Abstract
The aim of this pilot study was to determine health-related quality of life (HRQoL) in patients with history of medication overuse headache (MOH) after detoxification and a headache-specific inpatient rehabilitation program and to receive necessary information for future prospective studies.
HRQoL and headache-related disability were cross-sectionally measured by Short Form 36 (SF-36), Hospital Anxiety and Depression Scale (HADS), Migraine Disability Score (MIDAS), Coping Strategies Questionnaire (CSQ), and Symptom Checklist 90 revised (SCL-90-R). SF-36, HADS, and SCL-90-R data were compared to German population norms, stratified by age, sex, and comorbidities.
Fifty-one patients (72.5% females, mean age 47.3 years) were included with an average headache duration of 25.3 years. Moderate to high levels of headache were reported on the MIDAS VAS at 6.51 (range 0–10); SF-36 bodily pain was 40.3 (norm = 59.0, P < .001, 100 = best). Impaired functioning averaged at 78.4 (100 = no impairment) on the MIDAS. In contrast, SF-36 physical functioning was comparable to the norm (mean: 78.4, norm = 81.8, P = .63). All other SF-36 scales were significantly lower than expected from the norm (all P < .001). The scales depression, anxiety, obsessive-compulsive, and interpersonal sensitivity were significantly affected, whereas the levels of SCL-90-R schizophrenia nuclear and schizotypia were not lower than the norm. Coping with pain was moderate.
This pilot study is the first that presents a comprehensive and simultaneously specific assessment of health and quality of life of MOH patients after detoxification and inpatient rehabilitation. Moderate to high levels of pain and self-reported disability owing to headache were observed, whereas physical function on the SF-36 was not different from the expected level of the norm. Mental health was substantially affected in several dimensions, which had been described to reduce the ability to cope with pain. MOH patients seem to have high expectations of functionality, low symptomatology, and intact well-being.
Keywords: health, inpatient rehabilitation, medication overuse headache, outcome, quality of life
1. Introduction
Medication overuse headache (MOH) is a chronic headache syndrome associated with regular overuse of acute or symptomatic headache medication on at least 10 days per month (for triptans, opioids, ergotamin, combination of analgesics) or 15 (for simple analgesics, salicylate, non-steroidal anti-inflammatory drugs [NSAIDs]) during a period of a minimum of 3 months.[1] The definition of MOH is focused on the substance and duration of substance use, but not on the primary form of headache (e.g., tension-type headache or migraine), which is the underlying etiology for substance overuse. MOH is common among patients with chronic headache and has a prevalence of up to 1% in the adult population of different countries.[2,3] In the EU, the total annual cost of MOH among adults aged 18 to 65 years amounts to €37 billion reflecting the high public health impact.[4]
Compared to patients with migraine without medication overuse, MOH was associated with more depression, lower self-efficacy, and weak pain-coping, but more pain-induced disability when measured by self-assessment instruments.[5] MOH patients suffered from anxiety and depression and reduced quality of life as rated on the Short Form 36 (SF-36).[6,7]
The withdrawal of the overused acute headache medication is considered as the causal treatment of MOH.[8,9] A majority of MOH patients improve after discontinuation of the overused substances.[7] Average medication use after detoxification decreased by about 14% and average annual costs for medication by 24%.[10] Inpatient and outpatient detoxification programs are effective in reducing medication and in decreasing headache frequency.[11] Dropout rates in outpatient programs are higher than in inpatient programs.[11] In particular, patients with “complicated MOH” benefit from a structured detoxification program.[12] Nevertheless, there is a lack of studies providing evidence for specific treatment strategies.[13] However, consensus reflected in guidelines highlights withdrawal as the major strategy.[3,14]
After detoxification, the relapse rate of MOH patients is high, especially in the first year, but decreases thereafter.[15] Experts recommend interdisciplinary rehabilitation after the detoxification to ensure long-lasting effects.[16] Further goals of multidisciplinary treatment are education, improvement of therapy to reduce headache frequency, and to increase quality of life.[16] There is lacking evidence as to which therapeutic elements have to be part of a multidisciplinary treatment approach.
There is only little empirical research about intensive multidisciplinary headache treatment programs for MOH, migraine, and tension-type headache, but some multimodal treatment approaches were shown to be effective for MOH and primary headaches.[17,18] The study of Gunreben et al[18] presented pre- and postdata of intensive multidisciplinary pain program in terms of number of headache days per month, headache intensity, and depression. Pre- and post-data of standardized outcome instruments were not presented. At present, there are no published data on comprehensive health-related quality of life (HRQoL) from patients who underwent an intensive interdisciplinary MOH rehabilitation program after detoxification available.
