Abstract
Metabolomics is the study of small, organic molecules within biochemical pathways. With advancement of technology, nuclear magnetic resonance, gas chromatography, and mass spectrometry have allowed for the discovery and analysis of large databases of metabolites implicated in heart failure. The biochemical pathways involved in heart failure suggest that a metabolic switch occurs in the failing myocardium. Furthermore, serum and breath analysis of patients with heart failure have created metabolomic profiles of patients with systolic heart failure, which can be applied clinically for diagnosis and prognosis in this population. Metabolomics also explores the patient and environment interactions and unlocks the link between environmental exposures and the development of cardiovascular disease. Although a relatively new field, metabolomics is poised to become a clinically impactful field that develops novel biomarkers, and explores new therapeutic interventions in heart failure.
Keywords: Heart failure metabolomics: arginine, nitric oxide, myocardial energy utilization, acylcarnitine, TMAO
Introduction
Heart failure is a complex disease process that affects an increasing number of patients due to advancements in cardiac care and increased longevity of the aging population. From an epidemiological, macroscopic perspective, timely diagnosis and early interventions in heart failure are likely to have far reaching impact on health care economics and public health. Many discoveries in pathophysiology and pharmacotherapy have already improved mortality and morbidity in this population of patients in the past few decades. However, for all the progress in the diagnosis and management of this complex disease, there remain many unresolved mysteries, the unlocking of which could lead to profound understanding of heart failure pathogenesis, treatment, and prognosis. From a microscopic perspective, heart failure is a manifestation of metabolic derangements on the cellular, genetic, proteomic, and metabolic levels1.
Traditionally, genetic information is translated from DNA into RNA and transcribed into protein. The proteome is made of a variety of proteins that can then undergo post transcription modification, and through interactions with environmental factors, produce metabolites used for energy production. A metabolite is thus defined as any small organic molecule detectable in the human body with a molecular weight of 50–1500Da. The source of metabolite generation can include any biofluid such as blood, urine, saliva, and respiratory gases. Thus a rich collection of metabolites including peptides, oligonucleotides, sugars, nucleosides, organic acids, ketones, aldehydes, amines, amino acids, lipids, steroids, alkaloids, and small molecule drugs are included in the study of metabolomics. The metabolite may arise from external sources such as exposure to medications, toxins, or microbes, or it may be generated by the human host in the homeostatic process of energy utilization. With growing interest in this field and improved methods of identification, more than 40,000 unique metabolites have been identified in the Human Metabolome Database2. With this expansive list of metabolites, we are now poised with the opportunity to discover metabolomic biomarkers of the failing myocardium in order to facilitate in the early detection of heart failure3, appropriate targeted medical therapy of heart failure4, and offer prognostic insights into the progression of this disease. The study of metabolomics, then, represents another example of the spirit of translational research, bridging the gap from bench to bedside. This chapter will first examine the definition of a clinically useful biomarker, with respect to the biochemical utility of metabolomics, then delve into the methods of metabolomic profiling, examine several metabolomic pathways being pursued in heart failure (Table 1), present possible avenues of clinical application, and discuss challenges in the utilization of metabolomics.
Table 1.
Summary of metabolites in heart failure:
| Metabolite | Findings in heart failure |
| Nitric Oxide | Improves left ventricular dilation, vasodilation14–16 |
| Arginine | Bioavailability and methylation affects nitric oxide synthesis17–20 |
| Ketones | Myocardial metabolism switch from lipids to ketogenic state in heart failure23,26 |
| Long chain acylcarnitines | Myocardial metabolism switch in heart failure states24,25 |
| Breath analysis of pentane, acetone, nitric oxide | Heart failure patients may expel a unique “breathprint” 28–31 |
| TMAO (host-gut microbiome interactions) | Elevated levels implicated in poor prognosis in myocardial infarction and chronic and acute heart failure38,39,39,41–44,48 |
Metabolites as Biomarkers-A Prolific Profile
With advancements in technology, it is now feasible to generate large databanks of metabolites, and these extensive repositories of metabolites have become hypothesis generating in the pathogenesis of various diseases. For example, biochemical analysis of the human myocardium has revealed both its remarkable efficiency of energy utilization, as well as the pathological alterations in biochemical substrate utilization in the failing myocardium5. As a metabolically active organ, the heart is in constant need for fuel, which comes in the form of adenosine triphosphate (ATP). Generation of ATP occurs either via catabolism, or breakdown, of exogenous molecules circulating in the blood, such as glucose, fatty acids, amino acids, or of endogenous stores of energy such as triacylglycerols or glycogen 6. In the healthy human heart, energy production occurs via efficient production of ATP with rapid turnover occurring every 10 seconds such that the healthy heart metabolizes 30 grams of fat and 20 grams of carbohydrates daily 7.
