Abstract
Cardiac magnetic resonance (CMR) is a valuable tool for the evaluation of patients with, or at risk for, heart failure and has a growing impact on diagnosis, clinical management, and decision-making. Through its ability to characterize the myocardium using multiple different imaging parameters, it provides insight into the etiology of the underlying heart failure and its prognosis. CMR is widely accepted as the reference standard for quantifying chamber size and ejection fraction. Additionally, tissue characterization techniques such as late gadolinium enhancement (LGE) and other quantitative parameters such as T1-mapping, both native and with measurement of extracellular volume fraction, T2-mapping, and T2-* mapping have been validated against histology in a wide range of clinical scenarios. In particular, the pattern of LGE in the myocardium can help determine the underlying etiology of the heart failure. The presence and extent of LGE determines prognosis in many of the non-ischemic cardiomyopathies. The use of CMR should increase as its utility in characterization and assessment of prognosis in cardiomyopathies is increasingly recognized. Cardiovascular magnetic resonance, cardiomyopathy, heart failure, hypertrophic cardiomyopathy, sarcoidosis, amyloidosis
Keywords: Cardiovascular magnetic resonance, cardiomyopathy, heart failure, hypertrophic cardiomyopathy, sarcoidosis, amyloidosis
Cardiac magnetic resonance (CMR) is a valuable tool for the evaluation of patients with, or at risk for, heart failure and has a growing impact on diagnosis, clinical management, and decision-making (1,2). Through its ability to characterize the myocardium using multiple different imaging parameters, it provides insight into the etiology of the underlying heart failure and its prognosis. CMR is considered the reference standard for quantifying chamber size and ejection fraction. Additionally, tissue characterization techniques such as late gadolinium enhancement (LGE) and other quantitative parameters such as T1-mapping with measurement of extracellular volume fraction (ECV), T2-mapping, and T2-* mapping have been validated against histology in a wide range of clinical scenarios. In particular, the pattern of LGE in the myocardium can help determine the underlying etiology of the heart failure. Figure 1 shows examples of LGE in a spectrum of patients with non-ischemic cardiomyopathy. In this review, we will examine the role of CMR in the diagnosis and prognosis of non-ischemic cardiomyopathy.
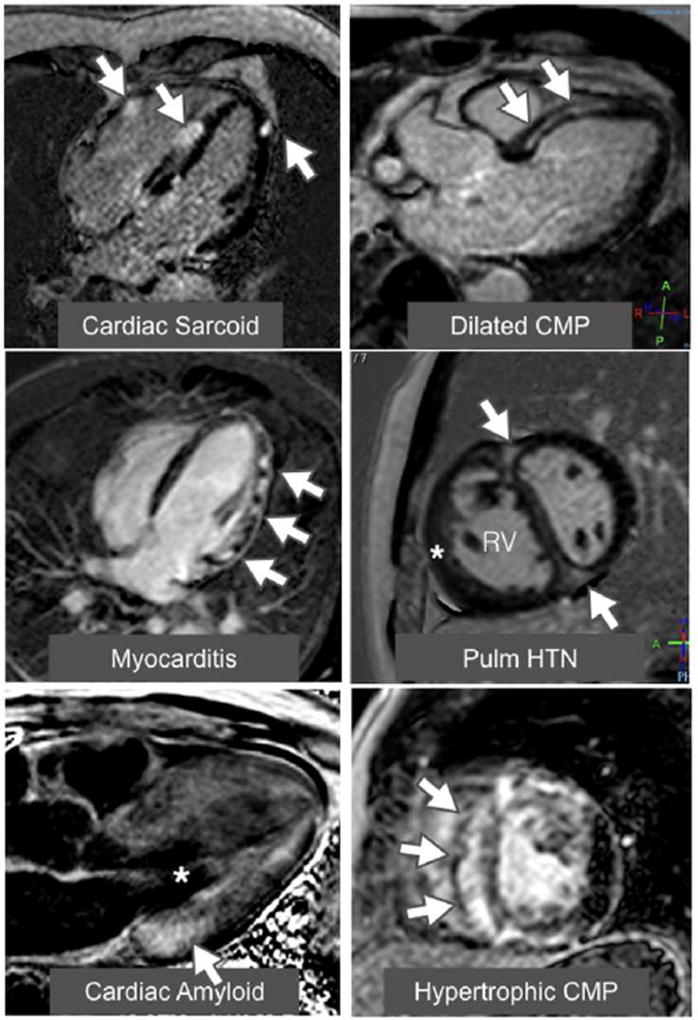
Figure 1. Examples of late gadolinium enhancement (LGE) in a variety of nonischemic cardiomyopathies.
The top left image shows a 4-chamber view of a patchy distribution of late mid wall and epicardial LGE in a patient with cardiac sarcoidosis. The top right image shows a 3-chamber view of a mid-wall stripe pattern of LGE in a patient with dilated cardiomyopathy. The middle left image shows a 4-chamber view of patchy epicardial and midwall LGE along the lateral wall in a patient with myocarditis. The middle right image shows a mid-ventricular short axis image in a patient with pulmonary hypertension with right ventricular (RV) dilation and hypertrophy (*) along with LGE in the anterior and inferior right ventricular insertion points (arrows). The bottom left image shows a 3-chamber view of a LGE image in an amyloid patient. The left ventricular blood pool is nulled (*) and there is subtle circumferential subendocardial LGE throughout the left ventricle (LV). The LGE is most pronounced at the base of LV within hypertrophied myocardium. The bottom right image shows a mid-ventricular short axis image in a patient with HCM with evidence of asymmetric septal hypertrophy with extensive mid-wall LGE within the hypertrophied myocardium.
Determining the etiology of a cardiomyopathy is of clinical importance, as it has implications with regards to the optimal treatment strategy and the prediction of prognosis. Although the recently proposed MOGE(S) [morphofunctional phenotype (M), organ(s) involvement (O), genetic inheritance pattern (G), etiological annotation (E) including genetic defect or underlying disease/substrate, and the functional status (S) of the disease] classification system may play an important role in the understanding of the cardiomyopathies in the future (3), for the purpose of this review, the non-ischemic cardiomyopathies have been divided into just four groups: dilated, genetic, inflammatory, and infiltrative. The genetic cardiomyopathies include disorders such has hypertrophic cardiomyopathy, left ventricular non-compaction, the muscular dystrophies, and arrhythmogenic right ventricular dysplasia. The inflammatory and/or autoimmune cardiomyopathies include cardiac sarcoidosis and conditions associated with connective tissue disorders such as systemic lupus erythematosus, rheumatoid arthritis, etc. The infiltrative cardiomyopathies include cardiac amyloidosis, cardiac siderosis, and Anderson-Fabry’s disease.
