (Figure 1a). The outdoor taps were 125 meters from the toilet facilities. We tested drinking water samples from each of these sources for total coliform bacteria counts. Samples from within the school had lower bacterial counts than drinking water samples collected throughout the community. However, there are no acceptable levels of coliform bacteria in drinking water according to international drinking water standards (WHO, 2011).
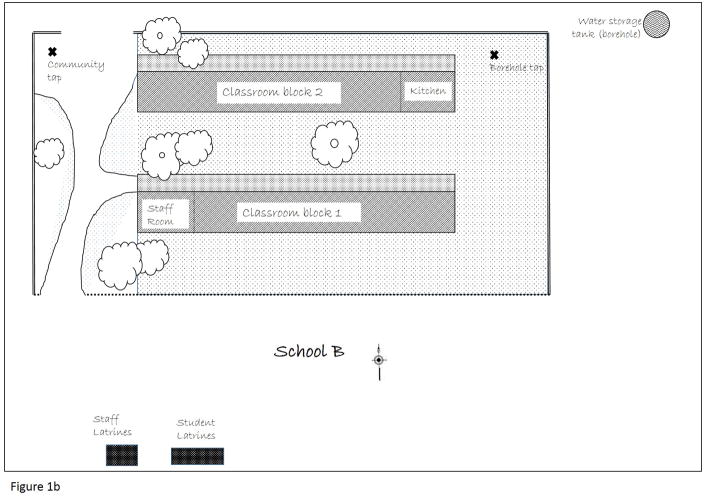
Figure 1.
Figure 1a. Physical layout of School A, including water sources (municipal tap and borehole storage tanks servicing outside sinks), and toilet facilities (staff toilets, student toilet blocks by gender, pit latrines beyond school grounds). Not to scale.
Figure 1b. Physical layout of School B, including water sources (community tap with surface water from local stream, borehole with tap beyond school grounds), and toilet facilities (community built pit latrines for students and faculty). Not to scale.
An Australian missionary organization provided the funds needed to erect the toilet facilities in School A in 2006. These facilities included three separate buildings. The first was an unfinished bathroom facility, used as a storage facility for grounds staff. The second and third buildings contained two sections. Each section had four stalls (not all in operation) and two sinks (also, not all in operation). Students had access to one-half of each building, with faculty and staff sharing the other half. Males used one building, females the other. Under this arrangement, eight female staff members shared four toilet stalls, two male staff shared another four stalls, and the 400 students shared the remaining eight stalls. Staff had disconnected all the sinks in the student sections of the toilet blocks. In addition to these bathroom facilities, with flushing toilets and sinks, four pit latrines were available to students, beyond the school grounds.
School B hosted approximately 200 students aged 6–13 years at the time of this study. The school had two water sources: a private borehole supplying untreated water, and untreated surface water diverted from local streams through a series of pipes installed by the community. Bacterial coliform counts from the tested drinking water samples were consistent with community samples. All were higher than bacterial levels from drinking water samples taken at School A, and above acceptable international standards. Toilet facilities were limited to four latrines erected by community members and parents. Most students elected to use the open bush surrounding the latrines. Staff had their own ventilated improved pit-latrine, which they kept locked. The single tap for hand washing was located 125 meters from the latrines, at the opposite side of the classroom buildings from the latrines (Figure 1b).
Structural Environment Intervention (School A)
Following an assessment of available resources and negotiations with school faculty and staff, it was determined that efforts would focus on the repair of tap facilities for hand washing. Given that functioning water taps were located a significant distance from the toilet facilities, with the classrooms situated between the toilets and the taps, providing a supply of soap and water closer to the toilet facilities would likely increase routine hand washing. Reconnecting the taps in the student assigned bathrooms would create an environment that facilitated hand washing after toilet use. Completing the third toilet block, to serve as the faculty toilets, would make eight more toilet stalls and four more sinks available for student use. A local plumber made all improvements. We provided bars of soap at all sinks.
We thoroughly cleaned the toilet facilities and decorated them with educational images displaying three steps to hand washing (rinsing with water, lathering with soap, and rinsing again, Figure 2). We placed the lyrics of a hand washing song composed by a teacher and a local member of the study team in the TshiVenda language on the toilet stall doors. These decorations aimed to serve as positive reinforcements reminding students that they should routinely practice proper hand washing. We used the songs and images in the educational component of the study.
Figure 2.
Photographic image of educational hand washing images placed in student toilet facilities in School A.