By understanding biochemical derangements occurring in heart failure, we can aim to produce metabolomic biomarkers as an indicator of presence of heart failure or gauge of the severity of cardiac metabolic dysregulation. Indeed, the concept of unique metabolomic patterns, or fingerprints, has already been validated in various small cohorts of patients with chronic heart failure 8. The concept that unique patterns of metabolomic expression and energy utilization occur in different etiologies of heart failure could revolutionize the diagnosis and management of heart failure in this era of personalized medicine.
Metabolomic Discovery
With the application of nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS), it is now feasible to efficiently analyze large volumes of metabolites derived from human samples, such as blood, urine, and gut contents. NMR allows for detection and quantification of metabolites based on chemical shifts in resonance frequency when subject to an electromagnetic field9,10. On the other hand, mass spectrometry (MS), identifies metabolites based on their unique mass/charge (m/z) ratio, and MS is often preceded by the use of gas chromatography, which separates metabolites based on solubility through a stationary media11,12. MS is more sensitive than NMR, and can detect a large quantity of metabolites. MS can also be used either in a “targeted” manner to detect prespecified molecules or in an “untargeted” manner without pre-specification for discovery of a large cohort of molecules13. The development and application of these techniques to the study of human myocardial metabolism has led to the creation of metabolomic patterns in healthy and pathologic cardiac states. The next section will address some of the examples of metabolomic studies in heart failure patients.
Metabolomic Biomarkers and Profiling
Nitric oxide production and arginine methylation
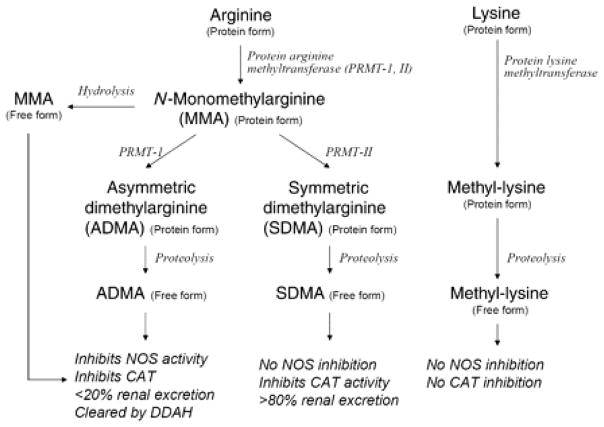
Nitric oxide (NO) is an important molecule that regulates vasodilation, left ventricular relaxation, and diastolic relaxation14–16. A series of animal and human studies have implicated alterations in NO synthesis in the pathogenesis of heart failure. NO is synthesized from its precursor L-arginine and oxygen by various NO synthases, and this process may be altered by arginine methylation in an epigenetic phenomenon. These alterations on NO synthesis are theorized to be a response to inflammation and oxidative stress. Alterations in the arginine regulation implicate its role in heart failure pathogenesis (Figure 3).
Figure 3. Scheme of arginine metabolic pathways in high throughput mass spectrometry arrays.
From Tang WHW, Tong W, Shrestha K, et al. Differential effects of arginine methylation on diastolic dysfunction and disease progression in patients with chronic systolic heart failure. Eur. Heart J. 2008;29:2506–13; with permission. Abbreviations: NOS, nitric oxide synthase isoforms; ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; MMA, N-mono-methylarginine; DDAH, dimethylarginine dimethylaminohydrolase; PRMT, protein arginine methyltransferases; CAT, cationic amino acid transport
In a single center study of chronic systolic heart failure patients with LVEF ≤35%, plasma samples taken from these patients were analyzed for concentration of plasma aminoterminal pro-B-type natriuretic peptide (NT-proBNP) and endogenous arginine metabolites including asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), and N-mono-methylarginine (MMA) 17. ADMA inhibits nitric oxide synthase (NOS) activity, while SDMA does not. This study found that both ADMA and SDMA plasma levels were positively correlated with echocardiographic estimates of LV filling pressures. In addition, the level of NT-proBNP was also correlated to all three arginine methylation products (MMA, ADMA, and SDMA), while no correlation was found with methyl-lysine, a metabolite with no nitric oxide inhibition, which was used as the internal control. Using Cox proportional hazard analysis, ADMA levels also increased the hazard ratio for death along, death or cardiac transplantation, and the combine endpoint of death, cardiac transplantation, or HF hospitalization. In addition, ADMA and MMA levels were lower in patients treated with beta-blocker, indicating response to therapy.
