Abstract
Previous studies suggested that oxidative stress is related to the onset and development of osteoporosis. Moreover, it was demonstrated that berberine has a protective effect against oxidative stress-induced injuries. In this study, we aimed to investigate the effect and mechanism of action of berberine on rats with induced osteoporosis. Sixty 8-week-old female Wistar rats were randomly divided into the following 6 groups: control saline-treated, osteoporosis saline-treated, 3 osteoporosis berberine-treated groups (Ber 5, 10, and 20 mg/kg/body weight, respectively), and osteoporosis alendronate-treated (ALD) group. Osteoporosis was induced by bilateral ovariectomy. All treatments were performed for 8 weeks. The bone mineral density (BMD), serum alkaline phosphatase (ALP), osteocalcin, calcium, phosphorus, superoxide dismutase (SOD), methylenedioxyamphetamine (MDA), and glutathione peroxidase (GSH-Px) level was determined in the rat femur tissue. The gene and protein expression of osteoprotegerin (OPG) and receptor activator of nuclear factor kappa-B ligand (RANKL) was analyzed by quantitative reverse transcription PCR and Western blot, respectively. The BMD, SOD and GSH-Px levels, and the expression of OPG were significantly lower in osteoporosis compared to control group (all P < 0.05). The serum levels of osteocalcin, ALP, and MDA, and the expression of RANKL were significantly higher in osteoporosis compared to control group (all P < 0.05). Berberine, especially the high doses of berberine, effectively increased SOD, GSH-Px, and OPG levels as well as decreased serum osteocalcin, ALP, MDA and RANKL levels in berberine-treated osteoporosis groups (all P < 0.05). To conclude, oxidative stress may promote the development of osteoporosis in rats through the RANK/RANKL/OPG pathway. The antioxidative effect of berberine reduces the development of osteoporosis in rats to some extent.
Keywords: Osteoporosis, oxidative stress, Wistar rats, berberine, OPG, osteoprotegerin, RANK, RANKL, receptor activator of nuclear factor kappa-B ligand
INTRODUCTION
Aging is the process of physical deterioration of the body and can be accompanied by various diseases, including osteoporosis, arthritis, cardiovascular atherosclerosis, Alzheimer’s disease, and malignant tumors. With increasing aging population in China as well as in the whole world, common diseases related to old age, such as osteoporosis, have been causing widespread concern. As a systemic metabolic disease, osteoporosis causes microarchitectural deterioration of bone tissue, leading to increased bone fragility and risk of bone fractures. In China, the prevalence of osteoporosis in adults over 40 years old is about 13.2% [1], which is also associated with increased medical costs.
Different factors contribute to the onset of osteoporosis, including genetic, nutritional, and lifestyle factors among others [2,3]. Mody et al. [4] showed that oxidative stress could inhibit osteoblast differentiation from rat bone-marrow derived cells, stimulate the differentiation of osteoclasts, and promote the development of osteoporosis. The exact mechanism of how oxidative stress inhibits the differentiation of osteoblasts and induces the apoptosis of osteogenic cells is still not clear. Previous studies suggested that extracellular signal-regulated kinase (ERK) and ERK-dependent activation of nuclear factor-kappa-B (NF-kB) play an important role in the inhibition of osteoblast differentiation and in the apoptosis of osteogenic cells [5,6]. Superoxides produced by osteoclasts are directly involved in the process of bone degradation. Superoxide radicals are synthetized by osteoclasts at the osteoclast-bone interface, and the inhibition of superoxide production in osteoclasts reduces bone resorption [7]. Using an RNA interference method to inhibit the production of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 1 (NOX1) in osteoclast precursor cells, Lee et al. [8] found that the production of reactive oxygen species (ROS) and differentiation of osteoclasts, which are mediated by receptor activator of NF-kB ligand (RANKL), are completely suppressed [8]. These results suggest that NOX1 in osteoclasts participates in ROS production, and that ROS further promote the differentiation of osteoclast precursor cells. In other words, oxidative stress stimulates the growth and differentiation of osteoclasts, while osteoclasts produce ROS.