Dilated Cardiomyopathies
Dilated cardiomyopathy likely represents an end-stage manifestation of multiple non-ischemic disorders that can damage the myocardium. Despite significant treatment advances, a recently published randomized-controlled trial which included over 8,000 heart failure patients with a left ventricular ejection fraction (LVEF)<40%, a significant number of whom had some form of non-ischemic cardiomyopathy, revealed that after a median follow up of just 27 months, the mortality rate was nearly 20% despite use of modern therapies (4). The management plan is often determined by patient symptoms, abnormalities on the electrocardiogram, and LVEF; however, this is an imperfect approach that does not adequately identify those patients who are unlikely to respond to medical therapy or at risk for sudden cardiac death. Recently, there has been growing interest in exploiting the role of myocardial fibrosis, an integral pathophysiologic component of dilated cardiomyopathy, as a biomarker for guiding patient management and determining prognosis. It is increasingly being understood that fibrosis can occur in two forms that can be detected by CMR (5): (A) irreversible replacement fibrosis which corresponds to the presence of LGE and (B) diffuse interstitial fibrosis which better corresponds to findings on T1-mapping. Replacement myocardial fibrosis is often present in the midwall of the interventricular septum and can be identified in approximately 30% of individuals with dilated cardiomyopathy using LGE imaging (6,7) and differentiates it from ischemic cardiomyopathy. The presence of LGE is associated with abnormalities in contractility and serves as a potential substrate for re-entrant ventricular arrhythmia(8). The presence of LGE identifies a cohort of patients who do not respond as well to optimal medical therapy(9), a finding independent of other standard clinical parameters such as QRS duration and pro N-terminal brain natriuretic peptide levels. The burden of LGE is independently and inversely associated with the change in LVEF that occurs following medical therapy.
Based on a prospective longitudinal study performed in 472 patients with dilated cardiomyopathy(10), patients with mid-wall LGE were significantly more likely to die (27% vs 11%) or have a significant arrhythmic event (30% vs 7%) when compared to those patients without LGE. The findings were independent of LVEF. These findings were subsequently confirmed in a larger, multicenter observational study (11) that showed that the presence and extent of LGE was predictive of all-cause mortality, but also suggested that abnormalities in native myocardial T1 relaxation times may be an even better and independent marker of poor outcomes in these patients. Although not limited to patients with non-ischemic cardiomyopathy, another study revealed that those with an LVEF >30% but who still had LGE involving more than 5% of their left ventricular mass were just as likely to die or receive an implantable cardioverter defibrillator (ICD) shock for ventricular tachycardia as those with an LVEF <30%(12). Conversely, those patients with LVEF <30% who had minimal or no LGE did just as well as those patients with LVEF>30%. The ability of LGE burden to risk stratify patients was independent of LVEF and the presence of inducible ventricular tachycardia during an electrophysiology study. Interestingly, the presence of >5% myocardial LGE burden was associated with an annualized event rate of death or significant arrhythmia of nearly 20%; whereas the absence of LGE was associated with only a 3% annualized event rate. In another study which prospectively enrolled 399 patients with dilated cardiomyopathy who had an LVEF>40%, the presence of a mid-wall pattern of LGE was present in 25% of individuals and was associated with a 9-fold increase in the risk of sudden cardiac death (SCD) or aborted SCD when compared against those without LGE (13). The potential role of LGE-CMR for identifying patients with non-ischemic cardiomyopathy who are at risk of SCD is of particular interest given the findings of recently published randomized controlled trial suggesting that ICD implantation guided by LVEF alone may not be associated with improved survival (14). Similarly, LGE may be helpful for predicting response to biventricular pacemaker resynchronization therapy. In a study of 559 patients with both ischemic and non-ischemic cardiomyopathy(15), LGE-guided biventricular pacemaker implantation was associated with a significant improvement in identifying patients most likely to benefit from biventricular pacing. Those patients who did not have LGE did significantly better following resynchronization therapy relative to those who had LGE and relative to those who underwent implantation without LGE guidance. Regardless of its ability to predict which patients may or may not respond to resynchronization therapy, the presence of LGE in the septal mid-wall was an independent predictor of morbidity and mortality in patients with dilated cardiomyopathy undergoing cardiac resynchronization therapy (16).
Genetic Cardiomyopathies
Hypertrophic Cardiomyopathy (HCM)
HCM is the most common genetic heart disease as it now is identified in up to 1 in 200 to 300 individuals with the advent of more advanced imaging and genetic testing (17). A mutation in a gene coding for any of the cardiac contractile proteins can lead to the phenotype of HCM. Although echocardiography is typically used for screening for HCM, CMR is more sensitive for the identification of more unusual sites of hypertrophy and for apical HCM (18). It is the most accurate way of measuring LV mass which is important as higher LV mass is associated with worse outcome (19). Similarly to echo, CMR can identify systolic anterior motion of the mitral valve, measure the LV outflow gradient using velocity encoded imaging, and assess mitral regurgitation.
In addition and most importantly, CMR offers the ability to identify and quantify myocardial fibrosis. Between half and two-thirds of patients with HCM may have LGE with a characteristic pattern of patchy involvement, particularly at the right ventricular septal insertion sites and in those walls with the greatest hypertrophy. A number of studies have examined the relationship between the presence of LGE and outcome in HCM. One such study of 711 patients followed for a mean of 3.5 years found no relationship between LGE and risk of SCD after adjusting for other risk factors(20). However, a recent meta-analysis of 5 studies including the latter study involving 2993 patients with a median follow-up of 3 years demonstrated that the presence of LGE was associated with a 3.4 fold increase in risk for sudden cardiac death (SCD), 1.8-fold increase in all-cause mortality, 2.9 fold increase in cardiovascular mortality and a trend to increase in heart failure death (21).
Due to the high prevalence of LGE, its presence alone cannot be used as an indication for ICD implantation as the risk of SCD in HCM is <1% per year.
Thus, the extent of LGE may have more discriminatory value than its presence. A 4-center study of 1293 patients followed for 3.3 years showed that LGE≥15% of LV mass was associated with a 2-fold increase in SCD event risk (22). The aforementioned meta-analysis showed that after adjusting for baseline characteristics, the extent of LGE was strongly associated with the risk of SCD with a hazard ratio of 1.36 per increase in LGE extent of 10% (21).
Evidence is mounting in regards to the diagnostic and prognostic role of interstitial fibrosis in HCM. One study demonstrated mildly elevated native T1 in HCM, highest in segments that subsequently demonstrate LGE(23). Native T1 is accurate in discriminating HCM from hypertensive heart disease (24). A study of HCM and idiopathic dilated CM showed that native T1 performed better than ECV at discriminating cardiomyopathies from normal (25). ECV is also useful as it is elevated in phenotypically positive HCM as well as genotype positive, phenotype negative individuals(26). Post-contrast T1 time was shown to be associated with nonsustained ventricular tachycardia in a study of 100 HCM patients and thus may be a marker of risk once studied in larger patient groups(27).
In addition to the extent, the location and/or pattern of LGE may be more predictive of adverse outcome than the presence of LGE alone and the role of native T1 and ECV remain to be fully elucidated. The ongoing NIH-funded natural history study “HCMR-Novel Predictors of Prognosis in Hypertrophic Cardiomyopathy” (NCT01915615) using CMR, genetics, and biomarker evaluation of 2750 patients with HCM is likely to offer further insight into identifying risk markers including LGE, native T1, and ECV (28).