Another study compared the plasma metabolites of patients admitted to the intensive care unit with advanced, acute decompensated heart failure to patients with stable chronic heart failure, to elucidate the role of arginine regulation in heart failure. ADMA levels were significantly higher in the acute decompensated population18. This study again suggests that arginine metabolism via ADMA, a nitric oxide synthase inhibitor, plays an important role in the endothelial dysfunction in patients with acute decompensated heart failure. Furthermore, Shao et al examined the global arginine bioavailability ratio (GABR), defined as the quotient between substrates (arginine) and products (orinthinine+ citrulline) of NOS detected by mass spectrometry, between the two heart failure populations. They found that GABR is significant lower in the acutely decompensated heart failure patients. Low GABR also has been implicated in major adverse cardiovascular events of death, myocardial infarction, and stroke19. In the heart failure population, low GABR has been associated with more severe left ventricular and right ventricular dysfunction as well as higher levels of plasma natriuretic peptide levels. Treatment with beta-blocker therapy improves GABR levels20. In summary, nitric oxide regulation, arginine bioavailability and methylation are important metabolomic pathways in the pathophysiology of cardiac dysfunction.
Ketones and Long Chain Acylcarnitine
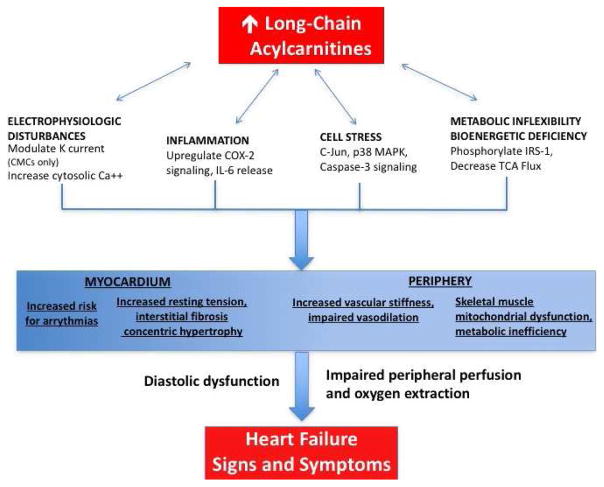
Energy utilization by the heart and alterations in cellular metabolism has been a topic of interest in heart failure research. Normally, fatty acids are the predominant source of energy for the myocardium. Acylcarnitines are the primary lipid substrate involved in fatty acid oxidation. Under normal metabolic conditions, β-oxidation produces 50 to 70 percent of ATP utilized by the myocardium21. Long-chain fatty acids, such as oleic and palmitic acids, are esterified into long-chain acylcarinitines (LCAC). LCAC then serve as lipid intermediates that transport carbon atoms into the mitochondrial for β-oxidation of fatty acids into triglycerides. In the failing myocardium, a metabolic switch from fatty acid utilization to oxygen-sparing carbohydrate metabolism has been observed22. This metabolic switch leads to accumulation of LCAC, which are postulated have pleotropic adverse effects on myocytes by way of promoting inflammation, increasing apoptotic signals, and generating ion channel dysregulation (Figure 4).
Figure 4. Proposed model for plasma long-chain acylcarnitine contributions to the heart failure phenotype.
From Hunter WG, Kelly JP, McGarrah RW 3rd, et al. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J. Am. Heart Assoc. 2016;5(8); with permission. Abbreviations: Ca++ indicates calcium; CMC, cardiomyocyte; COX, cyclooxygenase; IL, interleukin; IRS, insulin receptor substrate; K, potassium; MAPK, mitogen-associated protein kinase; TCA, tricarboxylic acid.
Using liquid chromatography-mass spectrometry in a non-diabetic advanced heart failure population of patients at time of heart transplantation or left ventricular assist device implantation, Bedi et al evaluated the energy utilization in this group of patients with severe cardiac dysfunction23. They found that failing myocardium increasingly utilized ketogenic β-hydroxybutyryl-CoA and β-hydroxybutyrate, while there was a decrease in the availability of lipid energetic substrates such as medium- and long-chain acylcarnitines.
Other studies have assessed the metabolic derangements in heart failure with regard to energy utilization. In support of the metabolic switch theory by which the failing myocyte preferentially uses glucose rather than fatty acids, metabolic profiles of patients with chronic systolic heart failure reveal alterations in fatty acid metabolism. Ahmad et al. evaluated a cohort of 453 patients with chronic systolic heart failure along with 41 end stage heart failure patients undergoing LVAD implantation24. Patients were randomized to exercise training versus usual care, and frozen plasma samples were collected and analyzed using mass spectrometry. LCAC (C16 and C18) were associated with lower peak VO2 as well as increased risk of all-cause mortality, all-cause hospitalization, and cardiovascular death or hospitalization. Additionally, levels of long-chain acylcarnitine also decreased after placement of LVAD, suggesting response to therapy.