The impairment of antioxidant defense system can lead to the development of osteoporosis. Although previous studies indicated that oxidative stress-induced osteoporosis might be associated with NF-kB and RANK/RANKL/osteoprotegerin (OPG) pathways, the specific mechanism is still not clear [9,10].
Berberine is the major constituent of Rhizoma Coptidis, also known as Coptis berberine. It has numerous positive effects, including those on congestive heart failure, blood glucose and blood lipids, as well as cholagogue and antiarrhythmic effects. Recent studies showed a protective effect of berberine on acute myocardial ischemia, i.e. it could reverse ventricular hypertrophy and inhibit ventricular remodeling. This process is related to the inhibitory effect of berberine on oxidative stress [11-13]. In addition, Zhou et al. [14] showed that berberine sulfate could attenuate the differentiation of osteoclasts.
Here we investigated the mechanism of oxidative stress in rats with induced osteoporosis and the effect and possible mechanism of action of berberine on osteoporosis in rats.
MATERIALS AND METHODS
Animals and groups
Sixty healthy female Wistar rats, 8 weeks old and with body weight 238 + 22 g, were provided by the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences. After 1 week of adaptive feeding, the rats were randomly divided into 6 groups, with 9 or 10 rats in each group, as follows: sham operation group (control group, normal rats), osteoporosis group, 3 groups of rats with induced osteoporosis and treated with different concentrations of berberine (ber 5 mg/kg body weight [b.w.], ber 10 mg/kg b.w., and ber 20 mg/kg b.w.) and a group of rats with induced osteoporosis and treated with alendronate [ALD] (7 mg/s kg b.w., per week). Berberine and ALD were administered orally, and sham and osteoporosis group received the same amount of vehicle buffer.
The animal protocol was approved by the Institutional Animal Care of our hospital. The animals were housed under controlled conditions (temperature, 23 ± 2°C; relative humidity 50 ± 10%; 12-hour light/dark cycle) and allowed free access to standard diet.
Osteoporosis model and treatments
Osteoporosis was induced by bilateral ovariectomy; the rats were anesthetized by intraperitoneal injection (IP) of 3% pentobarbital sodium (0.1 mL/g). The abdominal skin and muscles were opened, the ovarian arteries were ligated in the uterus, and bilateral ovariectomy was performed. The small adipose tissue around the ovary was resected in the control group. The drugs were administered orally with intragastric volume of 1 ml; berberine was administered daily, while ALD was administered weekly. The control and osteoporosis groups were treated with physiological saline. The treatments lasted for 8 weeks.
Bone mineral density (BMD)
After 8 weeks of treatment in each group, the rats were euthanized by decapitation. The soft tissues around the proximal end of the femur were removed carefully. The BMD of the femur was measured by dual-energy X-ray absorptiometry [DXA] (Prodigy DXA systems, GE Healthcare, Pittsburgh, PA, USA) and analyzed by Hologic Discovery DXA system (Hologic, Inc., Bedford, MA, USA).
Laboratory measurements
Serum alkaline phosphatase (ALP), osteocalcin, calcium, and phosphorus levels were determined using an automatic biochemical analyzer (Beckman Coulter, Miami, FL, USA). Malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and total protein in the femur tissue were detected with commercial kits according to the manufacturer’s instructions (Beyotime Biotech, Shanghai, China).