Left Ventricular Noncompaction (LVNC)
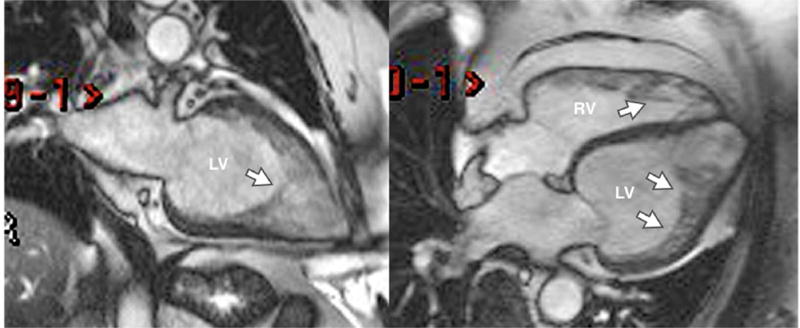
LV noncompaction cardiomyopathy is characterized by extensive LV trabeculations (Figure 2) and an increased risk of clinical heart failure, thrombosis, and mortality. The accurate diagnosis of LVNC by noninvasive imaging can be quite difficult due to substantial overlap between it and conditions such as dilated cardiomyopathy and even normal LV trabeculation. The role of CMR in the diagnosis of LV noncompaction continues to evolve. Peterson et al proposed CMR criteria of noncompacted to compacted myocardium ratio of 2.3 to 1 (29) and demonstrated a sensitivity of 86% and 99% in a study of 177 patients with and without cardiac disease. However, when using these criteria, up to 43% of individuals who get CMR as part of screening studies such as the Multi-Ethnic Study of Atherosclerosis can meet imaging criteria (30). Similarly, another screening study showed that up to 14.8% of normal individuals met at least 1 diagnostic criterion for LVNC (31). Thus, clinical criteria of symptomatic HF or LV dysfunction on CMR likely need to be included to improve the specificity of diagnosis. Jacquier et al proposed another set of criteria in a study of patients with LVNC compared to those with DCM, HCM, and controls (32). They found that a trabeculated LV mass greater than 20% of the LV mass was 94% sensitive and specific for the diagnosis of LVNC. LGE may be seen in LVNC, but it is infrequent enough that its presence is specific but not sensitive in making the diagnosis (33). However, its presence may be important for prognostic purposes. In a study of 113 patients followed for a mean of 4 years, the presence of LV dilatation, LV dysfunction, and LGE (seen only in 11 patients) were predictive of cardiac events whereas the degree of LV trabeculation was not (34). More CMR studies of this condition are needed to establish its role in this condition as the sensitivity is high, but the specificity remains problematic.
Figure 2. LV Noncompaction.
Diastolic still frames from cine images of a two chamber view (left) and four chamber view (right) are shown. The myocardium is thin and the left ventricle (LV) and right ventricle (RV) are heavily trabeculated (arrows).
Muscular Dystrophies
Although the muscular dystrophies are often characterized by skeletal muscle degeneration and progressive weakness, a major problem can be the development of a dilated cardiomyopathy which often goes undetected until it is in its advanced stages. Early detection allows for the potential to introduce therapy that could alter the natural history. Despite the fact that cardiomyopathy is an important cause of death in DMD, the majority of patients (~70%) in one cross-sectional study have an LVEF >55%; whereas, 20% of patients had an LVEF between 45–54%, 6% of patients had an LVEF of 35–44%, and only 3% had an LVEF <35%.
Because of the relative insensitivity of LVEF for identifying DMD boys at risk for heart failure, CMR techniques such as myocardial tagging offer the potential to detect abnormalities in systolic function before a reduction is LVEF or LV dilation occurs (35). Circumferential strain may be abnormal even in DMD patients <10 years old, and it continues to worsen with aging despite the preservation of LV ejection fraction. In those with LV dysfunction, circumferential strain may be abnormal even in the absence of LGE (36). These findings suggest that abnormalities in strain precede both the reduction of LVEF and also the development of myocardial scar.
In addition to abnormalities in circumferential strain, the presence of LGE (Figure 3) may also precede a decrease in LVEF (37). In one study (38), 36% of DMD patients had LGE, and the prevalence increased with age. Only 30% of patients with LVEF >55% had LGE; whereas 84% of those with LVEF <55% had LGE. Importantly, 10% of those individuals with LGE died during an average follow up of 11 months; whereas, only one patient without LGE died. The burden of LGE was also associated with an increased risk of death. The presence of LGE also identifies individuals at risk for progressive LV dysfunction as in those with LGE, the LVEF decreases an average of 2.2% annually (39). Figure 5 is an example of LGE in a patient with DMD.
Figure 3. Duchenne’s Muscular Dystrophy.
A short axis image of the mid ventricle obtained in a patient with Duchenne’s Muscular Dystrophy shows epicardial late gadolinium enhancement (LGE) along the lateral wall (white arrows) and mid-wall LGE in the septum.
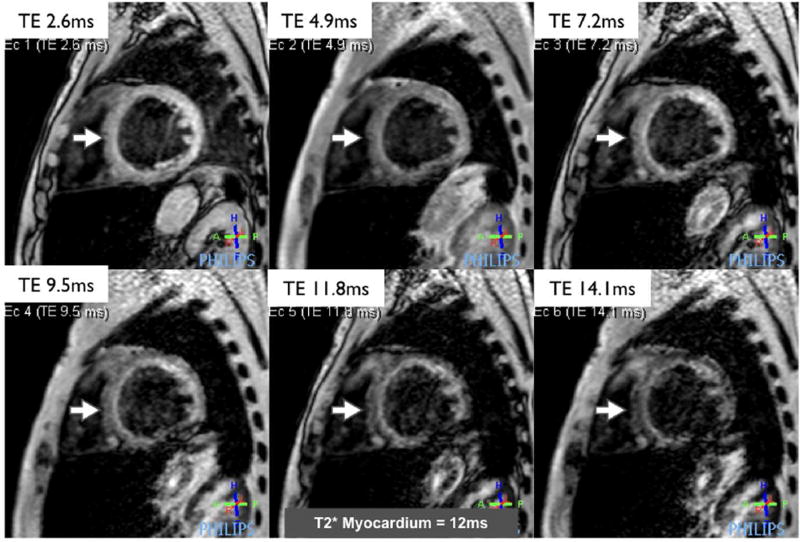
Figure 5. Cardiac siderosis.
A series of left ventricular short axis images acquired using a single breath-hold T2* pulse sequence in a patient with sickle cell anemia. A series of images are acquired at increasing echo times (TE). The signal intensity in the myocardium decreases as the TE increases. The rate of decay is used to calculate the T2* relaxation time, which is reduced in this patient and indicates the presence of significant myocardial iron overload.
Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC)
ARVC is an inherited cardiomyopathy that leads to ventricular arrhythmias and often causes SCD in the young. CMR is an important adjunct in making the diagnosis of ARVC, primarily due to its accuracy in assessing right ventricular dilatation and dysfunction (40). Diagnostic findings in this disease include RV dilatation and global or regional dysfunction including focal RV systolic bulging or aneurysm as defined by cine CMR and are carefully defined to meet Task Force Criteria that were redefined in 2010 (40). The finding of fatty infiltration in the RV free wall is not part of the diagnostic criteria as this is a nonspecific finding. The newer criteria are more restrictive than the older 1994 criteria and are more specific but may be less sensitive (41), although this continues to be debated in the literature. In certain cases, LGE of the RV free wall can be seen(42), although this can be difficult to identify definitively due to the thin RV wall in this disorder. LV involvement in ARVC is increasingly recognized(43). Quantitative functional RV imaging with strain measures by CMR feature tracking may objectify regional functional measures and improve upon diagnostic accuracy for ARVC compared to qualitative analysis of cine images (44). The hallmark of the diagnosis, however, remains RV dilatation and dysfunction.
