Abstract
The purpose of this study was to investigate the correlation of single nucleotide polymorphisms (SNPs) in Matrix metalloproteinase -2 (MMP-2) gene and the risk of age-related macular degeneration (AMD) in Chinese Han population.
A total of 126 AMD patients and 141 healthy controls participated in this study. Genotypes of MMP-2 gene polymorphisms were identified by the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). χ2test was used to detect the differences of genotypes and alleles frequencies between case and control groups. Relative risk of AMD was evaluated by odds ratios (ORs) with 95% confidence intervals (CIs).
Distribution of variant allele carriers (computed tomography + TT genotypes) of MMP-2 gene rs243865 SNP was significantly different between case and control groups, and might act as protective factors for the onset of AMD (P = .044, OR = 0.583, 95% CI = 0.344–0.987). Nevertheless, the T allele might reduce the AMD risk (P = .030, OR = 0.611, 95% CI = 0.390–0.956). However, no significant association existed between rs243865 and AMD risk in the subgroup analysis based on age. GA + AA genotypes of rs243866 SNP may associate with a decreased risk of AMD in the age≤65 years subgroup (P = .028, OR = 0.399, 95% CI = 0.174–0.915).
MMP-2 gene rs243865 and rs243866 SNPs associated with the risk of AMD. Further studies should be performed to confirm the results.
Keywords: age, AMD, MMP-2, polymorphisms
1. Introduction
Age-related macular degeneration (AMD), which leads to progressive vision impairment, is the main reason of the blindness in the elderly people worldwide.[1] Characteristics of AMD are as chronic and progressive degeneration of photoreceptors, retinal pigment epithelium, and Bruch membrane.[2] Neovascular AMD and non-neovascular AMD are the 2 subtypes of AMD.[3] In recent years, epidemiological study found that the morbidity of AMD is increased in China[4] and its incidence in females is higher than that in males in clinical. Moreover, the prevalence of AMD presents trend of increasing along with the age. AMD is one of complex diseases and influenced by the environment and genetic factors. So far, age, smoking, excess alcohol consumption, education level, and diet structure have been identified to be associated with AMD.[5] Genetic factors including a number of genes polymorphisms have been proved as the molecular basis for AMD.[6] Furthermore, many researches also report that the interaction of genetic factors and other risk factors are involved in the pathogenesis of AMD.[7] However, the etiology of AMD is not completely understood nowadays. Angiogenesis is considered one of the important mechanisms for AMD development. Angiogenesis is related to the extracellular matrix (ECM) remodeling which includes various proteolytic systems. Matrix metalloproteinases (MMPs) are 1 kind of the important enzyme in proteolytic systems and involve in many vascular diseases.[8]
MMPs is a zinc-dependent endopeptidases family with the function of resolving ECM proteins. MMP-2, a member of MMPs family also known as gelatinase A, is a membrane-bound protein which can be found in optic nerve head and the retina.[9]MMP-2 gene, located on chromosome 16 q12.2, includes 17 exons. MMP-2, essential for turnover of ECM,[10] can lead to optic nerve demyelination and other nerve changes.[11,12] It is reported that MMP-2 activity is associated with the collagen deposition and the formation of subretinal deposit.[13,14] It has been identified that the expression level of MMP-2 is upregulated in choroidal neovascularization in animal model.[15] The study performed by Hussain et al[16] suggested that the total level of pro-MMP-2 was raised in AMD. There are few studies analyzing the influence of MMP-2 gene polymorphisms on AMD progression.
In this case-control study, we investigated the association between the single nucleotide polymorphisms (SNPs) of MMP-2 gene and the risk of AMD in Chinese Han population. Our study will provide more theoretical basis for further researches on the pathogenesis of AMD.
2. Materials and methods
2.1. Study subjects
This study was approved by the Ethics Committee of Aerospace Central Hospital. Written informed consent was obtained from all the subjects after informed with the detail and purpose of this study. Processes of sample collection were performed in accordance with the Declaration of Helsinki.
