Abstract
Developmental conditions may impact the expression of immune traits throughout an individual’s life. Early-life challenges may lead to immunological constraints that are mediated by endocrine-immune interactions. In particular, individual differences in the ability to mount immune responses may be programmed by exposure to stressors or glucocorticoid hormones during development. To test this hypothesis, we experimentally elevated levels of the glucocorticoid hormone corticosterone during the nestling and fledgling periods in captive zebra finches (Taeniopygia guttata). We subsequently challenged birds with the antigen lipopolysaccharide (LPS) on days 60 and 100 post-hatch to determine if developmental exposure to elevated corticosterone impacted the later response to LPS. As measures of immune function, we quantified bacteria killing ability, haptoglobin concentrations, and LPS-specific antibody responses at multiple time points. We also measured circulating corticosterone concentrations during the experimental period and on day 60 before and after endotoxin challenge. During the experimental period, corticosterone treatment elevated corticosterone levels. Corticosterone treatment did not induce programming effects on immune function or corticosterone production. Independent of treatment, individuals with higher corticosterone concentrations during the nestling period had lower bacteria killing ability on day 36 and higher baseline corticosterone concentrations on day 60 post-hatch. These results suggest a limited role for corticosterone exposure during early life to mediate immunological constraints later in life. Manipulation of cortisol may be necessary to conclusively determine if developmental glucocorticoid exposure can program immune function in birds. To determine if developmental stress can program the immune response, exposure to environmentally relevant stressors should also be manipulated.
Keywords: bird, corticosterone, developmental programming, ecoimmunology, innate immunity, stress
Graphical abstract
Introduction
All living organisms are impacted by microbial pathogens (Hedrick, 2004). Thus, there is significant selection pressure to minimize the effects of microbial pathogens on survival and reproduction. Despite selection to respond appropriately to these pathogens, individuals within species exhibit striking variation in immune function. The intrinsic and extrinsic factors that contribute to variation among individuals in immune function have been a central area of research in the field of ecoimmunology since the inception of the field (Sheldon and Verhulst, ’96; Martin et al, 2011). One explanation for this variation arises from the concept of trade-offs (Sheldon and Verhulst, ‘96). Individuals have limited available resources that must be allocated and traded-off among competing demands, which can include immune defense, growth, and reproduction (Sheldon and Verhulst, ‘96). Trade-offs give rise to individual variation in immune function because the benefits and costs of these competing demands differ among individuals (Sheldon and Verhulst, ‘96). On the basis of life history and environmental conditions, individuals then adaptively modify investment in immune function and exhibit broad flexibility in immune responses (Ardia et al, 2011).
The degree of plasticity an individual can express for a given immunological trait, however, is generally limited by a combination of genetic and physiological constraints (DeWitt et al, ’98; Ardia et al, 2011). For example, artificial selection studies have revealed that genetic differences contribute substantially to variation among individuals in immune function (reviewed in: Ardia et al, 2011; Van der Most et al, 2011). Physiological state, including energetic reserves, oxidative stress, parasite infection, and age, has also been linked to individual variation in immune function (Folstad and Karter ’92; Sheldon and Verhulst, ’96; Koolhaas, 2008; Hill 2011). Moreover, conditions experienced early in life may influence the epigenetic state and constrain individual variation in immune function that is expressed in adulthood (Spencer et al, 2006; Macri et al, 2007; Merlot et al, 2008).
One physiological constraint on immune function that has been studied both in adulthood and from a developmental perspective is the complex network of interactions between the hypothalamic-pituitary-adrenal (HPA) axis and the immune system. Glucocorticoid hormones produced by the adrenals, such as cortisol and corticosterone, exert profound effects on the immune system. Acute, or short-term, exposure to glucocorticoids generally stimulates the immune response (Dhabhar and McEwen, ’99; Martin, 2009), whereas chronic exposure can depress the immune response (McEwen et al, ‘97). However, the impact of glucocorticoids is also dependent on the type of response measured. For example, glucocorticoids generally stimulate humoral immunity and depress cell-mediated immunity (Bartolomucci, 2007) or cause redistribution of leukocytes out of circulation and into tissues (Dhabhar et al, 1995). Exposure to elevated levels of glucocorticoid hormones during development can also exert sustained effects on adult immune function (reviewed in Merlot et al, 2008; Miller et al, 2011).
A number of studies have demonstrated that exposure to stressors or exogenous glucocorticoids during early postnatal life can impact immune responses over the short term (e.g., Saino et al, 2003; Loiseau et al, 2008; Stier et al, 2009; Schoech et al, 2011). However, few studies have tested for persistent effects of developmental exposure to glucocorticoids or stress on adult immune function, especially in non-mammalian model systems (Schmidt et al, 2015). Finally, previous studies have generally not tested for interactions between developmental and adult environmental conditions on adult immune responses (e.g., De Coster et al, 2011).
To test these ideas, we manipulated exposure to the glucocorticoid hormone corticosterone (CORT) during the nestling and fledgling periods and exposure to the endotoxin lipopolysaccharide (LPS) on days 60 and 100 post-hatch in a captive population of zebra finches (Taeniopygia guttata). We utilized a fully crossed design to test for potential interactive effects between the developmental and adult environments (Groothuis and Taborsky, 2015). We predicted that developmental CORT exposure would have long-term effects on our measures of immune function, and that the ability to respond to a simulated bacterial infection would be impacted by developmental history. We also expected that developmental CORT exposure would lead to increased production of CORT in response to LPS challenge later in life through a programming effect on the HPA axis (sensu Spencer et al, 2009).
Methods
Research animals and housing
Parents of birds used in the study were housed in wire cages (45 cm × 45 cm × 40 cm) in breeding pairs. Independent offspring were housed in larger single sex cages (92 cm × 38 cm × 43 cm) containing 4–10 individuals. Room lighting was maintained on a 13.5L: 10.5D light cycle. All birds were provided with ad libitum access to water, cuttlebone and a blend of red and white millet seeds. Birds were also supplemented once a week with millet spray and a breeding supplement containing dry egg food (ABBA 92A, ABBA Products, Hillside, NJ USA), hard-boiled chicken eggs, and vitamins (Avian Plus, Zoo Med Laboratories, San Luis Obispo, CA USA). Research was approved by the Oklahoma State University Institutional Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 2010).
CORT dosing and immune challenges
During the nestling period, young were assigned to either an elevated CORT treatment (N=74) or the control group (N=77). Within broods, we attempted to balance the number of young assigned to the CORT and control groups. Between days 12–15 post-hatch, birds in the CORT group were orally administered 0.124 mg/ml of CORT in 25 µl of peanut oil twice each day (between 9:30–10:30 and between 14:30–15:30). Between days 16 and 28 post-hatch, the CORT-treated birds received an increased dose of 0.163 mg/ml of CORT in 25 µl of peanut oil twice each day during the same time periods to account for increased mass (Spencer et al, 2009). Birds in the control group were given an equal volume of peanut oil throughout the treatment period (Spencer et al, 2009).