Inflammatory Cardiomyopathies
Sarcoidosis
Sarcoidosis is a multi-organ, inflammatory disorder characterized by non-caseating granulomatous infiltration. Clinical manifestations of cardiac involvement can range from none to SCD or advanced heart failure requiring heart transplantation. Although only 5% of sarcoidosis patients have clinical manifestations of cardiac sarcoidosis (CS), 25% have evidence of cardiac involvement at autopsy (45) and the primary presentation of CS can be SCD. Because CS is a patchy disorder and often involves only small amounts of myocardium without causing obvious abnormalities in LV function, commonly used tests such as the electrocardiogram, ambulatory monitoring, signal averaged electrocardiography, echocardiography, myocardial perfusion imaging, and electrophysiology testing do not reliably detect it (46). Even endomyocardial biopsy has a poor sensitivity due to myocardial sampling errors related to the patchy pattern of sarcoid infiltration (47).
CMR is becoming the preferred imaging modality for detecting CS and a consensus document published by the Heart Rhythm Society suggested that patients with sarcoidosis undergo CMR if an abnormality on an initial screening test is noted (48). Several groups have shown that CMR can readily identify individuals with CS because of its ability to identify small areas of myocardial involvement using LGE (49,50). Nearly 20% of individuals with extracardiac sarcoidosis have cardiac involvement based on CMR, even when LVEF is preserved (51). The true diagnostic performance of CMR in the detection of CS is unknown because there is no acceptable reference standard. Instead, the use of CMR in the setting of sarcoidosis is currently most often discussed in terms of its ability to risk stratify patients and to impact their treatment plan. The detection of CS using CMR has been shown to better identify patients at risk for cardiac death than the more commonly used diagnostic criteria published by the Japanese Ministry of Health and Welfare (JMHW) (52). The presence of LGE is associated with an increased risk of death or significant ventricular arrhythmia even in patients with preserved LVEF and the risk of adverse outcomes is modulated by the burden of myocardial damage and the presence of RV dysfunction (53). A recent meta-analysis (54) of 11 studies evaluating the role of CMR in patients with sarcoidosis revealed that the presence of LGE had an odds ratio of 7.4 for identifying patients at risk for a composite endpoint that included all-cause mortality and ventricular arrhythmogenic events. Furthermore, the absence of LGE is associated with a low risk of major cardiovascular events even when the LVEF is severely impaired (55). In addition to ventricular arrhythmias, sarcoidosis patients with LGE also have an increased burden of atrial fibrillation and atrial flutter (56).
Although the presence of LGE is a powerful predictor of risk in patients with sarcoidosis, it may not identify patients who have earlier stages of CS prior to the development of myocardial scar or overt inflammation. Abnormalities in myocardial T2-times may precede the development of LGE (57); however, more studies are needed to understand the clinical impact of this finding.
Systemic Lupus Erythematosus (SLE) and Other Connective Tissue Disorders
SLE is associated with a full range of cardiovascular complications including accelerated coronary disease and its associated complications such as myocardial infarction, microvascular disease, myocarditis, vasculitis (58), pericarditis, pulmonary hypertension, conduction disease, and valvular heart disease. Although these complications of SLE are well known, they are often clinically silent and require advanced imaging technologies such as CMR to detect them (59). In one study comparing the utility of echocardiography and CMR in SLE patients, 45% of all SLE patients with a prior history of cardiovascular complication had evidence of LGE on CMR, yet only 33% of those individuals with LGE on CMR were identified by echocardiography (60). The mechanism of accelerated coronary artery disease in SLE patients is not thought to be secondary to traditional risk factors. Varma et al used a novel, post-contrast high-resolution T1-W inversion recovery pulse sequence to evaluate the pattern and burden of coronary wall contrast enhancement in SLE patients. When compared to patients with coronary artery disease (CAD) those with SLE had a diffuse pattern of coronary wall contrast enhancement; whereas, CAD patients had a patchy pattern of enhancement, despite similar total enhancement burden (61). These findings suggest that SLE is associated with diffuse rather than focal coronary inflammation. In the same study, it was noted that SLE patients had global perfusion defects during hyperemia consistent with microvascular disease, rather than the regional perfusion defects more consistent with obstructive epicardial CAD. In another study that included 20 SLE patients with typical and atypical chest pain without evidence of epicardial coronary disease who underwent adenosine CMR, 44% had visible perfusion defects (62). When compared to controls, patients with SLE also had a significant reduction in myocardial perfusion reserve.
CMR can also detect lupus myocarditis (63). CMR was recently used to evaluate 32 SLE patients with new onset heart failure; 15% had evidence of acute myocarditis, 31% had evidence of transmural myocardial infarction, 28% had diffuse subendocardial scar suggestive of vasculitis, and 15% had LV dysfunction without evidence of inflammation or fibrosis. The burden of CMR abnormalities was correlated to lupus activity and duration. In another study (64), 37% of SLE patients had evidence of LGE that was typically small and most often found in the interventricular septum. Only those patients with a larger burden of LGE had evidence of functional abnormalities such as a decreased E/A ratio on echocardiography or reduced exercise tolerance. The presence of LGE has also been associated with heart block (65).
Interest is growing in quantifying the burden of myocarditis in SLE to guide treatment response (66). Initial approaches utilized T2-W and pre- and post- contrast T1-W images to calculate a T2-ratio as a marker of increased myocardial edema and early global relative contrast enhancement ratio as a marker of increased capillary leak indicative of myocardial inflammation (67). Using such measurements, it was evident that when compared to control subjects or individuals with inactive SLE, patients with active SLE had a higher T2-ratio and early global enhancement ratio. Although promising, these types of measurements are limited by imaging artifacts and absence of an absolute quantitative parameter. Others have advocated for techniques capable of measuring actual T2 and T1 relaxation times to identify lupus myocarditis and to monitor treatment response(68). In one study (69), patients with active SLE had increased myocardial T2 times suggesting the presence of myocardial edema/ inflammation when compared against patients with inactive SLE and control subjects. Furthermore, the T2 relaxation time improved with repeat imaging following clinical improvement. Others have shown similar findings with the use of T1-mapping and ECV techniques (70). Although CMR identifies myocardial involvement in SLE, there are no published studies examining the prognostic value of CMR or whether following treatment response using CMR is associated with improved outcomes in these patients.
CMR plays a similar role in other systemic inflammatory disorders such as rheumatoid arthritis, large and medium vessel vasculitides such as Takayasu’s arteritis, giant cell arteritis, polyarteritis nodosa, and Kawasaki disease; small vessel vasculitides such as microscopic polyangiitis; and necrotizing vasculitides including Wegener disease and Churg-Strauss syndrome. As a group, these vasculitides can be associated with aneurysm, dissection, and narrowing of arteries and tissue level complications such as myocardial ischemia and infarction. Like SLE, these disorders can also be associated with the development of myocarditis and, as described above, CMR is well suited to evaluate the full range of potential cardiovascular complications that occur in these patients.
Infiltrative Cardiomyopathies
Cardiac Amyloidosis (CA)
CA is a rare infiltrative disorder in which abnormally folded proteins are deposited within the myocardium. There are two major subtypes that must be distinguished from each other: light chain (AL) amyloidosis and transthyretin (ATTR) amyloidosis. AL amyloidosis is a plasma cell dyscrasia that is often treated with chemotherapy or stem cell transplantation; whereas, ATTR amyloidosis is due to the production of an abnormally folded transthyretin (i.e. prealbumin) in the liver and targeted treatments are in development. AL amyloidosis is rare and accounts for only a small percentage of patients with CA. On the other hand, the genetic mutations responsible for ATTR are present in 3–3.5% of American of African decent; however, the penetrance of the mutation is not known and the disease is almost certainly underdiagnosed. The annual mortality of both types of CA is relatively high, especially for AL amyloidosis. Given the development of new treatment strategies, there is a need to diagnose the disease during its earlier stages.