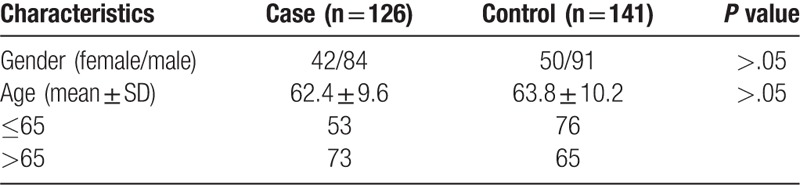
A total of 267 subjects consisting of 126 AMD patients and 141 healthy controls were enrolled in this study (Table 1). The patients with AMD were recruited during August 2013 to September 2015 from Ophthalmology Department of Aerospace Central Hospital. Fundus fluorescein angiography (FFA) and ophthalmic examinations were used to diagnose the AMD patients. Evaluation for AMD was performed according to the Age-Related Eye Disease Study (AREDS).[17] Patients with trauma, cancer, or abnormal liver or renal function tests were not included in this study. One hundred forty-one controls without AMD were recruited randomly from the Health Screening Center of Aerospace Central Hospital between August 2013 and September 2015. Individuals with degenerative myopia, angioid streaks, or drusen were excluded from this study. All of the subjects were Chinese Han population without blood relationship with each other. Frequencies of sex and age between case and control groups were good matched.
Table 1.
Demographic characteristics of the study subjects.
2.2. DNA extraction
Five milliliters of blood samples were obtained from all subjects via peripheral venous puncture. Genomic DNA was extracted by DNA extraction kits (QIAGEN, MD) following the instructions of the manufacturer. The genomic DNA was labeled and stored at −70°C until further analysis.
2.3. Genotyping
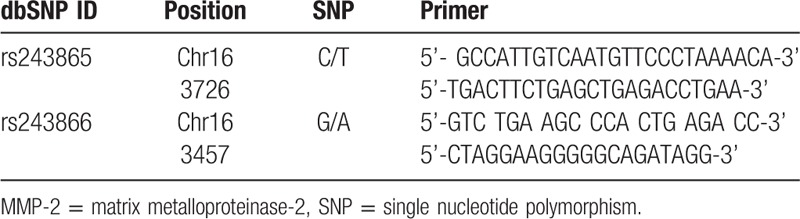
Region consisting of the MMP-2 SNPs was amplified using PCR. Primers were designed by Primer Premier 5 software according to the general primer design principles (Table 2). Total reaction system for PCR was 20 μL, which consisted of 3 μL DNA template, l μL forward primer, l μL reverse primer, 2.5 μL 10 × Buffer, 2 μL MgC12, 2.5 μL dNTPs, 1 μL Taq enzyme, 7 μL ddH2O. The reaction system was instantaneously centrifuged to mix well.
Table 2.
Primers of MMP-2 gene polymorphisms.
PCR conditions for rs243865 were initial denaturation at 94°C for 10 minutes, followed by 35 cycles of 94°C for 40 seconds, 62°C for 30 seconds, and 72°C for 45 seconds, and a final extension at 72°C for 3 minutes. The PCR conditions for rs243866 were initial denaturation at 94°C for 8 minutes followed by 40 cycles of 94°C for 60 seconds, 56°C for 30 seconds, 72°C for 50 seconds, and a final extension at 72°C for 5 minutes. PCR products were preserved at −20°C for standby application.
Restriction enzymes digestion was finished in 20 μL system, including 2 μL restriction enzyme, 8 μL PCR products, 2 μL 10 × Buffer, and 8 μL ddH2O. Digested products were separated by electrophoresis on 2.0% TBE agarose gel to confirm the size of amplicons.
2.4. Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences 18.0 for Windows (Chicago, IL). Hardy–Weinberg equilibrium (HWE) was used to assess the genotype distributions. Genotypic and allelic frequencies of MMP-2 polymorphisms were calculated by direct counting. Differences of genotype frequencies of MMP-2 gene polymorphisms between the case and control groups were compared by the χ2test. Effects of genotypes and alleles on AMD were evaluated by odds ratios (ORs) with 95% confidence intervals (CIs). Statistically significant differences were considered existing with P < .05.