The goal of this study was to quantify comprehensively as well as condition-specifically HRQoL for patients with MOH after detoxification and subsequent MOH rehabilitation program using standardized patient-reported outcome measures (PROMs). Data were compared to available normative values obtained by general population surveys. The hypothesis was that there were still health deficits in some health dimensions, especially in mental and psychosocial health scales, even after successful detoxification and subsequent rehabilitation. The findings help to get an idea of the health and impairment of those patients as well as the usefulness and feasibility of the generic and condition-specific PROMs. This should give a basis to plan future prospective studies, which quantify intervention effects.
2. Methods
2.1. Patients
This cross-sectional pilot study examined patients having participated in the Zurzach headache program (Zurzacher Kopfschmerz Programm, ZKP) at the rehabilitation center “RehaClinic,” Bad Zurzach, Switzerland, between July 2012 and June 2014, by means of a postal survey. The conditions for entry to the ZKP were diagnosis of MOH, confirmed by board-certified neurologists. Cross-sectional assessment was performed 0.5 to 2.5 years after the end of the ZKP.
Exclusion criteria for the ZKP and the study were: Abuse of benzodiazepines; serious psychiatric comorbidity such as psychosis or suicidality; severe somatic illness requiring specific treatment and preventing participation in the ZKP, for example, cancer, inflammatory rheumatic disease, serious other neurological diseases that prevented participation in the program (e.g., dementia); nonadherence for the correct intake of prescribed medication or regular participation in all therapies of the ZKP; insufficient German language skills to understand the study questionnaire. The study protocol was approved by the independent local ethics commission (Health Department in Aarau, Switzerland, EK AG 2008/026). Written, signed informed consent was obtained from all participants.
2.2. Intervention
Withdrawal of overused medication was performed in an acute hospital or, for a few exceptions, on an outpatient basis and with neurological supervision, directly before admission. This means that detoxification was completed before inpatient rehabilitation. During detoxification, all analgesics and triptans were stopped on the first day and were replaced by prednisone 100 mg per day for 5 days.[19,20] Prophylactic medication was started on the first day according to the treatment recommendations of the Swiss Headache Society.[21] If an analgesic reserve was needed, a substance from a class of acute headache medication other than the previously (over)used was provided, for example, an NSAID instead of a triptan.
Immediately after acute withdrawal, patients were admitted to the ZKP, a comprehensive and multimodal inpatient rehabilitation lasting 2 to 3 weeks which was established in 2010. The concept includes multidisciplinary therapies provided by a team of neurologists, behavioural and clinical psychologists, physical therapists, and nurses. The concept is standardized and in line with international recommendations.[16] Each week, patients had 3 physician visits and 2 to 3 consultations with psychologists, including patient education. The standardized weekly program also included daily physical therapy and aerobic exercise, 2 sessions of relaxation therapy, 2 to 3 sessions of medical massage, 2 to 3 sessions of acupuncture if applicable, individual use of thermal water, and medical training therapy. The treatment team met weekly to discuss the progress of the patients. During the period of this study, the physicians and therapists remained stable.
2.3. Measures
All medical records from neurologists and other physicians were obtained to confirm diagnosis of MOH, determine comorbidities, body height, and weight. Sociodemographic and disease-relevant data were measured using a standardized questionnaire that has already shown its usefulness in other studies.[22,23] The pain Numeric Rating Scale (NRS) was used, as it had been described to be easier to comprehend and to handle than the visual analogue scale (VAS), with comparable psychometric properties.[24,25]
The Short Form 36 (SF-36) is a self-assessment questionnaire that is used to measure health and HRQoL on 4 physical/somatic scales (physical functioning, role physical, bodily pain, general health) and 4 psychosocial/mental scales (vitality, social functioning, role emotional, mental health) comprising 36 items.[26,27] A complex linear-combination of all 8 scales provides the summary scales, with specific weights for the physical component summary (PCS) and different ones for the mental component summary (MCS).[27] Population survey-based normative data for the SF-36 exist and allow quantification stratified by sex, age (5-year classes), and comorbidity (present/absent).[28] These data had been collected in Germany, having comparable language and cultural properties, as in the German-speaking part of Switzerland.[28,29] The SF-36 is the best tested and most often used generic outcome measure having proven its validity and reliability in numerous studies.[30] Validity in measurement of pain, physical and social function, depression, and anxiety in chronic pain conditions have been proven.[24]
The Migraine Disability Assessment Score (MIDAS) was used to assess the condition-specific disability.[31,32] It records headache-related disability by measuring missing days because of headache in the last 3 months on 5 items: school or paid work: number of days missed, number of days with half performance or lower; household work: number of days missed, number of days with half performance or lower; family, social or leisure activities: number of days missed.[31] The number of days is summed up using items 1 to 5 and rescaled to a score of 0 = all days missed/disabled and 100 = full-working capacity on all days. In addition, the number of days with headache in the last 3 months is recorded, and pain is quantified on an NRS 0 to 10.