In a study that included both heart failure with preserved ejection fraction ≥45% (HFpEF) and heart failure with reduced ejection fraction <45% (HFrEF) patients, Hunter et al created metabolomic profiles of these patients to assess for differential alterations energy utilization25. They quantified levels of 60 metabolites consisting of 45 acylcarnitines and 15 amino acids, expanding upon an early study by Zordosky et al8. These authors find that elevated levels of LCAC were independently associated with worse functional status and higher mortality in both HFpEF and HFrEF patients. Additionally, the level of LCAC was significantly higher in patients with HFrEF compared to HFpEF, and both heart failure phenotypes had elevated levels of LCAC compared to normal controls. These studies further support the role of LCAC as a prognostic marker of disease severity in chronic systolic heart failure, as well as present potential targets for new therapeutic interventions in heart failure26.
Human and microbial byproducts
Beyond analysis of plasma metabolites, the study of metabolomics also includes the analysis of human and microbial products and byproducts. A few tantalizing studies have evaluated the exhaled gases of heart failure patients with respect to acetone, pentane, and other molecular excretion in the creation of a “breathprint” in chronic heart failure. The measurement of molecular concentrations via exhaled breath offers a major advantage in the non-invasive nature of the test, and several breath analysis test have already been approved by the FDA for the diagnosis of non-cardiac conditions such as Helicobacter pylori infection, asthma inflammation, and carbon monoxide poisoning27. Within the cardiovascular realm, breath analysis studies have implicated heart failure patients have elevated levels of expired pentane, which is generated from the perioxidation reaction of free radicals with cellular membrane lipids28. In a similar manner, the concentration of exhaled acetone has also been implicated as a biomarker of heart failure severity29. Exhaled nitric oxide is another potential prognostic biomarker which has correlated to higher pulmonary venous hypertension in patients with stable chronic systolic heart failure30. Drawing from the above studies, the feasibility of collecting exhaled breath samples in patients admitted with decompensated heart failure was validated in a recent proof of concept study31. Although breath analysis is subject to sampling discrepancy and require careful collection methods, the analysis of exhaled breath gases and small molecules offer a noninvasive assessment of heart failure severity. A unique “breathprint” raises the possibility that unique metabolomic breath profiles can be utilized to identify and prognosticate patients with systolic heart failure.
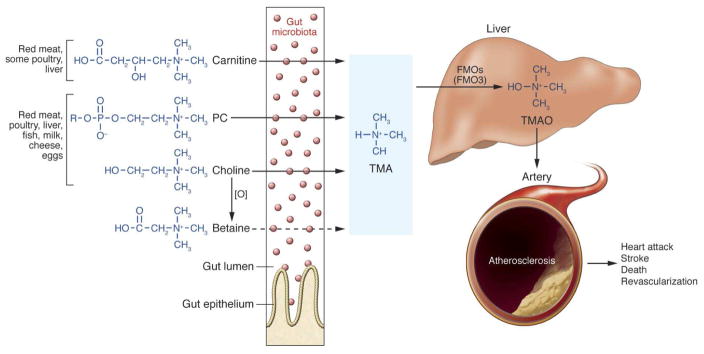
Another area of interest has been intestinal flora metabolism in the pathogenesis of cardio-renal disease and heart failure. The intestinal microbiome is composed of trillions of commensal bacteria that populate gut and aid in digestion and absorption of nutrients. The interaction between microbacterial metabolism and different human disease states have recently been studied in the pathogenesis of insulin resistance, obesity, and cardiovascular disease32–37. Wang et al. initially conducted hypothesis-generating studies to generate untargeted metabolomic maps to identify potential novel metabolites associated with cardiovascular risk38. Through this work, three novel metabolites of the phosphatidylcholine (PC; lecithin) metabolism: choline (m/z 104), betaine (m/z 116), and trimethylamine N-oxide, TMAO, (m/z 76) were implicated in the pathogenesis of cardiovascular disease. Phosphatidylcholine is the major dietary source of choline in omnivores. Betaine is a direct oxidation product of choline, and TMAO is hypothesized to arise from bacterial metabolism of choline via the intermediate trimethylamine (TMA), and subsequent hepatic oxidation via flavin monooxygenase 3 (FMO3), forming TMAO. Of these three metabolites, TMAO demonstrated the strongest correlation to cardiovascular disease, which further implicates the role of the human and gut microbiome interactions that influence cardiovascular risk. TMAO has been linked to accelerated atherosclerosis and major adverse cardiac events (death, myocardial infarction, and stroke, Figure 5) 39,40. TMAO has also been associated with poorer prognosis (death and myocardial infarction) at 2 years after myocardial infarction compared to GRACE score or other biomarkers in coronary artery disease including copeptin and natriuretic peptide, proenkephalin, mid-regional proadrenomedullin, pro-substance P41.