Social Intervention (Schools A and B)
Drawing from lesson plans developed by the South African Water Research Commission (http://www.wrc.org.za/Pages/Learning_School_lessonplans.aspx) for grades R or ‘Reception’ through 10 (ages 6–15), and working closely with school faculty, we developed and administered a multi-dimensional education program. Individual, age appropriate, classroom discussions and activities were conducted. For example, we asked students to consider how the customary practice of sharing one large bowl filled with soapy water to wash hands before meals might contribute to the spread of disease. We placed hand-washing posters in each classroom. Some teachers escorted their students to the hand washing facilities to develop self-efficacy further, but this was infrequent.
Thirty-minute school assemblies took place at both schools that emphasized community responsibilities and benefits associated with hand washing. Students performed short dramas that demonstrated how illnesses can spread to others when community members do not routinely wash their hands. Local collaborators suggested and developed this messaging, in an effort to bring indigenous knowledge and skill into the program design and implementation to improve local investment and sustainability (Jimenez et al., 2014). The assemblies also included the singing of the hand washing songs, described above.
Data collection and Analysis
Pre- and post-intervention observations of hand washing behaviours were conducted at both schools (Figure 3). Recommended hand washing technique involves the use of both hands, the use of an agent (soap), rubbing hands with the agent, rinsing with water, and drying. However, we could not always observe this technique directly. Given ethical concerns raised by a reviewing ethics committee, we did not observe hand washing in the toilet facilities. At the outside taps, the students frequently washed their hands in clusters, making it difficult to distinguish between complete hand washing with soap and merely hand rinsing. We measured hand washing as any rinsing of hands with water, resulting in wet hands upon exiting the toilet or tap facilities (there was no drying apparatus available). We noted, but did not measure, the amount of soap remaining at the end of an observation day.
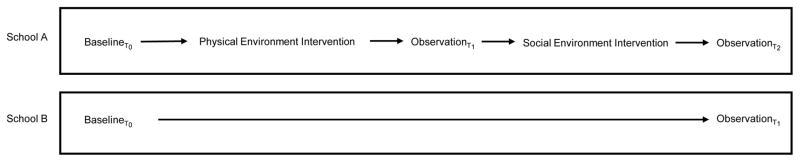
Figure 3.
Quasi-Experimental study design involving School A and B. School A received both the physical environment intervention (improved hygiene and sanitation facilities) and the social environment intervention (educational activities to address knowledge and cultural practices). School B received only the social environment intervention.
Structured observations were made on total toilet/latrine use, after toilet hand washing, and independent (not linked to toilet use) hand washing by “young” (Grade R-3) and “old” (Grade 4–7), male and female students. Observations involved total counts and did not exclude those children who washed their hands more than once. Observations took place during two break periods when most students utilized the sanitation facilities (9:30AM – 10:00AM and 12:00PM – 12:10PM). Measures of behaviours were limited to observations only, not self-report. Observation of hand washing is a common research method in varied settings. In comparisons with self-reported behaviours, observations are more accurate (Cousens, Kanki, Toure, Diallo, & Curtis, 1996; Curtis et al., 1993; Manun’Ebo et al., 1997; Ram et al., 2010). We recorded observations on two consecutive days, with total counts summed for all observation periods. We conducted an additional round of observations at School A between the structural and social environment interventions. This additional set of observations aimed to determine if structural environment modifications, independent of social environment factors (specifically knowledge transfer), altered hand-washing behaviours (see Figure 3, Observations T1 and T2 for School A).
We calculated behaviour change as percent change between baseline and post-structural environment intervention, and baseline and post-social environment intervention (total behaviour change). Paired t-tests determined the statistical significance of the percent change in behaviour. We did not measure knowledge, diarrhoea, or enteric pathogen infection at baseline or post-intervention. We assumed that knowledge levels would be consistent with those measured among secondary school students in the district, which was high with regards to diarrhoea pathogens and methods of prevention (Sibiya & Gumbo, 2013). Based on findings from the long-term prospective study being conducted in the community,1 self-reports of diarrhoea (as a health threat) are minimal, although enteric pathogen infections are common (Bessong, Nyathi, Mahopo, & Netshandama, 2014). We did not have the financial resources to measure enteric pathogen infections among the study population.