During its advanced stages, CA is often suggested on echocardiography by the presence of severe left ventricular hypertrophy with preserved systolic function, dilated atria, and restrictive physiology. CMR is emerging as a valuable tool for the detection of CA. In addition to the classical abnormalities seen during echocardiography, circumferential subendocardial LGE most pronounced at the base and mid-ventricle is present in 80% of patients with CA, with many of the other 20% of patients having alternative patterns of LGE. Diffuse subendocardial LGE has a specificity of nearly 95% for the diagnosis of CA (71,72). The interpretation of LGE images can often be difficult due to the diffuse nature of LGE and because the nulling time of the LV cavity can often be significantly altered. Typically, on inversion recovery imaging, the LV cavity has a significantly shorter inversion time than the normal myocardium. However, in cardiac amyloidosis, the LV cavity and myocardium often have very similar inversion times and occasionally the inversion time of the myocardium is shorter than that of the LV cavity. These characteristic alterations in inversion time can be readily recognized on inversion time scouts acquired after the administration of gadolinium-based contrast agents and have a very high sensitivity for the diagnosis of CA (73). The difference in inversion time between the LV cavity and the myocardium is also an important prognostic marker, as it provides insight into the burden of CA (74). Similarly, the transmurality of the LGE is another important and independent marker of patient prognosis (75), as the presence of transmural LGE is associated with a greater than 5-fold increase in mortality compared to those CA patients without LGE.
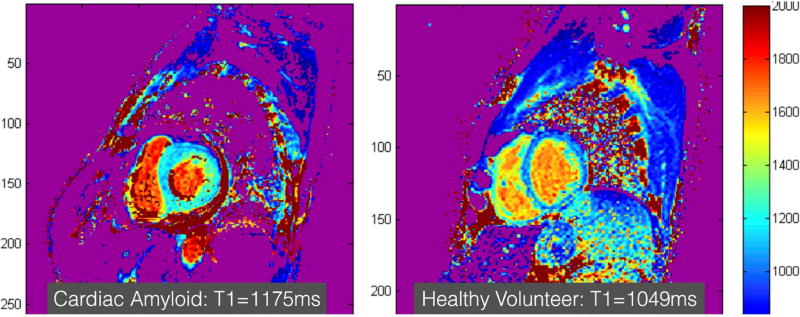
Because it is challenging to quantify the burden of LGE in patients with CA, there is a significant interest the use of quantitative ECV measurements. ECV is significantly elevated in CA patients and is inversely correlated with the QRS voltage and other commonly used biomarkers for CA (76). Similarly, because many patients with amyloidosis have renal disease and gadolinium-based contrast agents cannot be used, there has been significant interest in the utility of native T1-mapping techniques to diagnose CA (Figure 4). Native T1-times have been shown to be significantly elevated in patients with CA and correlated with other relevant biomarkers (77,78). Both native T1-mapping and ECV have also been shown to predict prognosis in these patients (79).
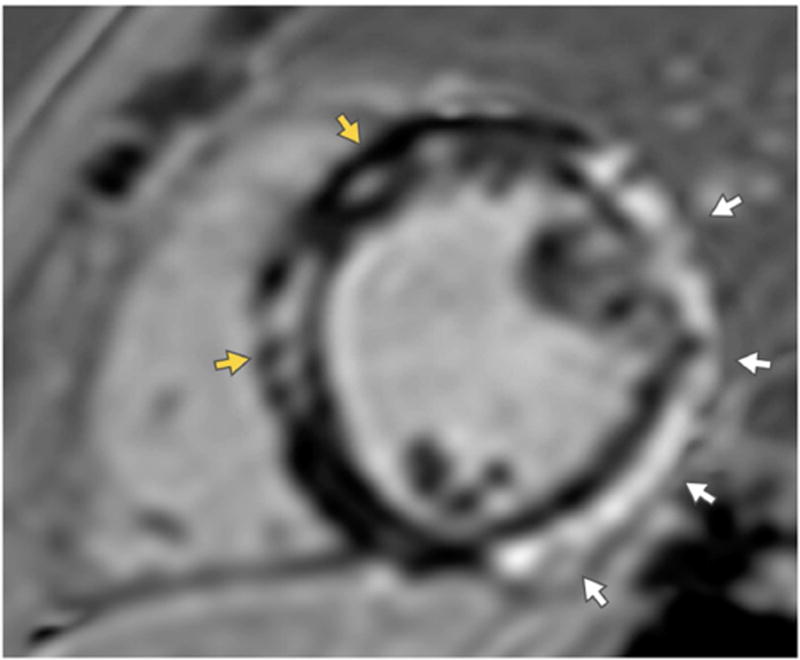
Figure 4. Cardiac amyloidosis.
Short axis images of the mid ventricle encoded with a native myocardial T1-color map generated from a series of images acquired at increasing repetition times acquired using MOdified Look Locker Image (MOLLI) pulse sequence is shown in a patient with cardiac amyloidosis (left) and a healthy volunteer (right). The T1-time shown in this figure are obtained from a region of interest drawn manually in the inter-ventricular septum. The T1-times can be even higher in patients with more extensive cardiac amyloidosis than the example shown here. Normal native T1 value on the particular scanner used to acquire these images is <1050ms.
Cardiac Siderosis
Cardiac siderosis or myocardial iron overload is rare but can occur in conditions such as thalassemia or hemochromatosis. Without treatment, it is associated with significant risk of death and heart failure (80). CMR is particularly well suited to quantitatively detect iron overload by taking advantage of the effect of iron deposits on the T2* relaxation time of surrounding protons in the myocardium (Figure 5). The T2* relaxation time linearly falls with increasing iron load (81). The reduction of T2* relaxation time in the presence of myocardial iron overload is only modestly associated with LVEF and is not associated with abnormalities of diastolic function. Cardiac siderosis patients with severe reductions in T2* relaxation time (<10ms) are at risk for ventricular tachycardia despite having a normal LVEF and diastolic function (82,83). A T2* relaxation time of <20ms has been proposed as a cutoff value for diagnosing cardiac siderosis, whereas, a value of <10ms is associated with poor prognosis and requires initiation of iron chelation therapy (84). T2* relaxation times can be serially monitored in patients who are receiving iron chelation therapy whenever the T2* relaxation time drops below a certain threshold. The therapy is continued until the T2* relaxation times rise above another threshold. Such a strategy has resulted in a significant decrease in cardiac morbidity and mortality in patients with thalassemia who require frequent blood transfusions (80).
Anderson-Fabry Disease (AFD)
Anderson-Fabry Disease is an X-linked lysosomal storage disease characterized by deficiency of α-galactosidase A. Early CMR studies of AFD showed LGE related to the extent of LVH, typically located in the basal inferolateral wall and in the midwall or subepicardium(85). T1 mapping in Anderson-Fabry disease demonstrates characteristically reduced native T1 in patients with phenotypic LVH compared to other hypertrophic diseases (86) (87) which generally have an elevated T1. AFD patients also demonstrate an intermediate reduction in native T1 before the onset of hypertrophy (88). CMR has been used to follow regression of LVH with enzyme replacement therapy in this disorder (89).
Conclusion
Although a discussion of all the available data related to the utility of CMR in non-ischemic cardiomyopathy is beyond the scope of this review, we have selected representative disease states to demonstrate its value. Its ability to characterize the myocardium using techniques such as LGE, T1-mapping, T2-mapping, T2*-imaging, and ECV provides important insights into the underlying etiology of a cardiomyopathy. It additionally can be used to help risk stratify patients, guide patient management plan, and evaluate treatment response. CMR should be routinely used in the workup of patients with non-ischemic cardiomyopathy.