Once birds reached the age of 60 days post-hatch, one-half of the birds in each of the two nestling treatment groups (CORT and control) were challenged with LPS (N=61) and the other birds were given a control injection (N=57). Again, we attempted to balance treatment groups within families. LPS challenged birds were injected with 1.0 mg LPS/kg body weight (Sigma-Aldrich L7261, St. Louis, MO, USA) in 50 µl of sterilized phosphate-buffered saline (PBS; Sigma-Aldrich P5368, St. Louis, MO, USA) subcutaneously in the abdomen, and birds in the control group were injected with 50 µl of PBS. The immune challenge was repeated on day 100 post-hatch.
Blood sample collection for measurement of CORT concentrations
To assess the effectiveness of the experimental manipulation in elevating circulating CORT concentrations, we collected blood samples (50 µl from the brachial vein) from all birds on day 14 post-hatch, 10 minutes after administering either CORT or peanut oil only, as appropriate (Khan and Robert, 2013). On day 60 post-hatch, we collected 2 blood samples (50 µl each) from all birds. The first blood sample was collected immediately before administering either the primary LPS immune challenge or an injection of PBS. The second blood sample was collected 1 hour after injection. Blood samples were centrifuged for 7 minutes at 1,845 × g, plasma was separated from the red blood cells, and samples were stored at −80°C until analysis. In response to LPS challenge, CORT levels should increase within 1 hour of challenge (Owen-Ashley et al, 2006). The blood samples collected on day 60 allowed us to assess if exposure to elevated levels of CORT during the nestling and fledgling periods affected the responsiveness of the hypothalamic-pituitary-adrenal axis to immune challenge.
Quantification of CORT concentrations
We quantified CORT concentrations using an enzyme immunoassay (EIA) from Enzo Life Sciences (cat. No. ADI-901-097, Farmingdale, NY, USA) and followed the kit instructions. The kit had been validated previously for use with zebra finches (Wada et al, 2007; Merrill and Grindstaff, 2015). To analyze samples, we diluted them 1:40 in 1% steroid displacement reagent and assay buffer. On each plate, we included a standard curve ranging from 20,000 to 32 pg/ml. Samples were assayed in duplicate and standards were assayed in triplicate. Plates were read on a Biotek ELx808 microplate reader at 405 nm. The assay detection limit is 27 pg (Enzo Life Sciences). The average intra- and inter-assay coefficients of variation were 6.9% and 22.4%, respectively.
Antibody response to LPS injection
Blood samples (∼50 µl) were collected from all birds on days 68 and 108 post-hatch to quantify anti-LPS antibody titers in both birds injected with LPS, and those not injected with LPS. Even birds not experimentally exposed to LPS may sometimes have LPS-reactive antibodies due to environmental exposure (Merrill and Grindstaff, 2014). Antibody titers were quantified with enzyme-linked immunosorbent assays (ELISAs) following previously described methods (Grindstaff et al, 2005; Grindstaff, 2008; Merrill and Grindstaff, 2014). In brief, plates (Nunc, Maxi-Sorp) were coated with 50 µg/mL LPS in carbonate buffer. Plasma samples were diluted 1:40 in 1% milk powder, PBS-Tween 20. The secondary antibody (goat, anti-bird IgG; Bethyl Labs, A140-110P) was diluted to 1:1000. This antibody may also detect other immunoglobulin isotypes, including IgM (Fassbinder-Orth et al, 2016). After a 30-minute incubation with the substrate buffer, plates were read at 405 nm. Each plate contained a serial dilution of a standard pool that covered the range of antibody titers in samples. Samples and standards were both run in duplicate. The average intra- and inter-plate coefficients of variation were 7.68% and 13.64%, respectively.
Haptoglobin
On day 100 post-hatch, blood samples were collected from all birds both immediately before injection with either PBS or LPS, and 12 hours later to quantify production of the acute phase protein haptoglobin in response to the injection (Hegemann et al, 2013). Subsequently, haptoglobin was quantified with a commercial kit (Tri-Delta Diagnostics, TP-801). We diluted plasma samples (5 µl) in 20 µl of the sample diluent and transferred 7.5 µl of diluted samples and standards in duplicate to the plates. We then added 100 µl of reagent 1 (hemoglobin). After shaking, plates were read at 630 nm in a plate reader and 140 µl of reagent 2, the chromogen, was added. After a 5-minute incubation at room temperature, the plate was then read for a second time at 630 nm. The absorbance values from measurements before addition of the chromogen were subtracted from the final absorbance values. The standard curve included haptoglobin concentrations of: 2.5, 1.25, 0.625, 0.313, 0.156, and 0.078 mg/ml. The average intra- and interplate coefficients of variation were 7.08% and 6.32%, respectively.
Bacteria Killing Ability
We quantified bacteria killing ability (BKA) of the plasma of birds using blood samples collected on days 14, 36 and 60 post-hatch. BKA on day 60 was quantified from the sample collected before immune challenge. BKA methods were derived from Matson et al, (2006), Millet et al, (2007) and Morrison et al, (2009). In brief, 5 µL of finch plasma were added to a combination of CO2-independent media (Gibco, Invitrogen) + 4mM L-glutamine (90 µL), and the E. coli (ATCC #8739) bacterial broth (10 µL), incubated for 20 min at 40°C, then pipetted out in 50 µL aliquots onto agar plates. All samples were run in duplicate, and after the solution was evenly distributed across the plates, the plates were then incubated overnight at 37°C. Four control plates, containing bacteria but no plasma, were included in each assay. The following day, the number of bacterial colonies were counted and compared to control plates in which the bacterial broth and 95 µL of PBS were incubated together without any plasma. Killing ability was calculated by subtracting the mean number of colonies for a bird’s two plates from the control mean, and then dividing that by the control mean. Intra-assay CV means were 13% for day 14 BKA, 10% for day 36 BKA, and 13% for day 60 BKA.
Statistical Analyses
To assess the impact of early-life CORT treatment on circulating CORT concentrations and measures of immune function, we used general linear mixed models (GLMMs). We included CORT treatment (Yes or No) as a fixed effect, maternal identity as a random effect, and hatch order, hatch date, sex, and clutch number (i.e., which clutch in the female’s life a given nestling originated from) as covariates in initial models for each dependent variable. Covariates that were significant or close to significant (p<0.06) were retained in the final model. We also examined the association between circulating CORT concentrations on day 14 and BKA on day 36, as well as between CORT concentrations on day 14 and both baseline and LPS-induced CORT concentrations on day 60 to determine if circulating baseline CORT concentrations on day 14 co-varied with BKA on day 36, and if CORT concentrations on day 14 were predictive of baseline and/or LPS-induced concentrations on day 60. In addition, we examined associations between baseline CORT concentrations on day 60 and day 36 BKA, day 68 anti-LPS antibody titers, and day 100 baseline haptoglobin and haptoglobin concentrations measured 12 hours after LPS immune challenge on day 100. We ran GLMMs with either day 14 or day 60 baseline CORT concentrations, CORT treatment, the interaction between CORT concentrations and CORT treatment, and any appropriate covariates as fixed effects, maternal identity as a random effect, and the measure of CORT or immune function of interest as the dependent variable.