3. Result
3.1. HWE test
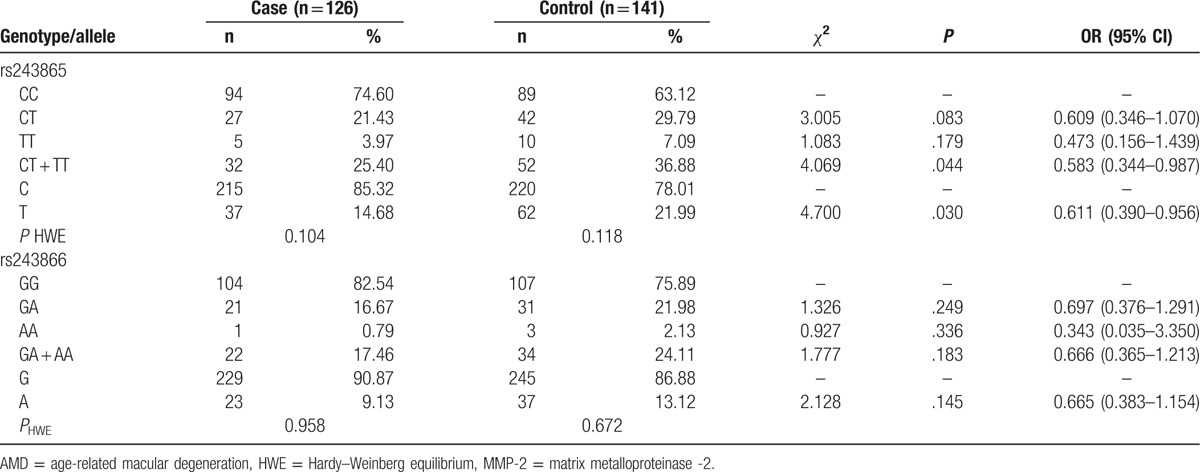
Genotype distributions of MMP-2 gene rs243865 and rs243866 SNPs were consistent with the HWE test respectively in cases and controls (Table 3). This result demonstrated that the subjects of present study could represent the general population.
Table 3.
Correlation of polymorphisms in MMP-2 gene and the risk of AMD.
3.2. Correlation of MMP-2 gene polymorphisms and AMD risk
CC, CT, and TT genotype frequencies of MMP-2 gene rs243865 SNP were 74.60%, 21.43%, 3.97% in AMD patients and 63.12%, 29.79%, 7.09% in healthy controls (Table 3). Variant allele carriers (CT + TT) were frequently observed in the case group, and the CT + TT genotypes might significantly be associated with the risk of AMD (P = .044, OR = 0.583, 95% CI = 0.344–0.987). Meanwhile, the T allele might protect against the development of AMD (P = .030, OR = 0.611, 95% CI = 0.390–0.956).
Both GA and AA genotypes of rs243866 SNP had higher frequencies in the controls. At the same time, A allele frequencies were respectively 9.13% in cases and 13.12% in controls. However, we failed to find any association between rs243866 SNP and the risk of AMD (P > .05).
3.3. Subgroup analysis of MMP-2 polymorphisms based on age
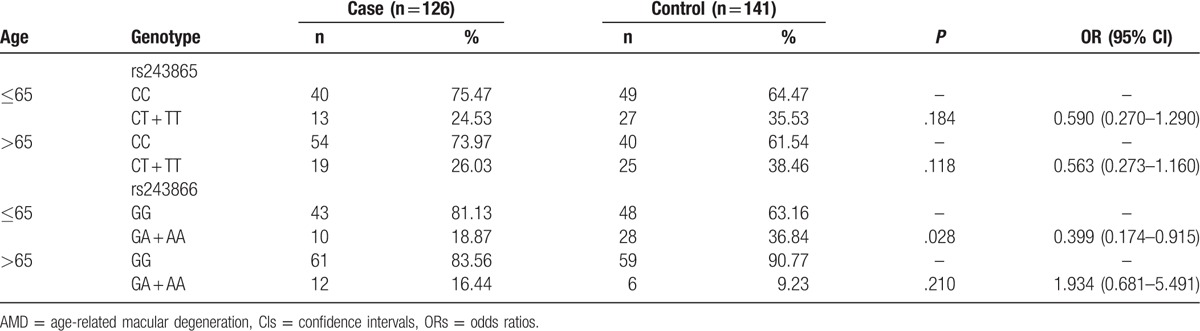
To detect the association of MMP-2 gene polymorphisms with the AMD risk, we performed the subgroup analysis based on age (Table 4). No significant association was observed between rs243865 genotypes and AMD risk both in the age≤65 years and > 65 years subgroups (P > .05). GA + AA genotypes of rs243866 polymorphism had a negative association with the AMD risk in the subgroup of age ≤ 65 years old (P = .028, OR = 0.399, 95% CI = 0.174–0.915). But rs243866 polymorphism did not relate to the occurrence of AMD in the age > 65 years subgroup (P > .05).
Table 4.