Figure 5. Nutrient/meta-organismal pathway associated with atherosclerosis and major adverse cardiovascular events.
From Tang WHW, Wang Z, Fan Y, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J. Am. Coll. Cardiol. 2014;64:1908–14; with permission. Abbreviations: PC, phosphatidylcholine; TMA, trimethylamine; TMAO, trimethylamine N-oxide; FMO, flavin monooxygenase; O, oxidation.
In addition to atherosclerotic heart disease, elevated levels of TMAO have also been identified as a prognostic biomarker in patients with systolic heart failure. Mice fed to high choline diets have more severe pulmonary edema, cardiac enlargement, and left ventricular ejection fraction42. In a large single center study, Tang et al. explored the incremental prognostic value of measuring TMAO levels in stable chronic systolic heart failure patients43. They found that TMAO correlates with BNP (R=0.23; p<0.001) and strong inverse correlation between TMAO and estimated glomerular filtration rate (eGFR, r=−0.55; p<0.001). Furthermore, elevated TMAO levels portended higher long-term mortality that is independent of traditional biomarkers of risk in the heart failure population such as BNP, eGFR, and markers of inflammation (hsCRP). Suzuki et al. analyzed the predictive value of TMAO in patients admitted with acute decompensated heart failure44. They found that the level of TMAO correlates with in-hospital mortality in patients admitted with acute heart failure when combined with clinical risk scores that include adjustment for renal function. Although the precise pathophysiologic contributions of TMAO in heart failure remain to be elucidated, the microbacterial-human gut metabolomic interaction presents a potential novel target for heart failure therapeutics both for chronic stable heart failure and in patients with acute heart failure admissions.
Clinical applications in Heart Failure
With improvements in technology and discoveries in new metabolomics pathways, our ever-expanding knowledge of metabolomics is paving the way for clinical application in the heart failure population. Several studies have already demonstrated a unique metabolomic profile in patients with heart failure 9,10,12,45. Although not yet in wide clinical use, it is feasible to design a metabolomic panel based on patient serum or breath analysis that can be used to gauge disease severity and prognosticate disease progression. Such a panel would aid in targeted and personalized treatment. A heart failure metabolomics panel could yield incremental benefit to traditional biomarkers used in heart failure such as NT-proBNP. In a study of novel metabolomics biomarkers analyzed by mass spectrometry profiling, Cheng et al demonstrated that a metabolomic panel of novel biomarkers including histidine, phenylalanine, spermidine, arginine, and phosphatidylcholine C34:4 have similar diagnostic value to B-type natriuretic peptide (BNP) but have higher prognostic value for combined endpoints of death and heart failure related hospitalization then BNP 12.
Metabolomic profiles can also be used to predict response to heart failure therapy. In small studies of patients undergoing ventricular assist device, trends in metabolomic biomarkers have indicated improvement in metabolomic profiles after left ventricular unloading 24. In patients undergoing cardiac resynchronization therapy, the assessment of metabolomic profiles have confirmed similar metabolomic derangements in patients with both ischemic and nonischemic systolic heart failure46. Although data appears controversial on baseline differences in metabolomic profiles between CRT-responders and CRT-non-responders, some small prospective studies have indicated that CRT-responders have baseline differences in plasma concentrations of isoleucine, phenylalanine, leucine, glucose, valine, and glutamate47. This suggests that baseline metabolomic differences may be able to predict response to CRT and further prognosticate progression of heart failure in patients with severe left ventricular dysfunction.
Challenges and Potentials
Although advancements in technology have propelled the study of metabolomics in heart failure, many challenges and questions remain. Due to the diversity and complexity of molecular pathways and human-microbiome interactions encompassed by the field of metabolomics, we are only beginning to tease out the intricate relationships between these biochemical pathways. Because of vast variations in biological diversity and assay variability, the field of metabolomics is subject to sampling error, and the discrimination of true signals from noise remains challenging. Standardization of data collection methods would be needed to improve sampling accuracy, and alterations in medication history, dietary patterns, and environmental exposures may influence metabolomic sampling 13. Furthermore, current methods of biomolecule profiling and discoveries are based on the assumption that the molecules implicated in heart failure pathogenesis are causative, but these molecules and pathways could simply be a down-stream effect of the metabolomic derangements in heart failure. Additionally, systemic versus local production of metabolites remains unclear. Metabolomic assays are currently being used in other disease states such as mitochondrial diseases and inborn errors of metabolism, however, the analytical variability of samples, the instability of sample collection, and errors in collection have proved challenging in large scale clinical use. Thus, the clinical application of metabolomics in heart failure remains limited at present time.