Quantitative reverse transcription PCR (RT-qPCR)
The mRNA expression of OPG and RANKL in the femur tissue was measured by RT-qPCR. The total RNA was extracted with the TRIzol (Life Technologies, Carlsbad, CA) reagent (a single-step RNA isolation procedure). The cDNA was synthesized by a reverse transcription reaction and amplified. The relative expression of OPG and RANKL mRNA was measured by SYBR Green Quantitative PCR (Takara, Tokyo, Japan). Primer 3 software was used for primer design. b-actin was used as an internal control. The primer sequences were as follows:
OPG upstream primer: 5’ TACAGCATCACTACGTA GGAC 3’; OPG downstream primer: 5’ ACGTCATGCGATCACAT ATCG 3’; RANKL upstream primer: 5’ GACAGGCACGGACT CGTA 3’; RANKL downstream primer: 5’ CGCTCATGCTAGTC GTCTA 3’; b-actin upstream primer: 5’ GAAATCGTGCGTGACAT TA 3’; and b-actin downstream primer: 5’ TAGGAGCCAGGGCA GTAA 3’.
The relative expression of mRNA was calculated by the 2-DDCT method.
Western blot
The OPG and RANKL protein expressions were detected by western blot. Ice-cold lysis buffer was added to prepared femur tissue. The samples were homogenized by grinding, centrifuged at 12,000 r/min for 2 minutes at 4°C, and the supernatant was separated for further analysis. The same amount of nuclear protein was loaded in 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) for protein separation. The proteins were transferred to polyvinylidene difluoride (PVDF) film, blocked in 5% skim milk overnight, and washed three times with Tween 20 Tris-buffered saline (TTBS). OPG (ab203061) and RANKL (ab169966) rabbit anti-rat primary antibodies (1:1000, Abcam plc, Cambridge, MA, USA) were added, incubated at 4°C overnight, and washed three times with TTBS. The secondary antibody, horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody, was added and cultured at room temperature for 2 hours. The proteins were visualized with RapidStep™ ECL Reagent (Merck Millipore, Darmstadt, Germany). An Odyssey® Imaging system (LI-COR Biosciences, Lincoln, NE, USA) and Quantity One® 1-D analysis software (Bio-Rad, Hercules, CA, USA) were used for semi-quantitative analysis.
Statistical analysis
PASW Statistics for Windows, Version 18.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis. Data were presented as mean ± standard deviation (SD). Analysis of variance (ANOVA) and Fisher’s Least Significant Difference (LSD) test were performed to test the difference between the groups. A value of P < 0.05 was considered statistically significant.
RESULTS
Weight comparison between groups
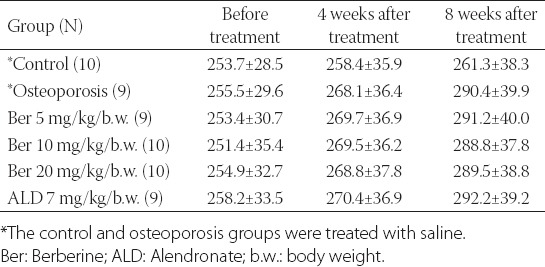
There was no difference in the rat body weight between the groups, prior to the treatment with saline, berberine, or ALD. After 8 weeks of the treatments, the weight in the osteoporosis, 3 berberine-treated groups (i.e. 5, 10, and 20 mg of berberine/kg b.w.), and ALD group was higher compared to the control saline group (290.3 g, 291.2 g, 288.8 g, 289.5 g, 292.2 g versus 261.3 g, respectively), but the differences were not statistically significant [Table 1].
TABLE 1.
Comparison of rat body weight between different groups
BMD comparison between groups
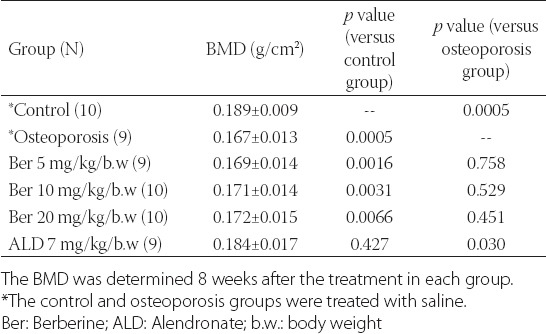
After 8 weeks of the treatments, the femur BMD in the osteoporosis group was lower compared to the control group (0.167 ± 0.013 versus 0.189 ± 0.009). The treatment with ALD significantly increased the femur BMD in the rats with induced osteoporosis compared to the control group (0.184 ± 0.017 versus 0.167 ± 0.013, P = 0.03). The treatment with berberine increased the BMD to some extent in 3 berberine-treated groups compared to the osteoporosis group, nevertheless, the BMD did not reach the normal values in these groups [Table 2].