Frequencies of genotypes in rs243865 and rs243866 for the patients with AMD and controls by age.
4. Discussion
In this case-control study, we found that the MMP-2 gene rs243865 and rs243866 SNPs might negatively associate with the risk of AMD. For rs243865 polymorphism, compared with the wild homozygote, CT + TT genotypes might associate with 0.583-fold decreased risk of AMD. T allele also reduced the risk of AMD approximately 0.611 fold. But CT and TT genotypes were not related to the risk of AMD respectively, although they had high frequencies in controls. That was in accordance with the previous study performed by Hall et al.[18] Rs243865 SNP CC genotype shows a greater prevalence in AMD patients, and may be a predictor for AMD in a Lithuanian population.[18] This SNP is unlikely to have a major role in AMD onset, but the genotype distributions were significantly different between dry AMD patients and healthy controls.[19] Meanwhile, rs243865 is widely detected in many other diseases. It is revealed that TT genotype reduces the incidence of esophageal squamous cell carcinoma (ESCC).[20] Low et al[21] showed that this SNP marginally related to intracranial aneurysm (IA) in a Japanese population. It also reduces the rheumatoid arthritis (RA) risk in a Chinese population.[22] Lacchini et al[23] found that CC genotype of rs243865 reduced the left ventricular mass index (LVMI) and left ventricular end-diastolic diameter in hypertensive patients. A recent study substantiates that CT + TT genotypes of rs243865 significantly downregulate the protein level of MMP-2.[24] Based on the above results, we suggested that rs243865 was associated with the onset of AMD. However, it was failed to find significant association between rs243865 and AMD risk in age subgroup analysis.
Rs243866 was another polymorphism of MMP-2 gene which was assessed in the present study. Variant genotypes were lower in AMD patients, but they did not significantly associate with the risk of AMD. In the people 65 or less than 65 years old, GA + AA genotypes associated with 0.399-fold decreased risk of AMD. This SNP is not detected in AMD in previous studies, but is explored in other diseases. Juiz et al[25] indicated that rs243866 implicated in the ECM remodeling. A previous study shows that A allele carriers relate to decreased risk of systolic heart failure in a Han Chinese population.[26] In s Mexican population, rs243866 A allele might be a predisposing factor for myocardial infarction.[27] Additionally, AA genotype carriers had a downregulated plasma level of pro-MMP-2, but the difference of pro-MMP-2 levels among the rs243866 genotypes had not statistical significance.[28] Although we obtained an evidence for the association between MMP-2 gene polymorphisms and AMD risk, the pathogenesis of AMD was still not completely understood. Further studies should be carried out to verify present study in other ethnicity.
To our knowledge, it is the first study to explore the correlation of MMP-2 gene polymorphisms with the risk of AMD in Chinese Han population. MMPs produced by retinal pigment epithelium cells take part in the homeostasis of components in eye tissues.[29] As a member of the MMPs family, MMP-2 gene plays a major role in the ECM degradation in angiogenesis, tissue repair, and so on.[30] It is proven that MMP-2 contributes to the development of choroidal neovascularization,[31] while neovascular is one of the direct mechanisms for the occurrence and development of AMD. MMP-2 localizes in the new vessel and envelops Bruch membrane, then contributes to the choroidal neovascular membranes’ progressive growth in AMD.[32] Nevertheless, Zeng et al[33] considered that the serum level of MMP-2 is elevated in polypoidal choroidal vasculopathy but not AMD patients. These inconsistent results may be caused by different sample size, study population, genetic background, and the individual difference.
In conclusion, MMP-2 gene correlates with the risk of AMD, and polymorphisms are conducive to the individual difference for the same disease. Specify results according to AMD type were not performed due to the small sample size that might reduce the statistical power. As one of complex diseases, AMD is affected by the interaction of confounding factors. However, our results cannot rule out the effects of other factors to the AMD. Each population has its specificity, the reports show that the morbidity of AMD in Caucasians is higher than that in Blacks; however, Blacks are easier to develop exudative AMD than Caucasians. The prevalence of AMD in Asians is similar to Caucasians.[34,35] It may derive from different genetic background and environmental factors. Therefore, more researches with larger and different samples should be performed to verify present results in other populations. Even so, we should not ignore the effects of our study, which will provide molecular basic for the study of AMD pathogenesis.