Results
School A
At baseline, we observed few students washing their hands after toilet use. More students were observed rinsing their hands (178 boys, 181 girls), though usually following eating rather than before, and without the use of soap. Young boys more routinely rinsed their hands than older boys, with an equal proportion of girls of both age groups rinsing their hands. Following structural environment modifications that reconnected the taps in the toilet facilities and repaired the broken taps at the outside sinks, hand washing, and specifically hand washing after toilet use, increased (Table 1; total counts: T0=359, T1=712; t=13.86, p=0.0052). These dramatic modifications in behaviour occurred following improvements in hygiene and sanitation facilities although behaviours continued to modestly improve following the social environment intervention (T2=1095, t=2.63; p=0.012).
Table 1.
Observational data on hand washing behaviors following toilet use and independent of toilet use, including percent change and paired t-test.
| Baseline | Post-Physical | % change | Post-Social | % change | Total % change | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| School A | School B | School A | School A | School A | School B | School A | School A | School B | ||
| Toilet Use | ||||||||||
| Boys | Grade R-3 | 14 | 9 | 76 | 443 | 80 | 20 | 5 | 471 | 122 |
| Grade 4–7 | 8 | 7 | 44 | 450 | 67 | 5 | 52 | 738 | −29 | |
| Girls | Grade R-3 | 66 | 2 | 87 | 32 | 107 | 21 | 23 | 62 | 950 |
| Grade 4–7 | 30 | 12 | 124 | 313 | 90 | 13 | −27 | 200 | 8 | |
| Paired T-test (p-value) | 3.3120 (0.0803) | 0.248 (0.410) | 7.4312 (0.0176) | 1.5253 (0.2667) | ||||||
| After Toilet Hand Washing | ||||||||||
| Boys | Grade R-3 | 2 | 0 | 39 | 1850 | 69 | 6 | 77 | 3350 | - |
| Grade 4–7 | 0 | 0 | 40 | 4000 | 66 | 0 | 65 | 6600 | 0 | |
| Girls | Grade R-3 | 2 | 0 | 38 | 1800 | 93 | 2 | 145 | 4550 | - |
| Grade 4–7 | 1 | 0 | 84 | 8300 | 86 | 0 | 2 | 8500 | 0 | |
| Paired T-test (p-value) | 31.3406 (0.001)* | 4.077 (0.0552) | 9.1372 (0.0118)* | - | ||||||
| Total Count of Hand Washing | ||||||||||
| Boys | Grade R-3 | 110 | 97 | 182 | 65 | 214 | 140 | 15 | 95 | 44 |
| Grade 4–7 | 68 | 46 | 132 | 94 | 219 | 65 | 66 | 222 | 41 | |
| Girls | Grade R-3 | 90 | 71 | 146 | 62 | 296 | 71 | 103 | 229 | 0 |
| Grade 4–7 | 91 | 35 | 252 | 177 | 366 | 48 | 45 | 302 | 37 | |
| Paired T-test (p-value) | 13.8564 (0.0052)* | 2.6303 (0.01192)* | 5.2135 (0.0349)* | 1.6612 (0.2386) | ||||||
Note:
indicates significance at level of α=0.05.
Soap was not available before the structural environment modifications at School A. However, once made available, students used the soap frequently. Due to the large number of hand washers during observation periods, many students, for the sake of time, continued to rinse their hands with water only rather than lathering fully with soap. As indicated above, we counted any rinsing of hands with water as hand washing.
School B
No students were observed washing their hands after latrine use at baseline (Table 1). A total of 249 students were observed washing their hands independent of latrine use, mostly following eating with their hands. Following the social environment intervention, eight students washed their hands after using the latrine. The number of students observed washing their hands independent of latrine use increased by 30% (T0=249; T2=324; t=1.66, p=0.239). Soap was not available before the social environment intervention. The staff introduced five bars of soap at the community tap following the social environment intervention.
In summary, before either intervention (structural or social) very few students washed their hands after using the sanitation facilities, with more routine hand washing occurring following meals. Students did not use soap, as it was not present. Following the structural environment intervention at School A (which introduced immediate access to running water in the toilet facilities and soap), more than 60% of students entering the toilet facilities washed their hands, and 712 counts of hand washing occurred independent of toilet use. Following the social environment intervention, 91% of students entering the toilet facility at School A washed their hands (t=5.21; p=0.035), whereas only 14% of students using the toilets at School B washed their hands (t=1.66; p=0.239). These data indicate that social environment interventions (i.e., education and cultural practices) alone, without alterations in the structural environment (i.e., improved access to soap and water), does not alter hand washing behaviours. Combined, adjustments in structural environments supported by educational programs to address the social environment significantly motivated routine risk reduction behaviours.