TABLE 2.
Comparison of bone mineral density (BMD) between different groups
Comparison of serum osteocalcin, ALP, calcium, and phosphorus levels between groups
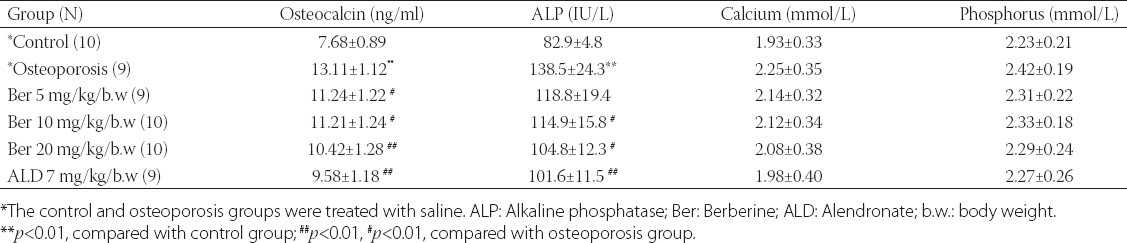
As shown in Table 3, osteoporosis led to a high expression of osteocalcin; the osteocalcin level in the osteoporosis group was significantly higher compared to the control group (13.11 ± 1.12 ng/ml versus 7.68 ± 0.89 ng/ml). The treatments with berberine and alendronate reduced the osteocalcin level in the rats with induced osteoporosis (all P < 0.05, Table 3); nevertheless, this result was not significant compared to the control saline group. Similarly, the ALP level was increased in the osteoporosis group (138.5 ± 24.3 versus 82.9 ± 4.8 in the control saline group, P < 0.01) and the treatments with berberine and alendronate reduced the ALP level in the rats with osteoporosis (all P < 0.05 except for the group treated with ber 5 mg/kg/b.w, P > 0.05). In addition, the serum calcium and phosphorus levels were increased in the osteoporosis group, and berberine and alendronate reduced their levels, but with no statistically significant difference compared with the control saline group [Table 3].
TABLE 3.
Serum osteocalcin, alkaline phosphatase, calcium, and phosphorus levels in each group, after the 8-week treatment
Expression of MDA, SOD and GSH-Px in rat femur tissue
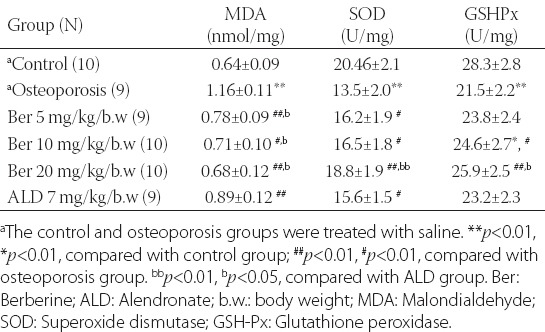
In the osteoporosis group, the expression of MDA in the femur tissue was markedly higher and the expression of SOD and GSH-Px was lower compared to the control group [Table 4]. The treatment with berberine significantly decreased the expression of MDA and increased the expression of SOD and GSH-Px in the berberine-treated groups. The expression of MDA, SOD and GSH-Px in the rats with induced osteoporosis and treated with the highest concentration of berberine (20 mg/kg/b.w) was not statistically different compared to the control saline group. Moreover, berberine demonstrated a higher antioxidative effect on the femur tissue in the rats with osteoporosis compared to alendronate.