To examine the effect of LPS treatment on day 60 CORT concentrations, haptoglobin concentrations and anti-LPS antibody titers, we ran GLMMs with LPS treatment (Yes or No) as a fixed effect, maternal identity as a random effect, and hatch order, hatch date, sex, clutch number and the interaction between CORT treatment and LPS treatment as covariates in initial models for each dependent variable. Parameters that were significant or close to significant (p<0.06) were retained in the final model. For all analyses in which we examined patterns of co-variation between physiological parameters, we used only measures for which we had at least 20 individuals in each CORT treatment so as not to draw inferences from small sample sizes. For all analyses we used maximum likelihood, and the denominator degrees of freedom were approximated using the containment method (Littell et al, 2006). We confirmed normality of residuals and homogeneity of variance, and all analyses were run in SAS 9.4 (Cary, NC). Antibody titer and haptoglobin data were log +1 transformed to achieve normality prior to analysis. Two outliers due to methodological error were removed from analyses, although inclusion of these values did not qualitatively or quantitatively impact results. Variation in sample sizes for each analysis (see Tables 1–3) reflects changes in the number of individuals due to mortality over time, plasma limitations, and removal of the two outliers.
Table 1.
Effects of developmental treatment with corticosterone (CORT) from days 12–28 post-hatch on circulating CORT and immune parameters.
| Dependent Variable |
CORT Treatment | Hatch Date | Hatch Order | Clutch # | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | N | Est | SE | P | N | Est | SE | P | N | Est | SE | P | N | Est | SE | P | |
| 14 | Baseline CORT | 100 | 0.63 | 0.05 | <0.001 | ||||||||||||
| BKA | 31 | 0.02 | 0.07 | 0.810 | 31 | 0.00 | 0.0 | 0.030 | 31 | 0.08 | 0.03 | 0.020 | |||||
|
| |||||||||||||||||
| 36 | BKA | 116 | 0.03 | 0.04 | 0.500 | 116 | 0.03 | 0.04 | 0.055 | ||||||||
|
| |||||||||||||||||
| 60 | Baseline CORT | 98 | 0.07 | 0.09 | 0.420 | ||||||||||||
| Stress CORT | 99 | 0.0 | 0.09 | 0.970 | 99 | 0.1 | 0.05 | 0.047 | |||||||||
| BKA | 25 | 0.01 | 0.05 | 0.850 | 25 | 0.00 | 0.0 | 0.001 | |||||||||
|
| |||||||||||||||||
| 68 | Anti-LPS Ab | 57 | 0.02 | 0.28 | 0.940 | ||||||||||||
|
| |||||||||||||||||
| 100 | Baseline Hp | 85 | 0.02 | 0.01 | 0.100 | 85 | 0.02 | 0.01 | <0.001 | ||||||||
| 12-Hr Hp | 83 | 0.0 | 0.01 | 0.940 | 83 | 0.00 | 0.00 | 0.001 | |||||||||
|
| |||||||||||||||||
| 108 | Anti-LPS Ab | 43 | 0.08 | 0.55 | 0.880 | 43 | 0.01 | 0.00 | 0.005 | ||||||||
CORT dosing elevated circulating CORT concentrations during the treatment period (i.e., day 14) but did not impact subsequent CORT concentrations, either baseline or concentrations produced in response to lipopolysaccharide (LPS) challenge (LPS CORT). Developmental CORT treatment also did not significantly affect immune parameters. BKA=Bacteria killing ability, Anti-LPS Ab=specific antibodies to LPS, Hp=haptoglobin, 12-Hr Hp=haptoglobin concentrations measured 12 hours after LPS challenge. Est is the model estimate (slope of the relationship) between parameters. Significant (p<0.05) effects are bolded.
Table 3.
Effect of lipopolysaccharide (LPS) treatment on circulating corticosterone (CORT) and immune parameters.
| Dependent Variable |
LPS Treatment | Hatch Date | Clutch # | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | N | Est | SE | P | N | Est | SE | P | N | Est | SE | P | |
| 60 | Stress CORT | 99 | 0.51 | 0.07 | <0.001 | 99 | 0.09 | 0.04 | 0.020 | ||||
|
| |||||||||||||
| 68 | Anti-LPS Ab | 57 | 0.22 | 0.29 | 0.440 | ||||||||
|
| |||||||||||||
| 100 | Baseline Hp | 85 | 0.00 | 0.01 | 0.840 | 85 | −0.02 | 0.01 | <0.001 | ||||
| 12-Hr Hp | 83 | 0.01 | 0.01 | 0.310 | 83 | −0.0 | 0.00 | 0.001 | |||||
|
| |||||||||||||
| 108 | Anti-LPS Ab | 43 | 1.26 | 0.52 | 0.020 | 43 | 0.01 | 0.00 | 0.002 | ||||
Birds challenged with LPS had significantly higher CORT concentrations one hour after challenge than control birds (LPS CORT) and significantly higher LPS-specific antibody titers (Anti-LPS Ab) on day 108 post-hatch. LPS challenges were administered on days 60 and 100 post-hatch. Hp=haptoglobin, 12-Hr Hp=haptoglobin concentrations measured 12 hours after LPS challenge. Est is the model estimate (slope of the relationship) between parameters. Significant (p<0.05) effects are bolded.
Results
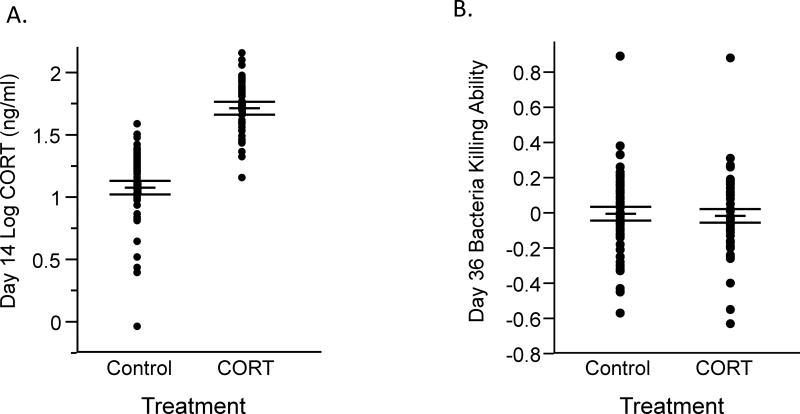
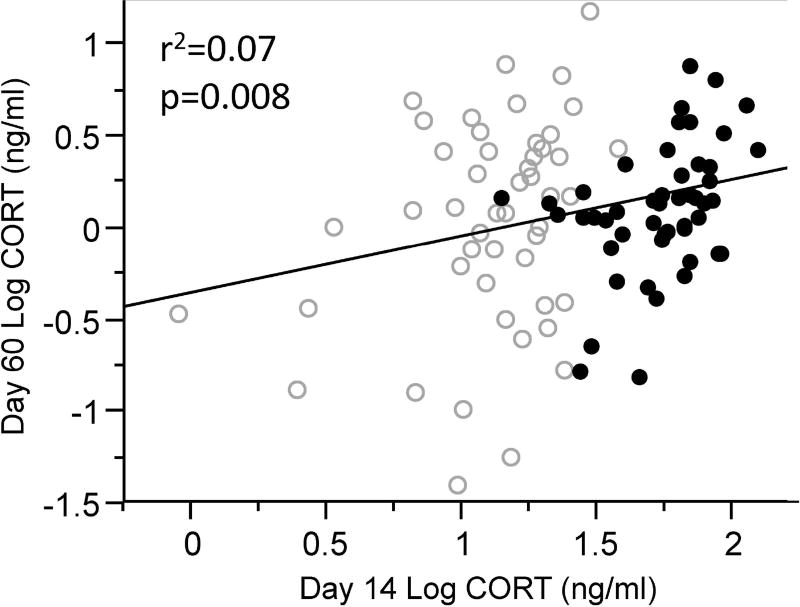
CORT treatment had a significant effect on baseline CORT concentrations at day 14 (Table 1, Fig. 2A), but did not impact any other measure (Table 1, Fig. 2B). When we examined the associations between circulating CORT and BKA, we found a negative association between baseline CORT concentrations on day 14 and BKA on day 36 (Table 2) as well as a borderline non-significant negative association between baseline CORT concentrations on day 60 and BKA on day 36 (Table 2). We found no association between baseline CORT concentrations on day 60 and anti-LPS antibody titers measured on day 68, (Table 2), nor between baseline CORT concentrations on day 60 and either measure of haptoglobin (Table 2). We found a strong positive association between baseline CORT concentrations on day 14 and baseline CORT concentrations on day 60 (Table 2, Fig. 3), but no relationship between baseline CORT concentrations on day 14 and LPS-induced CORT concentrations on day 60 (Table 2). Finally, we found no interaction effects between CORT treatment and circulating CORT concentrations for any measure (Table 2).