Discussion
The Global Public-Private Partnership for Hand Washing and the international ‘Global Handwashing Day’ initiative suggest that hand washing interventions are most effective when social and political will is dedicated to motivating individual behaviour change. However, many resource-poor communities struggle to access the resources they need for effective and sustained healthy hygiene practices given economic or infrastructure limitations. Economically strained communities have difficulty accessing soap and uncontaminated rinsing water. A recent study conducted among eight randomly selected remote and metropolitan secondary schools in the Vhembe district of Limpopo, South Africa, found that most students had a high level of knowledge of water borne disease and that most students engaged in basic hygiene practices (Sibiya & Gumbo, 2013). However, access to soap was limited in all schools and water supplies and sanitation facilities were inadequate, particularly in remote schools. Similarly, school children in Lagos, Nigeria, had high levels of knowledge, but lacked good hygiene and sanitation practices because of limited resources (Babalobi, 2013). Consistent with studies conducted in marginalized communities elsewhere (Briggs, 2005; Briggs & Mantini-Briggs, 2003), this study highlights that rational action of citizen agents is limited by the structural (infrastructure, economics, and politics) environment, such that citizens cannot necessarily manage their own health. Governments must make investments to develop sufficient hygiene and sanitation resources, at the very least in public schools and ideally in surrounding communities as well.
While schools provide a useful platform to deliver educational hand washing and other health interventions, we offer some caution. There is little critical scholarship to indicate that this approach is necessarily effective in reducing disease (Gard & Wright, 2014). In an editorial introducing the ‘Schools and public health’ collection in Critical Public Health, Michael Gard and Jan Wright (2014, p. 112) note that “reviews on school-based health interventions consistently find that measurable effect of any kind is rare and that this is true regardless of the particular focus of intervention.” Schools might not be the ideal avenue through which to provide such interventions, particularly in settings where resources are inconsistent (i.e., flush toilets at school, latrines at home; taps at school, buckets of water at home). While it would seem that education and public health share liberal notions of informed decision making, school health interventions are in fact advocating, prescribing, even coercing a certain choice (Bulled, 2015; Galitz & Robert, 2014; Gard & Pluim, 2014; Horton & Barker, 2009). Schools could serve as an ideal venue to teach children and their families the importance of advocating for improvements in community resources such as water and sanitation infrastructure that would enable sustained hygienic behaviours.
We recognize that this study does not provide long-term observations of hand washing behaviours. Consequently, the sustainability of altered behaviours and the upkeep of the altered structural environment to support such behaviours remains unknown. The novelty of newly available resources at School A may have stimulated the frequent use of the facilities (Gardner, de Bruijn, & Lally, 2011). Hand washing may decrease over time as students become more accustomed with the modifications made to the structural environment becomes less routinely available. In addition, the project is subject to participant or response bias. Students may have been more likely to wash their hands as they recognized that researchers were monitoring this practice. Furthermore, we did not distinguish between hand washing with and without soap. While this may be perceived as inflating the results, given that hand washing without soap is less effective in disease prevention, the process of data collection was consistent in both schools. The UNICEF call to action, “Advancing WASH in School Monitoring, 2015” recognizes hand washing facilities and soap availability as two independent variable suggesting, that while ideal, hand washing with clean water but without soap is an improvement over no hand washing, or hand washing in contaminated water.
Future studies should consider long-term observations of hand washing behaviours to determine if positive behaviours wane over time and what interventions might prove successful at continuing to stimulate behaviours, making them habitual. A large-scale trial from Burkina Faso suggested that changes in hand washing behaviour can be maintained in the longer term (three years) in a large community (a city of approximately 300,000 residents) (Curtis et al., 2001). Authors of this study concluded that using local research and locally appropriate channels of communication repeatedly, and for extended periods, contributed to sustained behaviour change. In the study presented here, we could only alter school environments not entire communities and for a limited time with the hope of sustained efforts by school staff. Many students returned home to latrine facilities with no hand washing facilities in close proximity. Future studies should examine the effects of the extended structural and social environment on students’ behaviours and consequent disease risk.