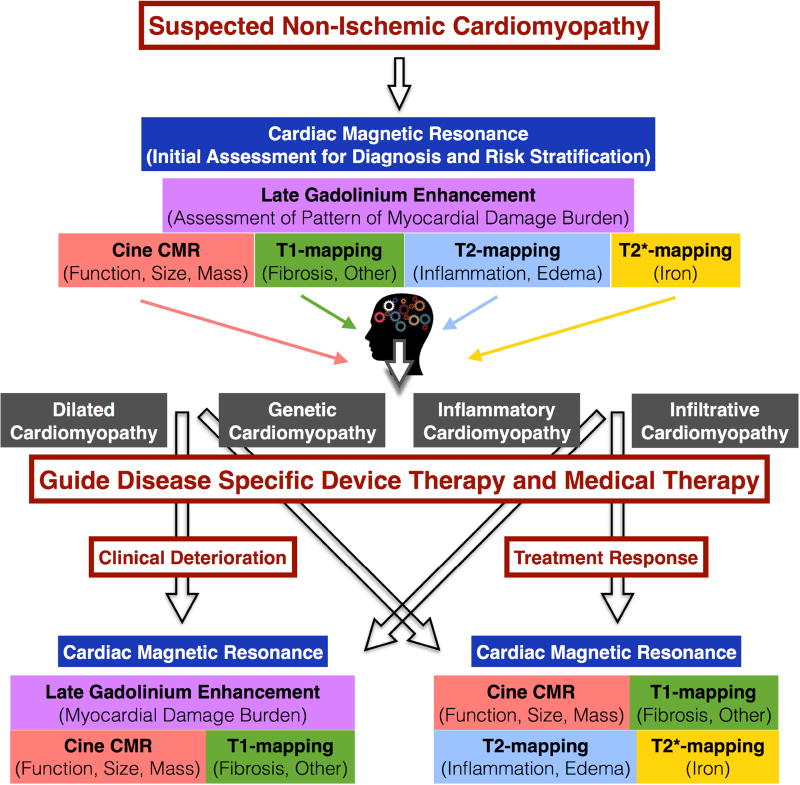
Central Illustration. Evaluation of Non-ischemic Cardiomyopathy Using Cardiac Magnetic Resonance.
This chart demonstrates a potential approach for incorporating the use of cardiac magnetic resonance for the initial evaluation and follow up of patients with cardiomyopathy.
Abbreviations
- CMR
cardiac magnetic resonance
- LGE
late gadolinium enhancement
- ECV
extracellular volume
- LVEF
left ventricular ejection fraction
- ICD
implantable cardioverter defibrillator
- SCD
sudden cardiac death
- HCM
hypertrophic cardiomyopathy
- LVNC
left ventricular noncompaction
- DMD
Duchenne’s muscular dystrophy
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- SLE
systemic lupus erythematosus
- CA
cardiac amyloidosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: ARP – Research support from Philips, Astellas, Myocardial Solutions; CMK – NIH U01HL117006-01A1, Consultant for Abbott
References
- 1.Abbasi SA, Ertel A, Shah RV, et al. Impact of cardiovascular magnetic resonance on management and clinical decision-making in heart failure patients. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2013;15:89. doi: 10.1186/1532-429X-15-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer CM. Role of Cardiac MR Imaging in Cardiomyopathies. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015;56(Suppl 4):39S–45S. doi: 10.2967/jnumed.114.142729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbustini E, Narula N, Tavazzi L, et al. The MOGE(S) classification of cardiomyopathy for clinicians. Journal of the American College of Cardiology. 2014;64:304–18. doi: 10.1016/j.jacc.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. The New England journal of medicine. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 5.Iles LM, Ellims AH, Llewellyn H, et al. Histological validation of cardiac magnetic resonance analysis of regional and diffuse interstitial myocardial fibrosis. European heart journal cardiovascular Imaging. 2015;16:14–22. doi: 10.1093/ehjci/jeu182. [DOI] [PubMed] [Google Scholar]
- 6.McCrohon JA, Moon JC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–9. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 7.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. European heart journal. 2005;26:1461–74. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 8.Iles L, Pfluger H, Lefkovits L, et al. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. Journal of the American College of Cardiology. 2011;57:821–8. doi: 10.1016/j.jacc.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 9.Leong DP, Chakrabarty A, Shipp N, et al. Effects of myocardial fibrosis and ventricular dyssynchrony on response to therapy in new-presentation idiopathic dilated cardiomyopathy: insights from cardiovascular magnetic resonance and echocardiography. European heart journal. 2012;33:640–8. doi: 10.1093/eurheartj/ehr391. [DOI] [PubMed] [Google Scholar]
- 10.Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. Jama. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 11.Puntmann VO, Carr-White G, Jabbour A, et al. T1-Mapping and Outcome in Nonischemic Cardiomyopathy: All-Cause Mortality and Heart Failure. JACC Cardiovascular imaging. 2016;9:40–50. doi: 10.1016/j.jcmg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Klem I, Weinsaft JW, Bahnson TD, et al. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. Journal of the American College of Cardiology. 2012;60:408–20. doi: 10.1016/j.jacc.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halliday B, Gulati A, Ali A, et al. Association Between Mid-Wall Late Gadolinium Enhancement and Sudden Cardiac Death in Patients with Dilated Cardiomyopathy and Mild and Moderate Left Ventricular Systolic Dysfunction. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.116.026910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kober L, Thune JJ, Nielsen JC, et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. The New England journal of medicine. 2016;375:1221–30. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 15.Leyva F, Foley PW, Chalil S, et al. Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2011;13:29. doi: 10.1186/1532-429X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leyva F, Taylor RJ, Foley PW, et al. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. Journal of the American College of Cardiology. 2012;60:1659–67. doi: 10.1016/j.jacc.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 17.Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. Journal of the American College of Cardiology. 2015;65:1249–54. doi: 10.1016/j.jacc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Rickers C, Wilke NM, Jerosch-Herold M, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005;112:855–61. doi: 10.1161/CIRCULATIONAHA.104.507723. [DOI] [PubMed] [Google Scholar]
- 19.Olivotto I, Maron MS, Autore C, et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Journal of the American College of Cardiology. 2008;52:559–66. doi: 10.1016/j.jacc.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 20.Ismail TF, Jabbour A, Gulati A, et al. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart. 2014;100:1851–8. doi: 10.1136/heartjnl-2013-305471. [DOI] [PubMed] [Google Scholar]
- 21.Weng Z, Yao J, Chan RH, et al. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovascular imaging. 2016;9:1392–1402. doi: 10.1016/j.jcmg.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Chan RH, Maron BJ, Olivotto I, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–95. doi: 10.1161/CIRCULATIONAHA.113.007094. [DOI] [PubMed] [Google Scholar]
- 23.Dass S, Suttie JJ, Piechnik SK, et al. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circulation Cardiovascular imaging. 2012;5:726–33. doi: 10.1161/CIRCIMAGING.112.976738. [DOI] [PubMed] [Google Scholar]
- 24.Hinojar R, Varma N, Child N, et al. T1 Mapping in Discrimination of Hypertrophic Phenotypes: Hypertensive Heart Disease and Hypertrophic Cardiomyopathy: Findings From the International T1 Multicenter Cardiovascular Magnetic Resonance Study. Circulation Cardiovascular imaging. 2015;8 doi: 10.1161/CIRCIMAGING.115.003285. [DOI] [PubMed] [Google Scholar]
- 25.Puntmann VO, Voigt T, Chen Z, et al. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovascular imaging. 2013;6:475–84. doi: 10.1016/j.jcmg.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Ho CY, Abbasi SA, Neilan TG, et al. T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circulation Cardiovascular imaging. 2013;6:415–22. doi: 10.1161/CIRCIMAGING.112.000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLellan A, Ellims AH, Prabhu S, et al. Diffuse Ventricular Fibrosis on Cardiac Magnetic Resonance Imaging Associates With Ventricular Tachycardia in Patients With Hypertrophic Cardiomyopathy. Journal of cardiovascular electrophysiology. 2016;27:571–80. doi: 10.1111/jce.12948. [DOI] [PubMed] [Google Scholar]