Figure 2.
Effect of treatment with corticosterone (CORT) during the nestling and fledgling periods from days 12–28 post-hatch on CORT concentrations during the treatment period (A) and bacteria killing ability after the end of the treatment period (B). CORT treatment significantly elevated CORT concentrations during the treatment period but did not significantly influence bacteria killing ability after the treatment period.
Table 2.
General linear mixed models for associations between circulating corticosterone (CORT), immune parameters and covariates.
| Response variable | Predictor variable | N | Estimate | SE | P |
|---|---|---|---|---|---|
| D36 BKA | CORT Treatment | 100 | −0.47 | 0.25 | 0.064 |
| Hatch Order | 100 | −0.03 | 0.02 | 0.063 | |
| Circulating Baseline CORT D14 | 100 | −0.35 | 0.13 | 0.006 | |
| Circ. Base. CORT D14 × CORT Trt | 100 | 0.25 | 0.16 | 0.128 | |
| CORT Treatment | 98 | 0.00 | 0.04 | 0.993 | |
| Hatch Order | 98 | −0.03 | 0.02 | 0.080 | |
| Circulating Baseline CORT D60 | 98 | −0.15 | 0.08 | 0.058 | |
| Circ. Base. CORT D60 × CORT Trt | 98 | 0.13 | 0.09 | 0.147 | |
|
| |||||
| D68 Anti-LPS Ab | CORT Treatment | 47 | 0.28 | 0.35 | 0.426 |
| Circulating Baseline CORT D60 | 47 | 0.42 | 0.60 | 0.428 | |
| Circ. Base. CORT D60 × CORT Trt | 47 | −0.23 | 0.74 | 0.754 | |
|
| |||||
| D100 Baseline Hp | CORT Treatment | 72 | 0.02 | 0.01 | 0.096 |
| Clutch Number | 72 | −0.03 | 0.01 | <.001 | |
| Circulating Baseline CORT D60 | 72 | −0.0 | 0.02 | 0.521 | |
| Circ. Base. CORT D60 × CORT Trt | 72 | −0.01 | 0.02 | 0.777 | |
|
| |||||
| D100 12Hr Hp | CORT Treatment | 70 | −0.01 | 0.01 | 0.711 |
| Hatch Date | 70 | 0.00 | 0.00 | <.001 | |
| Circulating Baseline CORT D60 | 70 | 0.03 | 0.02 | 0.089 | |
| Circ. Base. CORT D60 × CORT Trt | 70 | −0.01 | 0.02 | 0.538 | |
|
| |||||
| D60 Baseline CORT | CORT Treatment | 98 | 0.58 | 0.59 | 0.335 |
| Circulating Baseline CORT D14 | 98 | 0.72 | 0.32 | 0.001 | |
| Circ. Base. CORT D14 × CORT Trt | 98 | −0.19 | 0.38 | 0.625 | |
|
| |||||
| D60 Stress CORT | CORT Treatment | 99 | 0.84 | 0.61 | 0.171 |
| Clutch | 99 | 0.08 | 0.05 | 0.099 | |
| Circulating Baseline CORT D14 | 99 | 0.33 | 0.32 | 0.836 | |
| Circ. Base. CORT D14 × CORT Trt | 99 | −0.58 | 0.38 | 0.134 | |
Estimate is the regression coefficient for each predictor and response variable. Bacteria killing ability (BKA) on day 36 was negatively related to CORT concentrations measured on day 14 post-hatch. CORT concentrations measured on day 14 were positively related to baseline CORT concentrations measured on day 60 post-hatch. Base=baseline, Anti-LPS Ab=specific antibodies to LPS, Clutch number=number of clutches parents had produced, Hp=haptoglobin, 12-Hr Hp=haptoglobin concentrations measured 12 hours after LPS challenge, Trt=treatment. Significant (p<0.05) effects are bolded.
Figure 3.
Relationship between corticosterone (CORT) concentrations during the period of experimental manipulation (day 14) and after the experimental period (day 60). Points are coded by developmental treatment group. Open circles represent birds in the control group and filled circles represent birds in the group with experimentally elevated CORT levels.
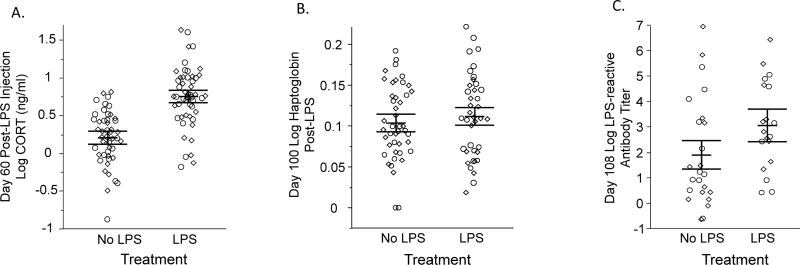
On day 60, birds injected with LPS had significantly higher CORT concentrations one hour after injection than control (PBS-injected) birds (Table 3, Fig. 4A). There was no effect of LPS treatment on either haptoglobin measure (Fig. 4B) or day 68 anti-LPS antibody titers (Table 3), but there was a significant effect of LPS treatment on day 108 anti-LPS antibody titers, in which birds challenged with LPS on days 60 and 100 exhibited a stronger anti-LPS antibody response on day 108 (Table 3, Fig. 4C).
Figure 4.