As the field of biomedical research has begun to discover the intricate biochemical pathways involved in disease pathogenesis, the field of medicine is moving toward more personalization of diagnostics, prognostics, and therapeutics. Metabolomics provides a more discrete dataset that is likely functionally impactful for human health and disease. Some studies have delved deeper into myocardial metabolism such as studies on ketone and acylcarnitines, while other discoveries, such as TMAO have given us insights onto the host and microbial interactions that are implicated in heart failure pathogenesis. The study of small molecular compounds and pathways involved in heart failure represents an area of active clinical investigation. The creation of individualized metabolomic profiles in heart failure patients could reveal novel pathways of heart failure pathogenesis, better define patients with a biochemical vulnerability for heart failure, and identify new targets of intervention. The study of metabolomics can also shed light onto the complex gene-environment interactions involved in heart failure onset and progression. We stand on the precipice of molecular discoveries that hold the potential to revolutionize the management of heart failure.
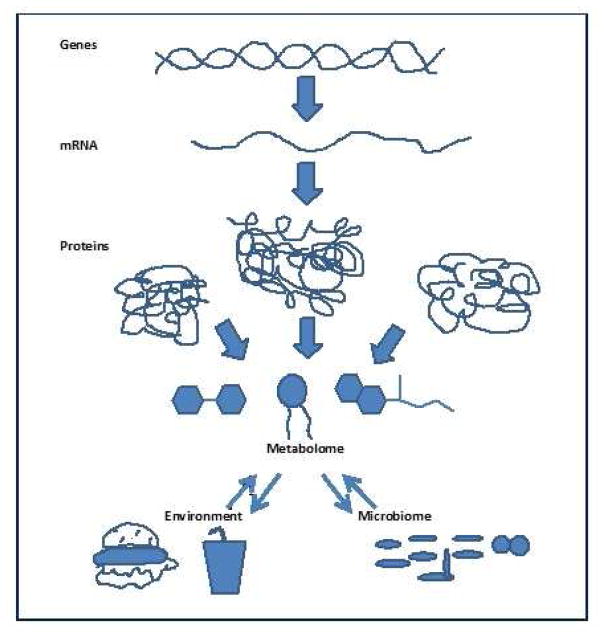
Figure 1.
Scheme of interactions of the metabolome with the genome, proteome, environment, and microbiome
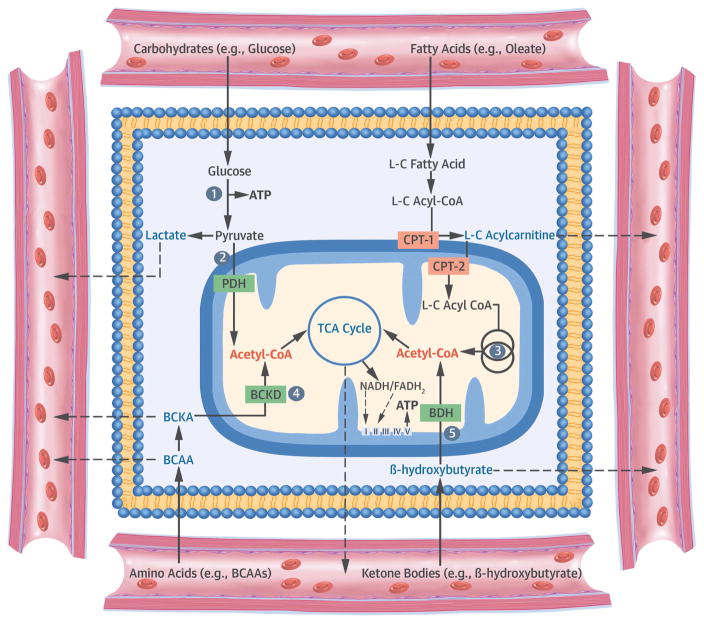
Figure 2. Cellular Metabolism and Implications of Metabolomic Profiling.