- 28.Kramer CM, Appelbaum E, Desai MY, et al. Hypertrophic Cardiomyopathy Registry: The rationale and design of an international, observational study of hypertrophic cardiomyopathy. American heart journal. 2015;170:223–30. doi: 10.1016/j.ahj.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen SE, Selvanayagam JB, Wiesmann F, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. Journal of the American College of Cardiology. 2005;46:101–5. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 30.Kawel N, Nacif M, Arai AE, et al. Trabeculated (noncompacted) and compact myocardium in adults: the multi-ethnic study of atherosclerosis. Circulation Cardiovascular imaging. 2012;5:357–66. doi: 10.1161/CIRCIMAGING.111.971713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weir-McCall JR, Yeap PM, Papagiorcopulo C, et al. Left Ventricular Noncompaction: Anatomical Phenotype or Distinct Cardiomyopathy? Journal of the American College of Cardiology. 2016;68:2157–2165. doi: 10.1016/j.jacc.2016.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacquier A, Thuny F, Jop B, et al. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. European heart journal. 2010;31:1098–104. doi: 10.1093/eurheartj/ehp595. [DOI] [PubMed] [Google Scholar]
- 33.Nucifora G, Aquaro GD, Pingitore A, Masci PG, Lombardi M. Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. European journal of heart failure. 2011;13:170–6. doi: 10.1093/eurjhf/hfq222. [DOI] [PubMed] [Google Scholar]
- 34.Andreini D, Pontone G, Bogaert J, et al. Long-Term Prognostic Value of Cardiac Magnetic Resonance in Left Ventricle Noncompaction: A Prospective Multicenter Study. Journal of the American College of Cardiology. 2016;68:2166–2181. doi: 10.1016/j.jacc.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 35.Ashford MW, Jr, Liu W, Lin SJ, et al. Occult cardiac contractile dysfunction in dystrophin-deficient children revealed by cardiac magnetic resonance strain imaging. Circulation. 2005;112:2462–7. doi: 10.1161/CIRCULATIONAHA.104.516716. [DOI] [PubMed] [Google Scholar]
- 36.Hor KN, Wansapura J, Markham LW, et al. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: a cardiac magnetic resonance tagging study. Journal of the American College of Cardiology. 2009;53:1204–10. doi: 10.1016/j.jacc.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker S, Florian A, Patrascu A, et al. Identification of cardiomyopathy associated circulating miRNA biomarkers in patients with muscular dystrophy using a complementary cardiovascular magnetic resonance and plasma profiling approach. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2016;18:25. doi: 10.1186/s12968-016-0244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hor KN, Taylor MD, Al-Khalidi HR, et al. Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: effect of age and left ventricular systolic function. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2013;15:107. doi: 10.1186/1532-429X-15-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tandon A, Villa CR, Hor KN, et al. Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in duchenne muscular dystrophy. Journal of the American Heart Association. 2015;4 doi: 10.1161/JAHA.114.001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. European heart journal. 2010;31:806–14. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vermes E, Strohm O, Otmani A, Childs H, Duff H, Friedrich MG. Impact of the revision of arrhythmogenic right ventricular cardiomyopathy/dysplasia task force criteria on its prevalence by CMR criteria. JACC Cardiovascular imaging. 2011;4:282–7. doi: 10.1016/j.jcmg.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Tandri H, Saranathan M, Rodriguez ER, et al. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. Journal of the American College of Cardiology. 2005;45:98–103. doi: 10.1016/j.jacc.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 43.te Riele AS, Tandri H, Bluemke DA. Arrhythmogenic right ventricular cardiomyopathy (ARVC): cardiovascular magnetic resonance update. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2014;16:50. doi: 10.1186/s12968-014-0050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prati G, Vitrella G, Allocca G, et al. Right Ventricular Strain and Dyssynchrony Assessment in Arrhythmogenic Right Ventricular Cardiomyopathy: Cardiac Magnetic Resonance Feature-Tracking Study. Circulation Cardiovascular imaging. 2015;8:e003647. doi: 10.1161/CIRCIMAGING.115.003647. discussion e003647. [DOI] [PubMed] [Google Scholar]
- 45.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–11. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 46.Freeman AM, Curran-Everett D, Weinberger HD, et al. Predictors of cardiac sarcoidosis using commonly available cardiac studies. The American journal of cardiology. 2013;112:280–5. doi: 10.1016/j.amjcard.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 47.Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. American heart journal. 1999;138:299–302. doi: 10.1016/s0002-8703(99)70115-8. [DOI] [PubMed] [Google Scholar]
- 48.Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart rhythm. 2014;11:1305–23. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 49.Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. Journal of the American College of Cardiology. 2005;45:1683–90. doi: 10.1016/j.jacc.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 50.Smedema JP, Snoep G, van Kroonenburgh MP, et al. The additional value of gadolinium-enhanced MRI to standard assessment for cardiac involvement in patients with pulmonary sarcoidosis. Chest. 2005;128:1629–37. doi: 10.1378/chest.128.3.1629. [DOI] [PubMed] [Google Scholar]
- 51.Patel AR, Klein MR, Chandra S, et al. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: an observational study. European journal of heart failure. 2011;13:1231–7. doi: 10.1093/eurjhf/hfr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–77. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murtagh G, Laffin LJ, Beshai JF, et al. Prognosis of Myocardial Damage in Sarcoidosis Patients With Preserved Left Ventricular Ejection Fraction: Risk Stratification Using Cardiovascular Magnetic Resonance. Circulation Cardiovascular imaging. 2016;9:e003738. doi: 10.1161/CIRCIMAGING.115.003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coleman GC, Shaw PW, Balfour PC, Jr, et al. Prognostic Value of Myocardial Scarring on CMR in Patients With Cardiac Sarcoidosis. JACC Cardiovascular imaging. 2017;10:411–420. doi: 10.1016/j.jcmg.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greulich S, Deluigi CC, Gloekler S, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovascular imaging. 2013;6:501–11. doi: 10.1016/j.jcmg.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 56.Cain MA, Metzl MD, Patel AR, et al. Cardiac sarcoidosis detected by late gadolinium enhancement and prevalence of atrial arrhythmias. The American journal of cardiology. 2014;113:1556–60. doi: 10.1016/j.amjcard.2014.01.434. [DOI] [PubMed] [Google Scholar]
- 57.Crouser ED, Ono C, Tran T, He X, Raman SV. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. American journal of respiratory and critical care medicine. 2014;189:109–12. doi: 10.1164/rccm.201309-1668LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mavrogeni SI, Kitas GD, Dimitroulas T, et al. Cardiovascular magnetic resonance in rheumatology: Current status and recommendations for use. International journal of cardiology. 2016;217:135–48. doi: 10.1016/j.ijcard.2016.04.158. [DOI] [PubMed] [Google Scholar]