Effect of challenge with lipopolysaccharide (LPS) on corticosterone (CORT) concentrations one hour after primary challenge (A), haptoglobin concentrations 12 hours after secondary challenge (B), and anti-LPS antibody titers 8 days after secondary challenge (C). Birds challenged with LPS had significantly higher CORT concentrations and elevated anti-LPS antibody titers relative to control birds, but LPS challenge did not significantly impact haptoglobin concentrations. Diamonds represent birds that received CORT treatment, and circles are those that did not receive CORT.
Discussion
Based on previous work documenting negative effects of early-life stress on adult measures of immune function and disease resistance in other organisms (reviewed in Martin, 2009; Merlot et al, 2008; Miller et al, 2011), we anticipated that elevation of CORT levels during the postnatal period would impact the ability of zebra finches to respond to a simulated bacterial challenge on days 60 and 100 post-hatch. However, we did not find evidence for an effect of CORT treatment group during development on subsequent immune responses. CORT concentrations measured during the treatment period were negatively related to BKA measured on day 36, which was eight days after the cessation of the CORT treatment (Fig. 2). Moreover, there was a non-significant negative relationship between baseline CORT concentrations on day 60 and BKA on day 36. These results suggest that elevated baseline CORT levels are negatively associated with BKA in young zebra finches. Second, we predicted that elevation of CORT levels during the postnatal period would result in increased production of CORT in response to LPS challenge on day 60 through a programming effect on the HPA axis (Spencer et al. 2009). LPS challenge on day 60 resulted in increased production of CORT 60 minutes following injection compared to control individuals that were not injected with LPS, but the magnitude of the response was not impacted by developmental CORT treatment (Fig. 2). In our analyses, we also accounted for the potential effects of hatching date, hatching order and the number of previous clutches produced by the mother. These covariates had significant effects on subsequent immune responses and deserve further experimental investigation. Hatching date was negatively related to both BKA on day 14 and haptoglobin concentrations 12 hours after challenge on day 100. Hatching date was positively related to anti-LPS antibody titers on day 108. Hatching order was negatively related to BKA on day 14, although this was largely driven by one nestling that hatched fifth in the brood. Finally, the number of clutches previously produced by the mother was positively related to CORT concentrations produced in response to LPS challenge on day 60 and negatively associated with baseline haptoglobin concentrations on day 100. Together, our findings provide evidence for dynamic, bi-directional relationships between CORT and measures of immune function, but limited evidence for programming effects of developmental CORT treatment.
The relationship between glucocorticoids and immune function is complex, and depends upon temporal exposure (i.e., acute versus chronic), magnitude of CORT production, and the specific immunological component (Dhabhar and McEwen ‘97; Martin, 2009; Shini et al, 2010). Moreover, the social, demographic, and ecological context can potentially impact relationships between the two. BKA indicates the potential of the whole blood or plasma to inactivate a potential microbial pathogen, and thus is a useful functional measure of the constitutive innate immune response (Boughton et al, 2011). Previous work has indicated that killing for this strain of E. coli (8739) is primarily complement dependent (Matson et al, 2006; Merrill unpublished data). Complement proteins are an important part of the constitutive innate immune system due to their ability to opsonize or lyse invading microbes (Esser, ‘94). Moreover, they can act as a link between innate and acquired immune defenses by lowering the threshold for B-cell activation and targeting antigen to lymphoid organs (Ochsenbein and Zinkernagel, 2000). Previous work on BKA with this strain of E. coli indicates that acute stressors typically result in a decrease in BKA (Matson et al, 2006; Merrill et al, 2012; Gao et al, 2017). Whether this change in BKA following stress is a product of immunosuppression or immunoredistribution is unclear, but the speed with which these changes can occur (7 min—Merrill et al, 2012) suggests that it may be immunoredistribution of immunological components in preparation for coping with an impending injury and associated influx of microbes (sensu Dhabhar, 2009; Martin, 2009).
The results from the present study are distinct from the ones described above, however, because the negative association we measured was between baseline CORT and BKA. Merrill et al, (2014) found a significant positive association between baseline CORT and BKA in wild male red-winged blackbirds (Agelaius phoeniceus) during the breeding season, and Merrill et al, (2015) found that the direction of the relationship between baseline CORT and BKA differed by month in rufous-collared sparrows (Zonotrichia capensis). The work on rufous-collared sparrows indicates that the association between baseline CORT and BKA may be impacted by other factors such as life-history stage. Finally, in work examining baseline CORT and BKA in molting brown-headed cowbirds (Molothrus ater), Ellis et al, (2012) found a negative association between the two measures in female cowbirds during molt, although they found no association between the two parameters prior to, or following, molt. The negative relationships between baseline CORT and BKA in young zebra finches could potentially reflect CORT-mediated down-regulation of BKA to reduce oxidative stress (sensu Dhabhar, 2009), or an investment strategy to free resources for other processes such as somatic growth or feather development. These non-mutually exclusive theories would require explicit testing. Additionally, one important difference between the prior studies and the current study is that these prior studies were all conducted in wild birds, whereas we studied zebra finches in captivity. The lower BKA in association with increased baseline CORT is unlikely to solely be a product of immunoredistribution because immunoredistribution is generally a short-term response to an acute stressor (Dhabhar and McEwen, 1997).
We found no effect of LPS treatment on specific anti-LPS antibody production on day 68, but there was a significant effect of LPS treatment on anti-LPS antibody production on day 108 (Fig. 2). This difference likely reflects that the challenge on day 60 was a primary exposure for the birds and primary antibody responses are characterized by slower, weaker antibody responses, primarily of the IgM isotype (Janeway et al, 2001). Thus, if we had collected blood samples at a later time point after the primary immune challenge on day 60 or had been able to more specifically quantify IgM, we might have detected an effect of LPS treatment on antibody titers. However, we did not find evidence at either time point that developmental CORT treatment affected the ability of birds to respond to LPS challenge by producing antibodies. This does not support the hypothesis that systemically elevated CORT levels can program immune responses (Taves et al, 2017).
Contrary to our predictions, we found no effect of either CORT or LPS exposure on haptoglobin production. Data from other species suggest that haptoglobin levels may increase, decrease, or remain the same after LPS challenge (Millet et al, 2007; Hegemann et al, 2013; Zylberberg, 2015; Bailly et al, 2016; Schultz et al, 2017). The haptoglobin response to LPS challenge has been demonstrated to vary among closely related species (Zylberberg, 2015), to vary across populations within a species (Bailly et al, 2016), and to vary seasonally within a species (Hegemann et al, 2013). Thus, it is difficult to generalize and describe the typical avian haptoglobin response to LPS challenge. Although there is some evidence that acute stressors can influence haptoglobin levels (e.g., Zylberberg, 2015), previous studies have not assessed the potential for CORT exposure during the nestling and fledgling periods to have a persistent effect on haptoglobin production.