From Ussher JR, Elmariah S, Gerszten RE, et al. The emerging role of metabolomics in the diagnosis and prognosis of cardiovascular disease. J. Am. Coll. Cardiol. 2016;68:2850–70; with permission. Abbreviations: ATP = adenosine triphosphate; BCAA = branched-chain amino acid; BCKA = branched-chain α-keto-acid; BDH = β-hydroxybutyrate dehydrogenase; CoA = coenzyme A; CPT = carnitine palmitoyltransferase; FADH2 = flavin adenine dinucleotide; L-C = long-chain; NADH = nicotinamide adenine dinucleotide; PDH = pyruvate dehydrogenase; TCA = tricarboxylic acid.
Key Points.
Metabolomics is the study of small, organic, molecules of biochemical pathways, some of which are strongly implicated in heart failure pathogenesis and progression
Modern developments in mass spectrometry and nuclear magnetic resonance (NMR) have enabled identification of ~40,000 human metabolites
The failing heart exhibits metabolic derangements, particularly in energy utilization and oxidation
Creation of metabolomic profiles may aid in the diagnosis, management, and prognosis of heart failure
Metabolomics extends to human and microbial products, further adding to the complex gene, protein, and environmental interactions in heart failure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- 1.Turer AT. Using metabolomics to assess myocardial metabolism and energetics in heart failure. J Mol Cell Cardiol. 2013;55:12–18. doi: 10.1016/j.yjmcc.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Wishart David S. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:801–807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Couto G, Ouzounian M, Liu PP. Early detection of myocardial dysfunction and heart failure. Nat Rev Cardiol. 2010;7:334–344. doi: 10.1038/nrcardio.2010.51. [DOI] [PubMed] [Google Scholar]
- 4.Griffin JL, Atherton H, Shockcor J, Atzori L. Metabolomics as a tool for cardiac research. Nat Rev Cardiol. 2011;8:630–643. doi: 10.1038/nrcardio.2011.138. [DOI] [PubMed] [Google Scholar]
- 5.Kolwicz SC, Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res. 2013;113:603–616. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ussher JR, Elmariah S, Gerszten RE, Dyck JRB. The Emerging Role of Metabolomics in the Diagnosis and Prognosis of Cardiovascular Disease. J Am Coll Cardiol. 2016;68:2850–2870. doi: 10.1016/j.jacc.2016.09.972. [DOI] [PubMed] [Google Scholar]
- 7.Taegtmeyer H, et al. Assessing Cardiac Metabolism. Circ Res. 2016;118:1659–1701. doi: 10.1161/RES.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zordoky BN, et al. Metabolomic fingerprint of heart failure with preserved ejection fraction. PloS One. 2015;10:e0124844. doi: 10.1371/journal.pone.0124844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deidda M, et al. Metabolomic approach to profile functional and metabolic changes in heart failure. J Transl Med. 2015;13 doi: 10.1186/s12967-015-0661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenori L, et al. Metabolomic fingerprint of heart failure in humans: A nuclear magnetic resonance spectroscopy analysis. Int J Cardiol. 2013;168:e113–e115. doi: 10.1016/j.ijcard.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Senn T, Hazen SL, Tang WHW. Translating metabolomics to cardiovascular biomarkers. Prog Cardiovasc Dis. 2012;55:70–76. doi: 10.1016/j.pcad.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng ML, et al. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: diagnostic and prognostic value of metabolomics. J Am Coll Cardiol. 2015;65:1509–1520. doi: 10.1016/j.jacc.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Cheng S, et al. Potential Impact and Study Considerations of Metabolomics in Cardiovascular Health and Disease: A Scientific Statement From the American Heart Association. Circ Cardiovasc Genet. 2017;10:e000032. doi: 10.1161/HCG.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couto GK, Britto LRG, Mill JG, Rossoni LV. Enhanced nitric oxide bioavailability in coronary arteries prevents the onset of heart failure in rats with myocardial infarction. J Mol Cell Cardiol. 2015;86:110–120. doi: 10.1016/j.yjmcc.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Azzam N, et al. Endothelial nitric oxide synthase polymorphism and prognosis in systolic heart failure patients. Nitric Oxide Biol Chem. 2015;47:91–96. doi: 10.1016/j.niox.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Bhushan S, et al. Nitrite therapy improves left ventricular function during heart failure via restoration of nitric oxide-mediated cytoprotective signaling. Circ Res. 2014;114:1281–1291. doi: 10.1161/CIRCRESAHA.114.301475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang WHW, et al. Differential effects of arginine methylation on diastolic dysfunction and disease progression in patients with chronic systolic heart failure. Eur Heart J. 2008;29:2506–2513. doi: 10.1093/eurheartj/ehn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao Z, et al. Pulmonary hypertension associated with advanced systolic heart failure: dysregulated arginine metabolism and importance of compensatory dimethylarginine dimethylaminohydrolase-1. J Am Coll Cardiol. 