TABLE 4.
Comparison of MDA, SOD and GSH-Px expression in rat femur tissue between different groups
mRNA and protein expression of OPG and RANKL
RT-qPCR and western blot results [Figure 1] showed that the expression of OPG in the osteoporosis group was lower compared to the control group. On the other hand, the expression of RANKL in the osteoporosis group was higher compared to the control group. Berberine increased the expression of OPG and decreased the expression of RANKL at the mRNA and protein level in a dose-dependent manner. The changes in the expression of OPG and RANKL between the osteoporosis and berberine-treated groups were considerable, especially in the group treated with the highest dose of berberine, where the differences in the OPG and RANKL expressions were statistically significant compared to the osteoporosis group. These results indicate that OPG and RANKL are associated with the mechanism of action of berberine in rats with osteoporosis.
FIGURE 1.
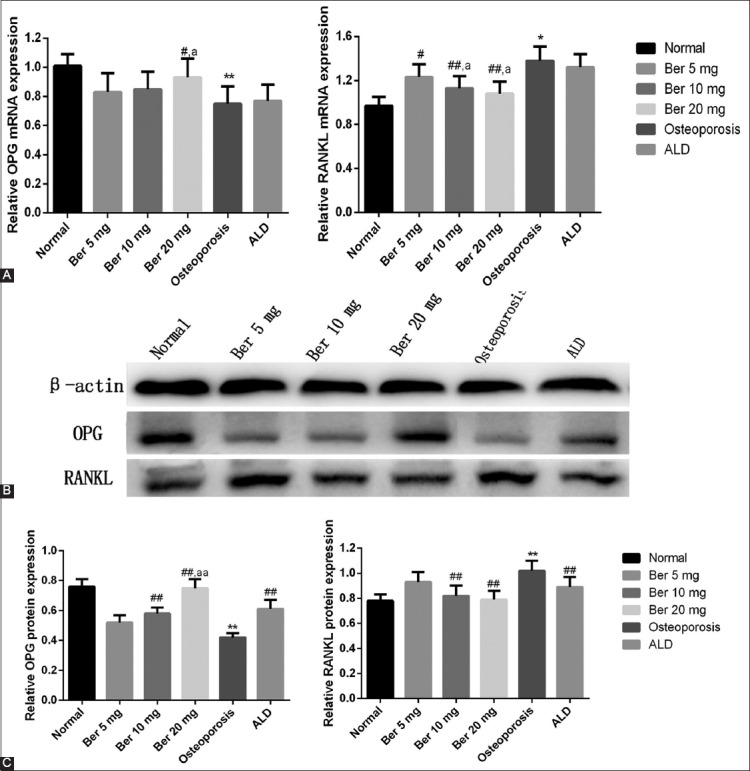
Protein and gene expressions of osteoprotegerin (OPG) and receptor activator of NF-κB ligand (RANKL) measured by Western blot and quantitative reverse transcription PCR (RT-qPCR), respectively. (A) Comparison of RT-qPCR results for OPG and RANKL between groups; (B and C) Comparison of Western blot results for OPG and RANKL between groups. **p<0.01, *p<0.01, compared with normal (control) group; ##p<0.01, #p<0.01, compared with osteoporosis group; aap<0.01, ap<0.05, compared with ALD group. Ber: Berberine; ALD: Alendronate.
DISCUSSION
In this study, we investigated the mechanism of oxidative stress in rats with induced osteoporosis by measuring the levels of different proteins possibly involved in the oxidative process. We found that the MDA level in the osteoporosis group was significantly higher compared to the control group, while the SOD and GSH-Px levels were significantly lower in the osteoporosis versus control group. These results indicate that the oxidative processes and antioxidative defense mechanism are unbalanced in osteoporosis, i.e., oxidative stress is increased and antioxidant system is impaired in this state. We further investigated the effect and possible mechanism of action of berberine in rats with induced osteoporosis. In our study, berberine decreased the MDA and osteocalcin levels in rats with osteoporosis, while it increased the SOD and GSH-Px levels. These results demonstrate that oxidative stress may be reduced by antioxidants and, consequently, the bone loss could be prevented or at least decreased.