During the acute phase response to immune challenge, activation of the hypothalamic-pituitary-adrenal axis occurs, resulting in elevated levels of glucocorticoids (Bateman et al, ’89; Besedovsky and del Rey, ’96; Owen-Ashley et al, 2006; Owen-Ashley and Wingfield, 2007). Spencer et al, (2009) used the same protocol for chronic exposure to CORT in zebra finches that we used in the current study, but in contrast to our findings, they documented programming effects of CORT exposure on the HPA axis. Birds exposed to elevated levels of CORT during the nestling and fledgling periods produced elevated levels of CORT in response to restraint stress at 60 days post-hatch (Spencer et al, 2009). In the current study, zebra finches produced a robust CORT response to LPS challenge on day 60, but we did not find evidence that the magnitude of this response was programmed by chronic exposure to CORT during development.
Although we found no effect of CORT treatment on day 60 baseline or LPS-induced CORT concentrations, we did find a positive relationship between baseline CORT concentrations during the treatment period (day 14) and baseline CORT concentrations on day 60 (Fig. 2). Spencer and colleagues (2009) did not detect an effect of developmental CORT treatment group on baseline CORT concentrations, but did not report CORT concentrations during the treatment period to determine if individuals with higher CORT levels in response to the treatment subsequently had elevated baseline CORT. It would be useful in future studies to directly quantify individual CORT levels during experimental treatment. Systemic treatment with CORT during the nestling and fledgling periods may not influence subsequent immune function or program the magnitude of the CORT response to an immune challenge for several potential reasons. First, the predominant glucocorticoid in immune tissues of zebra finches during development is cortisol, not CORT (Schmidt and Soma, 2008). Levels of cortisol in the Bursa of Fabricius decline during development, but are still elevated relative to levels in plasma and relative to levels of CORT at day 30 post-hatch (Schmidt and Soma, 2008). Additionally, cortisol levels are higher in the thymus than in the blood during this period (Schmidt and Soma, 2008; Taves et al, 2016). Targeted manipulations of cortisol in immune tissues may be necessary to conclusively determine if chronic glucocorticoid exposure during development can program immune function and interactions with the HPA axis in birds. Second, direct manipulations of CORT do not mirror endogenous responses to stressors because the adrenal medulla is not activated. In response to antigens such as LPS, both the HPA axis and the autonomic nervous system are activated, and catecholamines play a central role in the activation of the HPA axis (Karrow, 2006). Thus, experimental manipulations that account for the involvement of both the adrenal cortex and adrenal medulla in the endogenous response to stressors may be necessary to determine if chronic stress during development can program immune function. Third, chronic exogenous CORT treatment may initially elevate CORT levels as we observed on day 14, but may not result in sustained elevation of CORT levels due to negative feedback regulation (Young et al, ‘95, Romero, 2004). This emphasizes an additional weakness of utilizing exogenous CORT administration to experimentally mimic chronic stress exposure.
In humans, early-life stressors are associated with immune dysregulation later in life (reviewed in Fagundes et al, 2013). Acute inflammatory responses provide important protection against pathogens during the early stages of an immune response. However, down-regulation is necessary to suppress the inflammatory response, and prevent development of a chronic inflammatory state. One important negative feedback mechanism used to down-regulate the immune response is the release of glucocorticoids, which bind to receptors on immune cells such as macrophages and suppress further release of inflammatory cytokines (reviewed in Martin, 2009; Miller et al, 2011). It has been proposed that early-life stress programs macrophages both to release greater levels of pro-inflammatory cytokines in response to immune challenge and to be less sensitive to down-regulation by glucocorticoids (Miller et al, 2011). Thus, early-life stress becomes associated with a chronic inflammatory state (Miller et al, 2011). If the predominant glucocorticoid released systemically in response to stress in zebra finches is corticosterone, but the glucocorticoid responsible for programming effects on the immune response is cortisol (Taves et al, 2017), then this may protect zebra finches from any effects of chronic stress during development on the immune response, particularly the adaptive immune response.
Conclusions
We found no long-term programming effects of repeated CORT exposure during the nestling and fledgling periods on immune function or HPA axis activity. However, cortisol, rather than corticosterone, may be responsible for any programming effects on the adaptive immune response of zebra finches and future studies should manipulate cortisol levels in lymphoid organs. Additionally, chronic exposure to an environmentally relevant stressor in the field may have different effects on immune function and HPA axis activity. There was a negative relationship between baseline CORT levels and BKA, and a weak positive association between baseline CORT and haptoglobin response to LPS, indicating that baseline CORT levels may reflect investment strategies linked to down- or up-regulation of immune parameters. These patterns may be tied to different strategies of resource allocation or trade-offs, in which birds with elevated baseline CORT levels invest in microbicidal proteins at reduced levels, and acute-phase proteins at higher levels, depending on the stage of development. Our findings thus provide intriguing questions that require additional investigation into how specific glucocorticoids (i.e., cortisol vs. corticosterone) impact different immune tissues, and how developmental stage may impact associations between early-life challenges and expression of immune traits over time.
Figure 1.
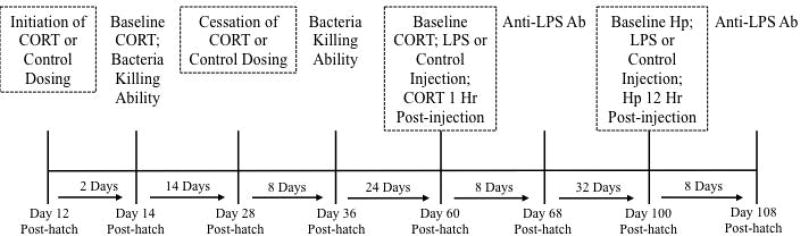
Timeline of experimental methods. At 12 days post-hatch, young were assigned to either an elevated corticosterone (CORT) treatment group or a control group. Dosing with CORT or the control vehicle continued until day 28 post-hatch. On day 14, we collected the first blood sample to quantify corticosterone concentrations and bacteria killing ability. We also measured bacteria killing ability from blood samples collected on days 36 and 60. On days 60 and 100, one-half of the individuals were challenged with lipopolysaccharide (LPS). We collected a blood sample before challenge to measure baseline CORT and a second blood sample one hour after challenge to measure LPS-induced CORT concentrations. We also collected blood samples on days 68 and 108 to quantify LPS-specific antibody (Ab) titers. Finally, on day 100 we collected a blood sample before LPS challenge to quantify baseline haptoglobin (Hp) concentrations and collected a second blood sample 12 hours later to quantify Hp concentrations in response to LPS.
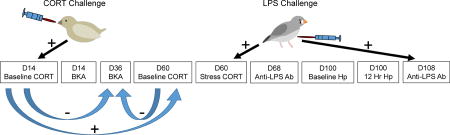
Figure 5.
Schematic illustration of results. During the nestling and fledgling periods, exposure to corticosterone (CORT) was experimentally manipulated and challenge with lipopolysaccharide (LPS) was experimentally manipulated on days 60 and 100 post-hatch. Straight, black arrows represent significant effects of these treatment groups on CORT concentrations and immune parameters. Curved, blue arrows represent significant relationships between traits. Developmental CORT exposure elevated CORT levels during the treatment period (D14 Baseline CORT). Birds challenged with LPS had higher CORT concentrations one hour after challenge (D60 LPS CORT) and higher LPS-specific antibody titers on day 108 (D108 Anti-LPS Ab). CORT concentrations on day 14 were positively related to baseline CORT concentrations on day 60. CORT concentrations on day 14 were negatively related to bacteria killing ability (BKA) on day 36. Hp=haptoglobin.