2012;59:1150–1158. doi: 10.1016/j.jacc.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang WHW, Wang Z, Cho L, Brennan DM, Hazen SL. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol. 2009;53:2061–2067. doi: 10.1016/j.jacc.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson Tang WH, et al. Diminished Global Arginine Bioavailability as a Metabolic Defect in Chronic Systolic Heart Failure. J Card Fail. 2013;19:87–93. doi: 10.1016/j.cardfail.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 22.Chokshi A, et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–2853. doi: 10.1161/CIRCULATIONAHA.111.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bedi KC, et al. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation. 2016;133:706–716. doi: 10.1161/CIRCULATIONAHA.115.017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad T, et al. Prognostic Implications of Long-Chain Acylcarnitines in Heart Failure and Reversibility With Mechanical Circulatory Support. J Am Coll Cardiol. 2016;67:291–299. doi: 10.1016/j.jacc.2015.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter WG, et al. Metabolomic Profiling Identifies Novel Circulating Biomarkers of Mitochondrial Dysfunction Differentially Elevated in Heart Failure With Preserved Versus Reduced Ejection Fraction: Evidence for Shared Metabolic Impairments in Clinical Heart Failure. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.115.003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolwicz SC, Airhart S, Tian R. Ketones Step to the Plate: A Game Changer for Metabolic Remodeling in Heart Failure? Circulation. 2016;133:689–691. doi: 10.1161/CIRCULATIONAHA.116.021230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cikach FS, Dweik RA. Cardiovascular biomarkers in exhaled breath. Prog Cardiovasc Dis. 2012;55:34–43. doi: 10.1016/j.pcad.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobotka PA, et al. Elevated breath pentane in heart failure reduced by free radical scavenger. Free Radic Biol Med. 1993;14:643–647. doi: 10.1016/0891-5849(93)90145-k. [DOI] [PubMed] [Google Scholar]
- 29.Marcondes-Braga FG, et al. Exhaled Acetone as a New Biomarker of Heart Failure Severity. Chest. 2012;142:457–466. doi: 10.1378/chest.11-2892. [DOI] [PubMed] [Google Scholar]
- 30.Schuster A, et al. Increased Exhaled Nitric Oxide Levels After Exercise in Patients With Chronic Systolic Heart Failure With Pulmonary Venous Hypertension. J Card Fail. 2012;18:799–803. doi: 10.1016/j.cardfail.2012.08.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samara MA, et al. Single Exhaled Breath Metabolomic Analysis Identifies Unique Breathprint in Patients With Acute Decompensated Heart Failure. J Am Coll Cardiol. 2013;61:1463–1464. doi: 10.1016/j.jacc.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumas ME, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dumas ME, Kinross J, Nicholson JK. Metabolic phenotyping and systems biology approaches to understanding metabolic syndrome and fatty liver disease. Gastroenterology. 2014;146:46–62. doi: 10.1053/j.gastro.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Ridaura VK, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koeth RA, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang WHW, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki T, Heaney LM, Jones DJL, Ng LL. Trimethylamine N-oxide and Risk Stratification after Acute Myocardial Infarction. Clin Chem. 2017;63:420–428. doi: 10.1373/clinchem.2016.264853. [DOI] [PubMed] [Google Scholar]
- 42.Organ CL, et al. Choline Diet and Its Gut Microbe Derived Metabolite, Trimethylamine N-Oxide (TMAO), Exacerbate Pressure Overload-Induced Heart Failure. Circ Heart Fail. 2016;9:e002314. doi: 10.1161/CIRCHEARTFAILURE.115.002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang WHW, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki T, Heaney LM, Bhandari SS, Jones DJL, Ng LL. Trimethylamine N-oxide and prognosis in acute heart failure. Heart Br Card Soc. 2016;102:841–848. doi: 10.1136/heartjnl-2015-308826. [DOI] [PubMed] [Google Scholar]
- 45.Du Z, et al. 1H-NMR-based metabolic analysis of human serum reveals novel markers of myocardial energy expenditure in heart failure patients. PloS One. 2014;9:e88102. doi: 10.1371/journal.pone.0088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Padeletti L, et al. Metabolomic does not predict response to cardiac resynchronization therapy in patients with heart failure. J Cardiovasc Med Hagerstown Md. 2014;15:295–300. doi: 10.2459/JCM.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 47.Nemutlu E, et al. Cardiac Resynchronization Therapy Induces Adaptive Metabolic Transitions in the Metabolomic Profile of Heart Failure. J Card Fail. 2015;21:460–469. doi: 10.1016/j.cardfail.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang WHW, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]