The process of differentiation of bone marrow-derived macrophages (BMM) into osteoclasts is mediated by the RANK/RANKL system. During this process, ROS are produced through a signaling cascade that involves tumor necrosis factor receptor associated factor 6 (TRAF6), Ras-related C3 botulinum toxin substrate 1 (Rac1) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 1 (No × 1). Under normal physiological circumstances, the production of ROS and antioxidant defense system are in equilibrium. Once the balance is impaired due to smoking, aging, estrogen deficiency or other factors, oxidative stress occurs in these cells. This may lead to extensive oxidative damage which negatively affects the growth, differentiation, and proliferation of the cells. If uncontrolled, the production of free radicals by osteoclasts can accelerate the damage of the calcified tissue and hamper the reconstruction of the bone. This further impairs the healing process and development of bone [15,16].
Using RANK-deficient mice, Dougall et al. [17] showed the important role of RANK in lymph node organogenesis and osteoclast differentiation [17]. In addition, it has been demonstrated that oxidative stress promotes cell senescence and apoptosis by activating different signal transduction pathways involving NF-kB, mitogen-activated protein kinases (MAPKs), tumor protein p53 (TP53), and heat shock factors (HSFs) [8,18,19].
Other studies showed that oxidative stress also damages fibronectin, one of the major components of bone extracellular matrix. This glycoprotein participates in the processes of migration, adhesion, proliferation, and differentiation of osteoblasts. Because the metabolic change of fibronectin is relatively slow compared to other components of the cell, fibronectin is affected by different non-enzymatic modifications, including the formation of oxygen-free radicals during the aging. Fibronectin molecules damaged by ROS lose their function in bone formation. Overall, this process is regulated by various factors, and although the exact mechanism is not clear, it appears that the regulation of fibronectin and RANKL is important for bone resorption [20].
Zhao et al. [21] also showed that the OPG/RANKL pathway is an important signal transduction pathway in osteoclast differentiation. Furthermore, in their review, Kobayashi et al. [22] indicated that the expression of RANKL increases with increased levels of oxidative stress, while the OPG expression and OPG/RANKL ratio decrease. This means that increased oxidative stress stimulates the RANKL/RANK interaction, while it inhibits the OPG/RANKL/RANK system. In this state, the formation, differentiation, and maturation of osteoclasts are promoted and bone resorption is decreased, eventually leading to the damage of bone tissue and development of osteoporosis. Consistent with the above-mentioned studies, the expression of RANKL was increased in our osteoporosis group, while the expression of OPG was decreased in this group compared to the controls. The RANKL/OPG ratio was also increased in the osteoporosis group (data not show). Furthermore, berberine decreased the expression of RANKL in the rats with induced osteoporosis and increased the OPG expression.
Moreover, Zhou et al. [14] showed that berberine sulfate could inhibit the differentiation of osteoclasts at the dose of 0.25, 0.5 and 1 mM, in BMM-derived osteoclast culture system [14]. In this study, we used an in vivo model for investigating osteoporosis. Although the berberine treatment did not improve the BMD significantly in the berberine-treated rats, it decreased the level of oxidative stress in these groups. However, whether clinical application of berberine could be beneficial in other instances, such as postmenopausal women with osteoporosis, needs further confirmation.