- 59.Knockaert DC. Cardiac involvement in systemic inflammatory diseases. European heart journal. 2007;28:1797–804. doi: 10.1093/eurheartj/ehm193. [DOI] [PubMed] [Google Scholar]
- 60.O'Neill SG, Woldman S, Bailliard F, et al. Cardiac magnetic resonance imaging in patients with systemic lupus erythematosus. Annals of the rheumatic diseases. 2009;68:1478–81. doi: 10.1136/ard.2008.098053. [DOI] [PubMed] [Google Scholar]
- 61.Varma N, Hinojar R, D'Cruz D, et al. Coronary vessel wall contrast enhancement imaging as a potential direct marker of coronary involvement: integration of findings from CAD and SLE patients. JACC Cardiovascular imaging. 2014;7:762–70. doi: 10.1016/j.jcmg.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishimori ML, Martin R, Berman DS, et al. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovascular imaging. 2011;4:27–33. doi: 10.1016/j.jcmg.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 63.Mavrogeni S, Karabela G, Stavropoulos E, et al. Heart failure imaging patterns in systemic lupus erythematosus. Evaluation using cardiovascular magnetic resonance. International journal of cardiology. 2014;176:559–61. doi: 10.1016/j.ijcard.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 64.Seneviratne MG, Grieve SM, Figtree GA, et al. Prevalence, distribution and clinical correlates of myocardial fibrosis in systemic lupus erythematosus: a cardiac magnetic resonance study. Lupus. 2016;25:573–81. doi: 10.1177/0961203315622275. [DOI] [PubMed] [Google Scholar]
- 65.Prochaska MT, Bergl PA, Patel AR, Moss JD, Archer SL. Atrioventricular heart block and syncope coincident with diagnosis of systemic lupus erythematosus. The Canadian journal of cardiology. 2013;29:1330 e5–1330 e7. doi: 10.1016/j.cjca.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saremi F, Ashikyan O, Saggar R, Vu J, Nunez ME. Utility of cardiac MRI for diagnosis and post-treatment follow-up of lupus myocarditis. The international journal of cardiovascular imaging. 2007;23:347–52. doi: 10.1007/s10554-006-9161-0. [DOI] [PubMed] [Google Scholar]
- 67.Abdel-Aty H, Siegle N, Natusch A, et al. Myocardial tissue characterization in systemic lupus erythematosus: value of a comprehensive cardiovascular magnetic resonance approach. Lupus. 2008;17:561–7. doi: 10.1177/0961203308089401. [DOI] [PubMed] [Google Scholar]
- 68.Hinojar R, Foote L, Sangle S, et al. Native T1 and T2 mapping by CMR in lupus myocarditis: Disease recognition and response to treatment. International journal of cardiology. 2016;222:717–26. doi: 10.1016/j.ijcard.2016.07.182. [DOI] [PubMed] [Google Scholar]
- 69.Singh JA, Woodard PK, Davila-Roman VG, et al. Cardiac magnetic resonance imaging abnormalities in systemic lupus erythematosus: a preliminary report. Lupus. 2005;14:137–44. doi: 10.1191/0961203305lu2050oa. [DOI] [PubMed] [Google Scholar]
- 70.Puntmann VO, D'Cruz D, Smith Z, et al. Native myocardial T1 mapping by cardiovascular magnetic resonance imaging in subclinical cardiomyopathy in patients with systemic lupus erythematosus. Circulation Cardiovascular imaging. 2013;6:295–301. doi: 10.1161/CIRCIMAGING.112.000151. [DOI] [PubMed] [Google Scholar]
- 71.Vogelsberg H, Mahrholdt H, Deluigi CC, et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. Journal of the American College of Cardiology. 2008;51:1022–30. doi: 10.1016/j.jacc.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 72.Selvanayagam JB, Hawkins PN, Paul B, Myerson SG, Neubauer S. Evaluation and management of the cardiac amyloidosis. Journal of the American College of Cardiology. 2007;50:2101–10. doi: 10.1016/j.jacc.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 73.White JA, Kim HW, Shah D, et al. CMR imaging with rapid visual T1 assessment predicts mortality in patients suspected of cardiac amyloidosis. JACC Cardiovascular imaging. 2014;7:143–56. doi: 10.1016/j.jcmg.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maceira AM, Joshi J, Prasad SK, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–93. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 75.Fontana M, Pica S, Reant P, et al. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation. 2015;132:1570–9. doi: 10.1161/CIRCULATIONAHA.115.016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Banypersad SM, Sado DM, Flett AS, et al. Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: an equilibrium contrast cardiovascular magnetic resonance study. Circulation Cardiovascular imaging. 2013;6:34–9. doi: 10.1161/CIRCIMAGING.112.978627. [DOI] [PubMed] [Google Scholar]
- 77.Karamitsos TD, Piechnik SK, Banypersad SM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovascular imaging. 2013;6:488–97. doi: 10.1016/j.jcmg.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 78.Fontana M, Banypersad SM, Treibel TA, et al. Native T1 mapping in transthyretin amyloidosis. JACC Cardiovascular imaging. 2014;7:157–65. doi: 10.1016/j.jcmg.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 79.Banypersad SM, Fontana M, Maestrini V, et al. T1 mapping and survival in systemic light-chain amyloidosis. European heart journal. 2015;36:244–51. doi: 10.1093/eurheartj/ehu444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2008;10:42. doi: 10.1186/1532-429X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. European heart journal. 2001;22:2171–9. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 82.Wood JC, Tyszka JM, Carson S, Nelson MD, Coates TD. Myocardial iron loading in transfusion-dependent thalassemia and sickle cell disease. Blood. 2004;103:1934–6. doi: 10.1182/blood-2003-06-1919. [DOI] [PubMed] [Google Scholar]
- 83.Leonardi B, Margossian R, Colan SD, Powell AJ. Relationship of magnetic resonance imaging estimation of myocardial iron to left ventricular systolic and diastolic function in thalassemia. JACC Cardiovascular imaging. 2008;1:572–8. doi: 10.1016/j.jcmg.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 84.Kirk P, Roughton M, Porter JB, et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation. 2009;120:1961–8. doi: 10.1161/CIRCULATIONAHA.109.874487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moon JC, Sachdev B, Elkington AG, et al. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. European heart journal. 2003;24:2151–5. doi: 10.1016/j.ehj.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 86.Sado DM, White SK, Piechnik SK, et al. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circulation Cardiovascular imaging. 2013;6:392–8. doi: 10.1161/CIRCIMAGING.112.000070. [DOI] [PubMed] [Google Scholar]
- 87.Thompson RB, Chow K, Khan A, et al. T(1) mapping with cardiovascular MRI is highly sensitive for Fabry disease independent of hypertrophy and sex. Circulation Cardiovascular imaging. 2013;6:637–45. doi: 10.1161/CIRCIMAGING.113.000482. [DOI] [PubMed] [Google Scholar]
- 88.Pica S, Sado DM, Maestrini V, et al. Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2014;16:99. doi: 10.1186/s12968-014-0099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hughes DA, Elliott PM, Shah J, et al. Effects of enzyme replacement therapy on the cardiomyopathy of Anderson-Fabry disease: a randomised, double-blind, placebo-controlled clinical trial of agalsidase alfa. Heart. 2008;94:153–8. doi: 10.1136/hrt.2006.104026. [DOI] [PubMed] [Google Scholar]