The Coping Strategies Questionnaire (CSQ) evaluates active and passive coping strategies and is internationally used in chronic pain conditions.[33–35] The German version is cross-culturally adapted and validated.[34] Catastrophizing and the 2 items “control over pain” and “ability to decrease pain” were included in this study because their clinical significance for MOH was considered to be most appropriate and the responsiveness to change was optimal.[24,36]
Psychopathological assessment was performed by the Hospital Anxiety and Depression Scale (HADS) and the Symptom Checklist 90 revised (SCL-90-R). This was done because the association between psychopathological symptoms and headache had been shown to be elevated in migraine patients when compared to controls without migraine, whereas this was not the case in patients with tension-type headache.[37]
The HADS is a questionnaire for patients of nonpsychiatric settings consisting of 14 items, 7 for anxiety and 7 for depression.[38,39] German population survey-based normative data are stratified by sex and age (10-year classes).[40]
The SCL-90-R is a self-report of psychopathological symptoms, especially for nonpsychiatric populations.[41,42] Patients rate distress on a 5-step Likert-scale on 90 items. Based on our clinical experience, the following specific scales that are assumed to be most important for MOH were selected: obsessive-compulsive, interpersonal sensitivity, schizotypia, and schizophrenic nuclear. The latter 2 scales are constructs based on a large population-based survey.[43] Depression and anxiety were already covered by the HADS. Population norms, stratified by sex and age using 10-year intervals from Germany, were used for comparison.[42] The normative data from the scale paranoid ideation were compared with the scale schizophrenia nuclear and those of the scale psychoticism were compared with the scale schizotypia.[44,45]
2.4. Statistical analysis
Unless indicated differently (e.g., for the pain NRS), all instrument scores were scaled from 0 = worst to 100 = best health/function/ability in accordance with the original scoring of the SF-36 to ease comparison with scores obtained from other instruments.[23] The specific “missing rules” of the instruments had to be fulfilled for determination of the scales. This means that at least 50% of the items had to be completed for each of the SF-36 scales and 6 of 7 for each of the HADS scales.[27,38] For the CSQ, MIDAS, and the SCL-90-R, no “missing rules” were specified in the original reports; thus, the “2 of 3 (67%) rule” was applied as described for other instruments.[29,34] Owing to the metric properties of the determination of the scales and to have a useful minimal number for metric statistics, a minimal number of n > 30 was set to be required with a wishful number of n ≥50 for this pilot study.[46]
All analyses were performed using the statistical software package IBM SPSS 22.0 for Windows (SPSS Inc, Chicago, IL). Two-tailed significance tests were performed by the nonparametric Wilcoxon test for continuous data to ensure appropriateness for all distributions, not only the Gaussian.
3. Results
3.1. Patients
Between July 2012 and June 2014, 106 patients with a confirmed MOH diagnosis were treated in the program, of which 83 (78%) could be contacted by telephone (max. 7 attempts). The remaining 23 received the assessment set without previous contact. Of those who were contacted by phone, 8 refused participation, and 7 had insufficient German language skills. Of the 91 mailed sets, 51 (56%) were returned with complete data. Of the 40 sets that were not returned, 4 patients withdrew willingness to participate; for 36 sets, no reasons for decline were given (Fig. 1). Time since the end of the pain program ranged between 0.5 and 2.5 years (Table 1).
Figure 1.
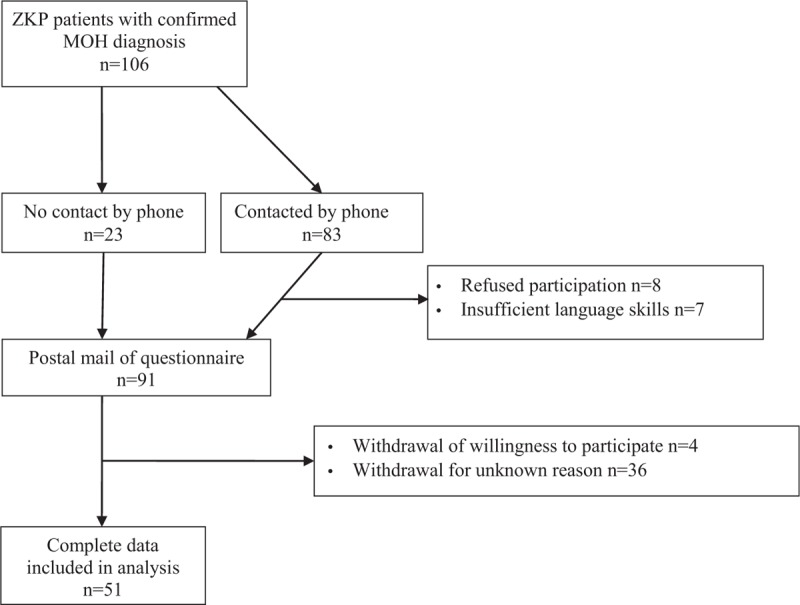
Patient selection. MOH = medication overuse headache, ZKP = Zurzach headache program (Zurzacher Kopfschmerz Programm).