Conclusion
Diarrhoea poses a major burden on health, significantly threatening the health and well-being of children in resource-poor settings. Hand washing is a cost effective, simple approach to reduce episodes of diarrhoea and enteric infections significantly reducing global morbidity and mortality. However, hand washing behaviours are complex and greatly influenced by environmental context. In this study, we showed that hand washing behaviours of young students in resource limited settings can be altered. However, we must not begin with the assumption that knowledge is lacking, or that with sufficient knowledge all barriers to healthy behaviours can be overcome as humans act as rationale beings, even without necessary resources. Reinforcing the findings from Akpabio and Takara (2014), we have shown that by including individual-level approaches (i.e., education) in multi-faceted hand-washing interventions aids to reinforce hygiene-related behaviours, but alone has limited effect. While structures and rational behaviours are intimately connected, by considering each separately allows for a reconsideration for how public health practice and policy could effectively address the health needs of populations. As evidenced here, the focus should shift from the management of individual behaviours to the provision of resources. The Sustainable Development Goal 6 acknowledges the importance of an enabling environment, capacity building, and the participation of local communities in water and sanitation management in efforts to ensure clean water and sanitation to everyone by 2030.
Acknowledgments
This work was funded by the National Science Foundation Research Experience for Undergraduates program, and by the Fogarty International Center of the NIH, award number D43 TW009259.
Footnotes
Notes
The Interactions of Malnutrition & Enteric Infections: Consequences for Child Health and Development (MAL-ED) study was a multi-sited, five-year cohort study, examining the relationship between malnutrition and enteric disease on childhood development funded by the Gates Foundation, via the National Institutes of Health (http://mal-ed.fnih.org/).
References
- Akpabio EM, Takara K. Understanding and confronting cultural complexities characterizing water, sanitation and hygiene in Sub-Saharan Africa. Water International. 2014;39(7):921–932. doi: 10.1080/02508060.2015.981782. [DOI] [Google Scholar]
- Babalobi B. Water, sanitation and hygiene practices among primary-school children in Lagos: a case study of the Makoko slum community. Water International. 2013;38(7):921–929. doi: 10.1080/02508060.2013.851368. [DOI] [Google Scholar]
- Bailie RS, Stevens M, McDonald EL. The impact of housing improvement and socio-environmental factors on common childhood illnesses: a cohort study in Indigenous Australian communities. Journal of Epidemiology and Community Health. 2012;66(9):821–831. doi: 10.1136/jech.2011.134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessong PO, Nyathi E, Mahopo TC, Netshandama V. Development of the Dzimauli Community in Vhembe District, Limpopo Province of South Africa, for the MAL-ED Cohort Study. Clinical Infectious Diseases. 2014;59(suppl 4):S317–S324. doi: 10.1093/cid/ciu418. [DOI] [PubMed] [Google Scholar]
- Black R, Lopez de Romana G, Brown K, Bravo N, Bazalar O, Kanashiro H. Incidence and etiology of infantile diarrhea and major routes of transmission in Huascar, Peru. American Journal of Epidemiology. 1989;129(4):785–799. doi: 10.1093/oxfordjournals.aje.a115193. [DOI] [PubMed] [Google Scholar]
- Bradshaw D. South African National Burden of Disease Study: Estimates of Provincial Mortality. Johannesburg, South Africa: 2006. [Google Scholar]
- Briggs CL. Communicability, Racial Discourse, and Disease. Annual Review of Anthropology. 2005;34:269–291. [Google Scholar]
- Briggs CL, Mantini-Briggs C. Stories in the Time of Cholera: Racial Profiling in a Medical Nightmare. Berkeley: University California Press; 2003. [Google Scholar]
- Briones-Chavez C, Torres-Zevallos H, Canales M, Stamato CM, O’Riordan TG, Terashima A. Differences in prevalence of geohelminth infections between indigenous and settler populations in a remote Amazonian region of Peru. Tropical Medicine & International Health. 2013;18(5):615–618. doi: 10.1111/tmi.12077. [DOI] [PubMed] [Google Scholar]
- Bulled N. Prescribing HIV Prevention: Bringing culture into global health communication. Walnut Creek, CA: Left Coast Press; 2015. [Google Scholar]