Footnotes
Abbreviations: AMD = age-related macular degeneration, AREDS = age-related eye disease study, CIs = confidence intervals, ECM = extracellular matrix, ESCC = esophageal squamous cell carcinoma, FFA = fundus fluorescein angiography, HWE = Hardy–Weinberg equilibrium, IA = intracranial aneurysm, LVMI = left ventricular mass index, MMP-2 = matrix metalloproteinase-2, MMPs = matrix metalloproteinases, ORs = odds ratios, PCR-RFLP = polymerase chain reaction-restriction fragment length polymorphism, RA = rheumatoid arthritis, SNPs = single nucleotide polymorphisms.
The authors have no conflicts of interest to disclose.
References
- [1].Lim LS, Mitchell P, Seddon JM, et al. Age-related macular degeneration. Lancet 2012;379:1728–38. [DOI] [PubMed] [Google Scholar]
- [2].Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med 2008;358:2606–17. [DOI] [PubMed] [Google Scholar]
- [3].Cheung LK, Eaton A. Age-related macular degeneration. Pharmacotherapy 2013;33:838–55. [DOI] [PubMed] [Google Scholar]
- [4].Qi HJ, Li XX, Zhang JY, et al. Efficacy and safety of ranibizumab for wet age-related macular degeneration in Chinese patients. Int J Ophthalmol 2017;10:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Meng Q, Huang L, Sun Y, et al. Effect of high-density lipoprotein metabolic pathway gene variations and risk factors on neovascular age-related macular degeneration and polypoidal choroidal vasculopathy in China. PLoS One 2015;10:e0143924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Holliday EG, Smith AV, Cornes BK, et al. Insights into the genetic architecture of early stage age-related macular degeneration: a genome-wide association study meta-analysis. PLoS One 2013;8:e53830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Naj AC, Scott WK, Courtenay MD, et al. Genetic factors in nonsmokers with age-related macular degeneration revealed through genome-wide gene-environment interaction analysis. Ann Hum Genet 2013;77:215–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Amin M, Pushpakumar S, Muradashvili N, et al. Regulation and involvement of matrix metalloproteinases in vascular diseases. Front Biosci (Landmark Ed) 2016;21:89–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].De Groef L, Van Hove I, Dekeyster E, et al. MMPs in the neuroretina and optic nerve: modulators of glaucoma pathogenesis and repair? Invest Ophthalmol Vis Sci 2014;55:1953–64. [DOI] [PubMed] [Google Scholar]
- [10].Schierwagen R, Leeming DJ, Klein S, et al. Serum markers of the extracellular matrix remodeling reflect antifibrotic therapy in bile-duct ligated rats. Front Physiol 2013;4:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hosokawa T, Nakajima H, Doi Y, et al. Increased serum matrix metalloproteinase-9 in neuromyelitis optica: implication of disruption of blood-brain barrier. J Neuroimmunol 2011;236:81–6. [DOI] [PubMed] [Google Scholar]
- [12].Tasaki A, Shimizu F, Sano Y, et al. Autocrine MMP-2/9 secretion increases the BBB permeability in neuromyelitis optica. J Neurol Neurosurg Psychiatry 2014;85:419–30. [DOI] [PubMed] [Google Scholar]
- [13].Yue H, Hu K, Liu W, et al. Role of matrix metalloproteinases in radiation-induced lung injury in alveolar epithelial cells of Bama minipigs. Exp Ther Med 2015;10:1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Elliot SJ, Catanuto P, Espinosa-Heidmann DG, et al. Estrogen receptor beta protects against in vivo injury in RPE cells. Exp Eye Res 2010;90:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Berglin L, Sarman S, van der Ploeg I, et al. Reduced choroidal neovascular membrane formation in matrix metalloproteinase-2-deficient mice. Invest Ophthalmol Vis Sci 2003;44:403–8. [DOI] [PubMed] [Google Scholar]
- [16].Hussain AA, Lee Y, Zhang JJ, et al. Disturbed matrix metalloproteinase activity of Bruch's membrane in age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52:4459–66. [DOI] [PubMed] [Google Scholar]