CONCLUSION
To sum up, oxidative stress may promote the development of osteoporosis through the RANK/RANKL/OPG pathway, and antioxidants such as berberine can mitigate the progress of osteoporosis. Nevertheless, we made this conclusion based on the rat model of osteoporosis and berberine should be evaluated for adverse as well as beneficial effects in clinical trials. In addition, the specific mechanism of oxidative stress in osteoporosis should be further investigated.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Zhen D, Liu L, Guan C, Zhao N, Tang X. High prevalence of vitamin D deficiency among middle aged and elderly individuals in northwestern China: Its relationship to osteoporosis and lifestyle factors. Bone. 2015;71:1–6. doi: 10.1016/j.bone.2014.09.024. https://doi.org/10.1016/j.bone.2014.09.024. [DOI] [PubMed] [Google Scholar]
- [2].Chen SJ, Liao WC, Huang KH, Lin CL, Tsai WC, Kung PT, et al. Chronic obstructive pulmonary disease and allied conditions is a strong independent risk factor for osteoporosis and pathologic fractures: A population based cohort study. QJM. 2015;108(8):633–40. doi: 10.1093/qjmed/hcv012. https://doi.org/10.1093/qjmed/hcv012. [DOI] [PubMed] [Google Scholar]
- [3].Weaver CM, Alexander DD, Boushey CJ, Dawson Hughes B, Lappe JM, LeBoff MS, et al. Calcium plus vitamin D supplementation and risk of fractures: An updated meta analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27(1):367–76. doi: 10.1007/s00198-015-3386-5. https://doi.org/10.1007/s00198 015 3386 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31(4):509–19. doi: 10.1016/s0891-5849(01)00610-4. https://doi.org/10.1016/S0891 5849 (01) 00610 4. [DOI] [PubMed] [Google Scholar]
- [5].Gyoneva L, Hovell CB, Pewowaruk RJ, Dorfman KD, Segal Y, Barocas VH. Cell matrix interaction during strain dependent remodelling of simulated collagen networks. Interface Focus. 2016;6(1):20150069. doi: 10.1098/rsfs.2015.0069. https://doi.org/10.1098/rsfs. 2015.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kim BJ, Shin KO, Kim H, Ahn SH, Lee SH, Seo CH, et al. The effect of sphingosine 1 phosphate on bone metabolism in humans depends on its plasma/bone marrow gradient. J Endocrinol Invest. 2016;39(3):297–303. doi: 10.1007/s40618-015-0364-x. https://doi.org/10.1007/s40618 015 0364 x. [DOI] [PubMed] [Google Scholar]
- [7].Vääräniemi J, Halleen JM, Kaarlonen K, Ylipahkala H, Alatalo SL, Andersson G, et al. Intracellular machinery for matrix degradation in bone resorbing osteoclasts. J Bone Miner Res. 2004;19(9):1432–40. doi: 10.1359/JBMR.040603. https://doi.org/10.1359/JBMR.040603. [DOI] [PubMed] [Google Scholar]
- [8].Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, et al. A crucial role for reactive oxygen species in RANKL induced osteoclast differentiation. Blood. 2005;106(3):852–9. doi: 10.1182/blood-2004-09-3662. https://doi.org/10.1182/blood 2004 09 3662. [DOI] [PubMed] [Google Scholar]
- [9].Pellegrini GG, Morales CC, Wallace TC, Plotkin LI, Bellido T. Avenanthramides prevent osteoblast and osteocyte apoptosis and induce osteoclast apoptosisin vitroin an Nrf2 independent manner. Nutrients. 2016;8(7):E423. doi: 10.3390/nu8070423. https://doi.org/10.3390/nu8070423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pietschmann P, Mechtcheriakova D, Meshcheryakova A, Föger Samwald U, Ellinger I. Immunology of osteoporosis: A mini review. Gerontology. 2016;62(2):128–37. doi: 10.1159/000431091. https://doi.org/10.1159/000431091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee IA, Hyun YJ, Kim DH. Berberine ameliorates TNBS induced colitis by inhibiting lipid peroxidation, enterobacterial growth and NF kB activation. Eur J Pharmacol. 2010;648(1-3):162–70. doi: 10.1016/j.ejphar.2010.08.046. https://doi.org/10.1016/j.ejphar.2010.08.046. [DOI] [PubMed] [Google Scholar]