- Clasen T, Roberts I, Rabie T, Schmidt W, Cairncross S. Interventions to improve water quality for preventing diarrhoea. Cochrane Database of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD004794.pub2. [DOI] [PubMed] [Google Scholar]
- Clasen T, Schmidt WP, Rabie T, Roberts I, Cairncross S. Interventions to improve water quality for preventing diarrhoea: systematic review and meta-analysis. BMJ. 2007;334(7597):782. doi: 10.1136/bmj.39118.489931.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens S, Kanki B, Toure S, Diallo I, Curtis V. Reactivity and repeatability of hygiene behaviour: structured observations from Burkina Faso. Social Science and Medicine. 1996;43(9):1299–1308. doi: 10.1016/0277-9536(95)00380-0. 0277953695003800 [pii] [DOI] [PubMed] [Google Scholar]
- Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: A systematic review. Lancet Infectious Diseases. 2003;3(5):275–281. doi: 10.1016/s1473-3099(03)00606-6. [DOI] [PubMed] [Google Scholar]
- Curtis V, Cousens S, Mertens T, Traore E, Kanki B, Diallo I. Structured observations of hygiene behaviours in Burkina Faso: validity, variability, and utility. Bulletin of the World Health Organization. 1993;71(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- Curtis V, Kanki B, Cousen S, Diallo I, Kpozehouen A, Sangare M, et al. Evidence of behaviour change following a hygiene promotion programme in Burkina Faso. Bulletin of the World Health Organization. 2001;79(6):518–527. [PMC free article] [PubMed] [Google Scholar]
- Curtis V, Kanki B, Mertens T, Traore E, Diallo I, Tall F, et al. Potties, pits and pipes: Explaining hygiene behaviour in Burkina Faso. Social Science and Medicine. 1995;41(3):383–393. doi: 10.1016/0277-9536(94)00341-p. [DOI] [PubMed] [Google Scholar]
- Demarest J, Pagsuyoin S, Learmonth G, Mellor J, Dillingham R. Development of a Spatial and Temporal Agent-Based Model for Studying Water and Health Relationships: The Case Study of Two Villages in Limpopo, South Africa. Journal of Artificial Societies and Social Simulation. 2013;16(4) doi: 10.18564/jasss.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWAF. Water Services National Information System (WS-NIS) Sanitation Backlog. 2010 Retrieved from: http://intertest.dwaf.gov.za/dir_ws/wsnis/default.asp?
- Ehiri J, Azubuike M, Ubbaonu C, Anyanwu E, Ibe K, Ogbonna M. Critical control points of complementary food preparation and handling in eastern Nigeria. Bulletin of the World Health Organization. 2001;79(5):423–433. [PMC free article] [PubMed] [Google Scholar]
- Ejemot-Nwadiaro RI, Ehiri JE, Meremikwu MM, Citchley JA. Hand washing for preventing diarrhoea. Cochrane Database of Systematic Reviews. 2008;(1):CD004265. doi: 10.1002/14651858.CD004265.pub2. [DOI] [PubMed] [Google Scholar]
- Fewtrell L, Kaufmann R, Kay D, Enanoria W, Haller L, Colford JJ. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: A systematic review and meta-analysis. Lancet Infectious Diseases. 2005;5(1):42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- Galitz T, Robert D. Governing bullying through the new public health model: A Foucauldian analysis of a school anti-bullying programme. Critical Public Health. 2014;24(2):182–195. [Google Scholar]
- Gard M, Pluim C. Schools and Public Health: Past, Present, Future. Lanham, MD: Rowman and Littlefield; 2014. [Google Scholar]
- Gard M, Wright J. Schools and critical public health: towards dialogue, collaboration and action. Critical Public Health. 2014;24(2):109–114. doi: 10.1080/09581596.2014.888872. [DOI] [Google Scholar]
- Gardner B, de Bruijn GJ, Lally P. A systematic review and meta-analysis of applications of the Self-Report Habit Index to nutrition and physical activity behaviours. Annals of Behavioral Medicine. 2011;42(2):174–187. doi: 10.1007/s12160-011-9282-0. [DOI] [PubMed] [Google Scholar]
- Graf J, Meierhofer R, Wegelin M, Mosler HJ. Water disinfection and hygiene behaviour in an urban slum in Kenya: impact on childhood diarrhoea and influence of beliefs. International Journal of Environmental Health Research. 2008;18(5):335–355. doi: 10.1080/09603120801966050. [DOI] [PubMed] [Google Scholar]
- Grimason AM, Masangwi SJ, Morse TD, Jabu GC, Beattie TK, Taulo SE, Lungu K. Knowledge, awareness and practice of the importance of hand-washing amongst children attending state run primary schools in rural Malawi. International Journal of Environmental Health Research. 2014;24(1):31–43. doi: 10.1080/09603123.2013.782601. [DOI] [PubMed] [Google Scholar]