- [17].Davis MD, Gangnon RE, Lee LY, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol 2005;123:1484–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hall JB, Chou ST, Louis CJ. The expression of Lewis antigens in neoplasms of the gastrointestinal tract. Pathology 1989;21:239–47. [DOI] [PubMed] [Google Scholar]
- [19].Ortak H, Demir S, Ates O, et al. The role of MMP2 (-1306C > T) and TIMP2 (-418 G > C) promoter variants in age-related macular degeneration. Ophthalmic Genet 2013;34:217–22. [DOI] [PubMed] [Google Scholar]
- [20].Zhang L, Xi RX, Zhang XZ. Matrix metalloproteinase variants associated with risk and clinical outcome of esophageal cancer. Genet Mol Res 2015;14:4616–24. [DOI] [PubMed] [Google Scholar]
- [21].Low SK, Zembutsu H, Takahashi A, et al. Impact of LIMK1, MMP2 and TNF-alpha variations for intracranial aneurysm in Japanese population. J Hum Genet 2011;56:211–6. [DOI] [PubMed] [Google Scholar]
- [22].Sun R, Huang Y, Zhang H, et al. MMP-2, TNF-alpha and NLRP1 polymorphisms in Chinese patients with ankylosing spondylitis and rheumatoid arthritis. Mol Biol Rep 2013;40:6303–8. [DOI] [PubMed] [Google Scholar]
- [23].Lacchini R, Jacob-Ferreira AL, Luizon MR, et al. Common matrix metalloproteinase 2 gene haplotypes may modulate left ventricular remodelling in hypertensive patients. J Hum Hypertens 2012;26:171–7. [DOI] [PubMed] [Google Scholar]
- [24].Singh N, Hussain S, Sharma U, et al. The protective role of the -1306C>T functional polymorphism in matrix metalloproteinase-2 gene is associated with cervical cancer: implication of human papillomavirus infection. Tumour Biol 2016;37:5295–303. [DOI] [PubMed] [Google Scholar]
- [25].Juiz NA, Cayo NM, Burgos M, et al. Human polymorphisms in placentally expressed genes and their association with susceptibility to congenital trypanosoma cruzi infection. J Infect Dis 2016;213:1299–306. [DOI] [PubMed] [Google Scholar]
- [26].Hua Y, Song L, Wu N, et al. Polymorphisms of MMP-2 gene are associated with systolic heart failure risk in Han Chinese. Am J Med Sci 2009;337:344–8. [DOI] [PubMed] [Google Scholar]
- [27].Perez-Hernandez N, Vargas-Alarcon G, Martinez-Rodriguez N, et al. The matrix metalloproteinase 2-1575 gene polymorphism is associated with the risk of developing myocardial infarction in Mexican patients. J Atheroscler Thromb 2012;19:718–27. [DOI] [PubMed] [Google Scholar]
- [28].Hua Y, Song L, Wu N, et al. Polymorphisms of MMP-2 gene are associated with systolic heart failure prognosis. Clin Chim Acta 2009;404:119–23. [DOI] [PubMed] [Google Scholar]
- [29].Treumer F, Klettner A, Baltz J, et al. Vectorial release of matrix metalloproteinases (MMPs) from porcine RPE-choroid explants following selective retina therapy (SRT): towards slowing the macular ageing process. Exp Eye Res 2012;97:63–72. [DOI] [PubMed] [Google Scholar]
- [30].Freije JM, Balbin M, Pendas AM, et al. Matrix metalloproteinases and tumor progression. Adv Exp Med Biol 2003;532:91–107. [DOI] [PubMed] [Google Scholar]
- [31].Yang SJ, Jo H, Kim JG, et al. Baicalin attenuates laser-induced choroidal neovascularization. Curr Eye Res 2014;39:745–51. [DOI] [PubMed] [Google Scholar]
- [32].Steen B, Sejersen S, Berglin L, et al. Matrix metalloproteinases and metalloproteinase inhibitors in choroidal neovascular membranes. Invest Ophthalmol Vis Sci 1998;39:2194–200. [PubMed] [Google Scholar]
- [33].Zeng R, Wen F, Zhang X, et al. Serum levels of matrix metalloproteinase 2 and matrix metalloproteinase 9 elevated in polypoidal choroidal vasculopathy but not in age-related macular degeneration. Mol Vision 2013;19:729–36. [PMC free article] [PubMed] [Google Scholar]
- [34].Kawasaki R, Yasuda M, Song SJ, et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology 2010;117:921–7. [DOI] [PubMed] [Google Scholar]
- [35].Yang K, Liang YB, Gao LQ, et al. Prevalence of age-related macular degeneration in a rural Chinese population: the Handan Eye Study. Ophthalmology 2011;118:1395–401. [DOI] [PubMed] [Google Scholar]