- [12].Li P, Ren J, Duan C, Lin C, Liu J. Effects of four components of Rhizoma Corydalis on anoxia and peroxidation injuries in neonatal cardiomyocytes. [Article in Chinese] Zhongguo Zhong Yao Za Zhi. 2010;35(1):84–8. doi: 10.4268/cjcmm20100118. [DOI] [PubMed] [Google Scholar]
- [13].Huang Z, Chen S, Zhang G, Xu S, Huang W, Han Y, et al. Protective effects of berberine and phentolamine on myocardial reoxygenation damage. Chin Med Sci J. 1992;7(4):221–5. [PubMed] [Google Scholar]
- [14].Zhou L, Song F, Liu Q, Yang M, Zhao J, Tan R, et al. Berberine sulfate attenuates osteoclast differentiation through RANKL induced NF kB and NFAT pathways. Int J Mol Sci. 2015;16(11):27087–96. doi: 10.3390/ijms161125998. https://doi.org/10.3390/ijms161125998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zheng B, Li G, Chen WC, Deasy BM, Pollett JB, Sun B, et al. Human myogenic endothelial cells exhibit chondrogenic and osteogenic potentials at the clonal level. J Orthop Res. 2013;31(7):1089–95. doi: 10.1002/jor.22335. https://doi.org/10.1002/jor. 22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fu C, Xu D, Wang CY, Jin Y, Liu Q, Meng Q, et al. Alpha lipoic acid promotes osteoblastic formation in H2O2 treated MC3T3 E1 cells and prevents bone loss in ovariectomized rats. J Cell Physiol. 2015;230(9):2184–2201. doi: 10.1002/jcp.24947. https://doi.org/10.1002/jcp. 24947. [DOI] [PubMed] [Google Scholar]
- [17].Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13(18):2412–24. doi: 10.1101/gad.13.18.2412. https://doi.org/10.1101/gad. 13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Joo JH, Huh JE, Lee JH, Park DR, Lee Y, Lee SG, et al. A novel pyrazole derivative protects from ovariectomy induced osteoporosis through the inhibition of NADPH oxidase. Sci Rep. 2016;6:22389. doi: 10.1038/srep22389. https://doi.org/10.1038/srep22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hemshekhar M, Thushara RM, NaveenKumar SK, Manoj P, Sundaram MS, Kemparaju K, et al. Bone degeneration, inflammation and secondary complications of arthritis: Potential targets and their natural inhibitors. Mini Rev Med Chem. 2017 doi: 10.2174/1389557517666170315144233. https://doi.org/10.2174/1389557517666170315144233. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [20].Abdollahi M, Larijani B, Rahimi R, Salari P. Role of oxidative stress in osteoporosis. Therapy. 2005;2(5):787–96. DOI: 10.1586/14750708.2.5.787. [Google Scholar]
- [21].Zhao H, Xu H, Qiao S, Lu C, Wang G, Liu M, et al. Boldine isolated from Litsea cubeba inhibits bone resorption by suppressing the osteoclast differentiation in collagen induced arthritis. Int Immunopharmacol. 2017;51:114–23. doi: 10.1016/j.intimp.2017.08.013. DOI: 10.1016/j.intimp.2017.08.013. [DOI] [PubMed] [Google Scholar]
- [22].Kobayashi Y, Udagawa N, Takahashi N. Action of RANKL and OPG for osteoclastogenesis. Crit Rev Eukaryot Gene Expr. 2009;19(1):61–72. doi: 10.1615/critreveukargeneexpr.v19.i1.30. https://doi.org/10.1615/CritRevEukarGeneExpr.v19.i1.30. [DOI] [PubMed] [Google Scholar]