- Hoque B, Mahalanabis D, Alam M, Islam M. Postdefecation handwashing in Bangladesh: practice and efficiency perspectives. Public Health. 1995;109(1):15–24. doi: 10.1016/s0033-3506(95)80071-9. [DOI] [PubMed] [Google Scholar]
- Hoque B, Mahalanabis D, Pelto B, Alam M. Research methodology for developing efficient handwashing options: An example from Bangladesh. Journal of Tropical Medicine and Hygiene. 1995;98(6):469–475. [PubMed] [Google Scholar]
- Horton S, Barker JC. “Stains” on their self-discipline: Public health, hygiene, and the disciplining of undocumented immigrant parents in the nation’s internal borderlands. American Ethnologist. 2009;36(4):784–798. doi: 10.1111/j.1548-1425.2009.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton G. Benefits and Costs of the Water Sanitation and Hygiene Targets for the Post-2015 Development Agenda: Post-2015 Consensus. Geneva, Switzerland: 2015. Retrieved from: http://www.copenhagenconsensus.com/publication/post-2015-consensus-water-and-sanitation-assessment-hutton. [Google Scholar]
- Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, … Musgrove P, editors. Disease Control Priorities in Developing Countries. 2. Washington (DC): World Bank; 2006. [PubMed] [Google Scholar]
- Jimenez A, Cortobius M, Kjellen M. Water, sanitation and hygiene and indigenous peoples: A review of the literature. Water International. 2014;39(3):277–293. doi: 10.1080/02508060.201.903453. [DOI] [Google Scholar]
- Kaltenthaler E, Waterman R, Cross P. Faecal indicator bacteria on the hands and the effectiveness of hand-washing in Zimbabwe. Journal of Tropical Medicine and Hygiene. 1991;94(5):358–363. [PubMed] [Google Scholar]
- Kroeger A, Schulz S, Witte B, Skewes-Ramm R, Etzler A. Helminthiasis and cultural change in the Peruvian rainforest. Journal of Tropical Medicine and Hygiene. 1992;95(2):104–113. [PubMed] [Google Scholar]
- Lanata C, Huttly S, Yeager B. Diarrhea: Whose feces matter? Reflections from studies in a Peruvian shanty town. Pediatric Infectious Disease Journal. 1998;17(1):7–9. doi: 10.1097/00006454-199801000-00003. [DOI] [PubMed] [Google Scholar]
- LeBaron C, Furutan N, Lew J, Allen J, Gouvea V, Moe C, et al. Viral agents of gastroenteritis. Public health importance and outbreak management. Morbidity Mortality Weekly Report. 1990;39(RR-5):1–24. [PubMed] [Google Scholar]
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE … Unicef. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- Luby SP, Agboatwalla M, Feikin DR, Painter J, Billhimer W, Altaf A, Hoekstra RM. Effect of handwashing on child health: a randomised controlled trial. Lancet. 2005;366(9481):225–233. doi: 10.1016/s0140-6736(05)66912-7. [DOI] [PubMed] [Google Scholar]
- Mainassara HB, Tohon Z. Assessing the Health Impact of the following Measures in Schools in Maradi (Niger): Construction of Latrines, Clean Water Supply, Establishment of Hand Washing Stations, and Health Education. Journal of Parasitology Research. 2014;1:1–8. doi: 10.1155/2014/190451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manun’Ebo M, Cousens S, Haggerty P, Kalengaie M, Ashworth A, Kirkwood B. Measuring hygiene practices: a comparison of questionnaires with direct observations in rural Zaire. Tropical Medicine & International Health. 1997;2(11):1015–1021. doi: 10.1046/j.1365-3156.1997.d01-180.x. [DOI] [PubMed] [Google Scholar]
- Mara D. Water, sanitation and hygiene for the health of developing nations. Public Health. 2003;117:452–456. doi: 10.1016/S0033-3506(03)00143-4. [DOI] [PubMed] [Google Scholar]
- McDonald E, Bailie R. Hygiene improvement: essential to improving child health in remote Aboriginal communities. Journal of Paediatric Child Health. 2010;46(9):491–496. doi: 10.1111/j.1440-1754.2010.01846.x. [DOI] [PubMed] [Google Scholar]
- Mellor J, Abebe L, Ehdaie B, Dillingham R, Smith J. Modeling the sustainability of a ceramic water filter intervention. Water Research. 2014;49:286–299. doi: 10.1016/j.watres.2013.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor J, Smith J, Samie A, Dillingham R. Coliform Sources and Mechanisms for Regrowth in Household Drinking Water in Limpopo, South Africa. Journal of Environmental Engineering (New York) 2013;139(9):1152–1161. doi: 10.1061/(asce)ee.1943-7870.0000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosler HJ. A systematic approach to behavior change interventions for the water and sanitation sector in developing countries: a conceptual model, a review, and a guideline. International Journal of Environmental Health Research. 2012;22(5):431–449. doi: 10.1080/09603123.2011.650156. [DOI] [PubMed] [Google Scholar]