Highlights.
Juvenile corticosterone (CORT) was elevated to test for short- and long-term effects on immunity.
Bactericidal ability, haptoglobin, and specific antibody were not affected by CORT treatment.
Bactericidal ability was negatively related to baseline CORT.
Acknowledgments
We would like to thank the editors of the special issue, Rachel Bowden, Susannah French, and Gregory Demas for the invitation to contribute to the issue and two anonymous reviewers for their thoughtful comments. We would also like to thank Matt Anderson, Kent Andersson, Sara Dawson, Katerina Faust, Lisa Hughes, Madeleine Naylor, Sarah Oppenborn, Alecia Rains, and Matthew Waselik for their assistance in performing several of the methods. Funding for this research was provided by the National Institutes of Health (Grant # 1R15HD066378-01).
Literature cited
- Ardia DR, Parmentier HK, Vogel LA. The role of constraints and limitation in driving individual variation in immune response. Funct Ecol. 2011;25:61–73. [Google Scholar]
- Bailly J, Garnier S, Khimoun A, Arnoux E, Eraud C, Goret J-Y, Luglia T, Gaucher P, Faivre B. Reduced inflammation in expanding populations of a neotropical bird species. Ecol Evol. 2016;6:7511–7521. doi: 10.1002/ece3.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A. Social stress, immune functions and disease in rodents. Front Neuroendocrinol. 2007;28:28–49. doi: 10.1016/j.yfrne.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Bateman A, Singh A, Kral T, Solomon A. The immune-hypothalamic-pituitary-adrenal axis. Endocr Rev. 1989;10:92–112. doi: 10.1210/edrv-10-1-92. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A. Immune–neuro–endocrine interactions: facts and hypothesis. Endocr Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- Boughton R, Joop G, Armitage S. Ecological immunology: methods for field experiments. Funct Ecol. 2011;25:81–100. [Google Scholar]
- De Coster G, Verhulst S, Koetsier E, De Neve L, Briga M, Lens L. Effects of early developmental conditions on innate immunity are only evident under favourable adult conditions in zebra finches. Naturwissenschaften. 2011;98:1049–1056. doi: 10.1007/s00114-011-0863-3. [DOI] [PubMed] [Google Scholar]
- DeWitt TJ, Sih A, Wilson DS. Costs and limits of phenotypic plasticity. Trends Ecol Evol. 1998;13:77–81. doi: 10.1016/s0169-5347(97)01274-3. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: A potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution: dynamics and hormonal mechanisms. J Immunol. 1995;154:5511–5527. [PubMed] [Google Scholar]
- Ellis VA, Merrill L, Wingfield JC, O’Loghlen AL, Rothstein SI. Changes in immunocompetence and other physiological measures during molt in brown-headed cowbirds (Molothrus ater) Auk. 2012;129:231–238. [Google Scholar]
- Esser AF. The membrane attack complex of complement - assembly, structure and cytotoxic activity. Toxicology. 1994;87:229–247. doi: 10.1016/0300-483x(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun. 2013;27:8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbinder-Orth CA, Wilcoxen TE, Tran T, Boughton RK, Fair JM, Hofmeister EK, Grindstaff JL, Owen JC. Immunoglobulin detection in wild birds: effectiveness of three secondary anti-avian IgY antibodies in direct ELISAs in 41 avian species. Methods Ecol Evol. 2016;7:1174–1181. doi: 10.1111/2041-210X.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. Am Nat. 1992;139:603–622. [Google Scholar]
- Gao S, Sanchez C, Deviche PJ. Corticosterone rapidly suppresses innate immune activity in the house sparrow (Passer domesticus) J Exp Biol. 2017;220:322–327. doi: 10.1242/jeb.144378. [DOI] [PubMed] [Google Scholar]
- Grindstaff JL. Maternal antibodies reduce costs of an immune response during development. J Exp Biol. 2008;211:654–660. doi: 10.1242/jeb.012344. [DOI] [PubMed] [Google Scholar]
- Grindstaff JL, Demas GE, Ketterson ED. Dietary protein restriction affects egg size and number but does not reduce maternal antibody transfer in Japanese quail (Coturnix japonica) J Anim Ecol. 2005;74:1051–1058. [Google Scholar]
- Groothuis TGG, Taborsky B. Introducing biological realism into the study of developmental plasticity in behaviour. Front. Zool. 2015;12:S6. doi: 10.1186/1742-9994-12-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick SM. The acquired immune system: A vantage from beneath. Immunity. 2004;21:607–615. doi: 10.1016/j.immuni.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Hegemann A, Matson KD, Versteegh MA, Villegas A, Tieleman BI. Immune response to an endotoxin challenge involves multiple immune parameters and is consistent among the annual-cycle stages of a free-living temperate zone bird. J Exp Biol. 2013;216:2573–2580. doi: 10.1242/jeb.083147. [DOI] [PubMed] [Google Scholar]
- Hill GE. Condition-dependent traits as signals of the functionality of vital cellular processes. Ecol Lett. 2011;14:625–634. doi: 10.1111/j.1461-0248.2011.01622.x. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M, Shlomchik M. Immunobiology: the immune system in health and disease. Fifth. New York, NY: Garland Publishing; 2001. [Google Scholar]
- Karrow NA. Activation of the hypothalamic-pituitary-adrenal axis and autonomic nervous system during inflammation and altered programming of the neuroendocrine—immune axis during fetal and neonatal development: Lessons learned from the model inflammagen, lipopolysaccharide. Brain, Behav Immun. 2006;20:144–158. doi: 10.1016/j.bbi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Khan N, Robert K. Does sex matter? Differential responses to corticosterone administration in the zebra finch. Zool. 2013;116:293–299. doi: 10.1016/j.zool.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM. Coping style and immunity in animals: making sense of individual variation. Brain Behav Immun. 2008;22:662–667. doi: 10.1016/j.bbi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger K, Schabenberger O. SAS system for mixed models. Cary, NC, USA: SAS Institute Inc; 2006. [Google Scholar]
- Loiseau C, Sorci G, Dano S, Chastel O. Effects of experimental increase of corticosterone levels on begging behavior, immunity and parental provisioning rate in house sparrows. Gen Comp Endocrinol. 2008;155:101–108. doi: 10.1016/j.ygcen.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Macri S, Pasquali P, Bonsignore LT, Pieretti S, Cirulli F, Chiarotti F, Laviola G. Moderate neonatal stress decreases within-group variation in behavioral, immune and HPA responses in adult mice. PLoS One. 2007;2:e1015. doi: 10.1371/journal.pone.0001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB. Stress and immunity in wild vertebrates: timing is everything. Gen Comp Endocrinol. 2009;163:70–76. doi: 10.1016/j.ygcen.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Martin LB, Hawley DM, Ardia DR. An introduction to ecological immunology. Funct Ecol. 2011;25:1–4. [Google Scholar]
- Matson KD, Tieleman BI, Klasing KC. Capture stress and the bactericidal competence of blood and plasma in five species of tropical birds. Physiol Biochem Zool. 2006;79:556–564. doi: 10.1086/501057. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Rev. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- Merlot E, Couret D, Otten W. Prenatal stress, fetal imprinting and immunity. Brain Behav Immun. 2008;22:42–51. doi: 10.1016/j.bbi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Merrill L, Angelier F, O’Loghlen AL, Rothstein SI, Wingfield JC. Sex-specific variation in brown-headed cowbird immunity following acute stress: a mechanistic approach. Oecologia. 2012;170:25–38. doi: 10.1007/s00442-012-2281-4. [DOI] [PubMed] [Google Scholar]
- Merrill L, Gonzalez-Gomez PL, Ellis VA, Levin II, Vasquez RA, Wingfield JC. A blurring of life-history line: Immune function, molt and reproduction in a highly stable environment. Gen Comp Endocrinol. 2015;213:65–73. doi: 10.1016/j.ygcen.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Merrill L, Grindstaff JL. Maternal antibody transfer can lead to suppression of humoral immunity in developing zebra finches (Taeniopygia guttata) Physiol Biochem Zool. 2014;87:740–751. doi: 10.1086/677218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L, Grindstaff JL. Pre and post-natal antigen exposure can program the stress axis of adult zebra finches: Evidence for environment matching. Brain Behav Immun. 2015;45:71–79. doi: 10.1016/j.bbi.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L, Levinson SD, O’Loghlen AL, Wingfield JC, Rothstein SI. Bacteria-killing ability is negatively linked to epaulet size, but positively linked to baseline corticosterone, in male Red-winged Blackbirds (Agelaius phoeniceus) Auk. 2014;131:3–11. [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet S, Bennett J, Lee KA, Hau M, Klasing KC. Quantifying and comparing constitutive immunity across avian species. Dev Comp Immunol. 2007;31:188–201. doi: 10.1016/j.dci.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Morrison ES, Ardia DR, Clotfelter ED. Cross-fostering reveals sources of variation in innate immunity and hematocrit in nestling tree swallows Tachycineta bicolor. J Avian Biol. 2009;40:573–578. [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Eighth. Washington, DC: National Academies Press; 2010. [Google Scholar]
- Ochsenbein AF, Zinkernagel RM. Natural antibodies and complement link innate and acquired immunity. Immunol Today. 2000;21:624–630. doi: 10.1016/s0167-5699(00)01754-0. [DOI] [PubMed] [Google Scholar]
- Owen-Ashley NT, Turner M, Hahn TP, Wingfield JC. Hormonal, behavioral, and thermoregulatory responses to bacterial lipopolysaccharide in captive and free-living white-crowned sparrows (Zonotrichia leucophrys gambelii) Horm Behav. 2006;49:15–29. doi: 10.1016/j.yhbeh.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Owen-Ashley NT, Wingfield JC. Acute phase responses of passerine birds: characterization and seasonal variation. J Ornithol. 2007;148:S583–S591. [Google Scholar]
- Romero LM. Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol. 2004;19:249–255. doi: 10.1016/j.tree.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Saino N, Suffritti C, Martinelli R, Møller AP. Immune response covaries with corticosterone plasma levels under experimentally stressful conditions in nestling barn swallows (Hirundo rustica) Behav Ecol. 2003;14:318–325. [Google Scholar]
- Schmidt KL, Kubli SP, MacDougall-Shackleton EA, MacDougall-Shackleton SA. Early-life stress has sex-specific effects on immune function in adult song sparrows. Phys Biochem Zool. 2015;88:183–194. doi: 10.1086/680599. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Soma KK. Cortisol and corticosterone in the songbird immune and nervous systems: local vs. systemic levels during development. Am J Physiol Regul Integr Comp Physiol. 2008;295:R103–R110. doi: 10.1152/ajpregu.00002.2008. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Rensel MA, Heiss RS. Short- and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: A review. Curr Zool. 2011;57:514–530. [Google Scholar]
- Schultz EM, Hahn TP, Klasing KC. Photoperiod but not food restriction modulates innate immunity in an opportunistic breeder, Loxia curvirosta. J Exp Biol. 2017;220:722–730. doi: 10.1242/jeb.149898. [DOI] [PubMed] [Google Scholar]
- Sheldon BC, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Shini S, Huff GR, Shini A, Kaiser P. Understanding stress-induced immunosuppression: Exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult Sci. 2010;89:841–851. doi: 10.3382/ps.2009-00483. [DOI] [PubMed] [Google Scholar]
- Spencer KA, Evans NP, Monaghan P. Postnatal stress in birds: A novel model of glucocorticoid programming of the hypothalamic-pituitary-adrenal axis. Endocrinol. 2009;150:1931–1934. doi: 10.1210/en.2008-1471. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Martin S, Mouihate A, Pittman QJ. Early-life immune challenge: defining a critical window for effects on adult responses to immune challenge. Neuropsychopharmacol. 2006;31:1910–1918. doi: 10.1038/sj.npp.1301004. [DOI] [PubMed] [Google Scholar]
- Stier KS, Almasi B, Gasparini J, Piault R, Roulin A, Jenni L. Effects of corticosterone on innate and humoral immune functions and oxidative stress in barn owl nestlings. J Exp Biol. 2009;212:2085–2091. doi: 10.1242/jeb.024406. [DOI] [PubMed] [Google Scholar]
- Taves MD, Losie JA, Rahim T, Schmidt KL, Sandkam BA, Ma C, Silversides FG, Soma KK. Locally elevated cortisol in lymphoid organs of the developing zebra finch but not Japanese quail or chicken. Dev Comp Immunol. 2016;54:116–125. doi: 10.1016/j.dci.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Taves MD, Hamden JE, Soma KK. Local glucocorticoid production in lymphoid organs of mice and birds: functions in lymphocyte development. Horm Behav. 2017;88:4–14. doi: 10.1016/j.yhbeh.2016.10.022. [DOI] [PubMed] [Google Scholar]
- Van der Most P, de Jong B, Parmentier HK, Verhulst S. Tradeoff between growth and immune function: a meta-analysis of selection experiments. Funct Ecol. 2011;25:74–80. [Google Scholar]
- Wada H, Hahn TP, Breuner CW. Development of stress reactivity in white-crowned sparrow nestlings: Total corticosterone response increases with age, while free corticosterone response remains low. Gen Comp Endocrinol. 2007;150:405–413. doi: 10.1016/j.ygcen.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Young EA, Kwak SP, Kottak J. Negative feedback regulation following administration of chronic exogenous corticosterone. J Neuroendocrinol. 1995;7:37–45. doi: 10.1111/j.1365-2826.1995.tb00665.x. [DOI] [PubMed] [Google Scholar]
- Zylberberg M. Common measures of immune function vary with time of day and sampling protocol in five passerine species. J Exp Biol. 2015;218:757–766. doi: 10.1242/jeb.111716. [DOI] [PubMed] [Google Scholar]