- Murray C, Lopez A. The global burden of disease: A comprehensive assessment of mortality and disability from diseases, injuries and risk factors in 1990 and projected to 2020. Boston: Harvard University Press; 1996. [Google Scholar]
- Murray C, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, … Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. doi: 10.1016/s0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Ndiritu J, Odiyo J, Makungo R, Ntuli C, Mwaka B. Yield-reliability analysis for rural domestic water supply from combined rainwater harvesting and run-of-river abstraction. Hydrological Sciences Journal. 2011;56(2):238–248. [Google Scholar]
- Oria R, Pinkerton R, Lima A, Guerrant R. DALYs and Diarrhea. In: Preedy V, Watson R, editors. Handbook of Disease Burdens and Quality of Life Measures. New York: Springer; 2010. pp. 1221–1232. [Google Scholar]
- Ram P, Halder A, Granger S, Jones T, Hall P, Hitchcock D, … Luby S. Is structured observation a valid technique to measure handwashing behavior? Use of acceleration sensors embedded in soap to assess reactivity to structured observation. American Journal of Tropical Medicine and Hygiene. 2010;83(5):1070–1076. doi: 10.4269/ajtmh.2010.09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt R, Bryan F, Jermini M, Chilufya E, Hakalima A, Zyuulu M, et al. Hazards and critical control points of food preparation in homes in which persons had diarrhoea in Zambia. Journal of Food Protection. 1997;60(2):161–171. doi: 10.4315/0362-028X-60.2.161. [DOI] [PubMed] [Google Scholar]
- Shahid N, Greenough W, Samadi A, Huq M, Rahaman N. Handwashing with soap reduces diarrhoea and spread of bacterial pathogens in a Bangladesh village. Journal of Diarrhoeal Disease Research. 1996;14(2):85–89. [PubMed] [Google Scholar]
- Sibiya JE, Gumbo JR. Knowledge, Attitude and Practices (KAP) survey on water, sanitation and hygiene in selected schools in Vhembe District, Limpopo, South Africa. International Journal of Environmental Research and Public Health. 2013;10:2282–2295. doi: 10.3390/ijerph10062282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Water Project. Water scarcity and the importance of water. 2017 Retrieved February 20, 2017, from www.thewaterproject.org/water-scarcity.
- Traore E, Cousens S, Curtis V, Mertens T, Tall F, Traore A, et al. Childhood defecation behaviour, stool disposal practices, and childhood diarrhoea in Burkina Faso: Results from a case-control study. Journal of Epidemiology and Community Health. 1994;48(3):270–275. doi: 10.1136/jech.48.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF. Committing to Child Survival: A Promise Renewed Progress Report 2014. New York: 2014. Retrieved from: http://files.unicef.org/publications/files/APR_2014_web_15Sept14.pdf. [Google Scholar]
- UNICEF. Advancing WASH in Schools Monitoring, 2015. New York, USA: 2015. [Google Scholar]
- UNICEF. Diarrhoea remains a leading killer of young children, despite the availability of a simple treatment solution. 2017 Retrieved February 20, 2017, from https://data.unicef.org/topic/child-health/diarrhoeal-disease/
- UNICEF/WHO. 25 years of progress on sanitation and drinking water - 2015 update and MGD assessment. Geneva, Switzerland: 2015. [Google Scholar]
- US Department of Health Services. Food Code 1999, Publication Number PB99-115925:43. 1999. Recommendations of the US Public Health Services. [Google Scholar]
- WHO. Water Sanitation and Health (WSH): Health through safe drinking water and basic sanitation. Geneva, Switzerland: 2016. Retrieved from http://www.who.int/water_sanitation_health/mdg1/en/ [Google Scholar]
- WHO. Water Sanitation Health: Microbia Aspects. Geneva, Switzerland: 2011. Retrieved from: http://www.who.int/water_sanitation_health/publications/2011/9789241548151_ch07.pdf?ua=1. [Google Scholar]
- WHO. Diarrhea Fact Sheet. Geneva, Switzerland: 2013. [Accessed 8 August, 2013]. Retrieved from http://www.who.int/mediacentre/factsheets/fs330/en/index.html. [Google Scholar]
- WHO/UNICEF. Levels and trends in child mortality. Geneva, Switzerland: 2012. [Google Scholar]
- WHO/UNICEF. Joint Monitoring Programme (JMP) for Water Supply and Sanitation (wssinfo.org) 2017 Retrieved February 20, 2017, from http://data.worldbank.org/indicator/SH.STA.ODFC.ZS.