Table 1.
Sociodemographic and disease-relevant data (n = 51).
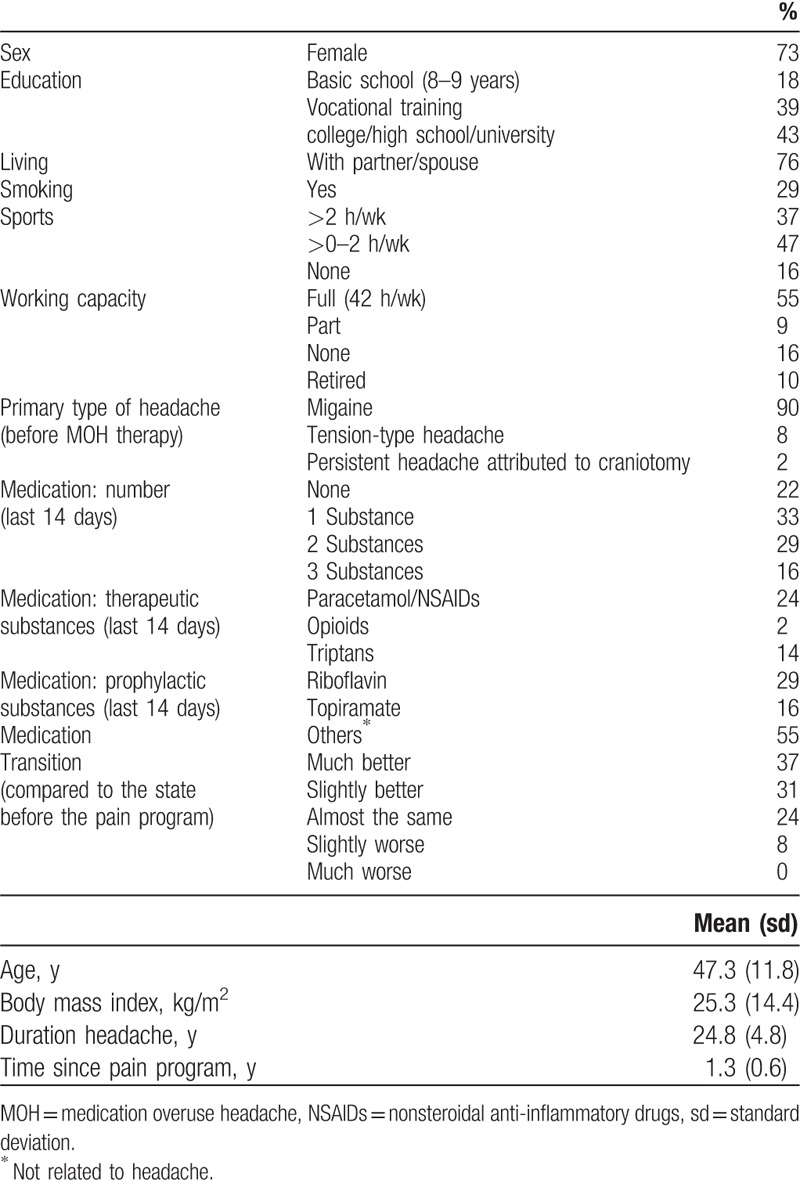
The median study participant was female, middle-aged, lived with a partner or spouse, was educated to the level of vocational training or less, worked full-time, did not smoke, and performed sports up to 2 hours/week (Table 1). The individuals had suffered from headache for 25 years, had taken 1 substance against headache in the last 2 weeks before assessment, and felt much better or slightly better in comparison to their state before the pain program.
In the last 14 days before assessment, 2 or 3 different pain medications (either acute or prophylactic) were taken by 45% of patients. A minority still took acute headache medication: 24% paracetamol/NSAIDs, 14% triptans, and 2% opioids. Prophylactic medication (e.g., riboflavin or topiramate) was taken by 45%.
3.2. Outcome
Pain intensity levels of the pain scales were moderate at the time of the assessment (mean: 3.3/10 points = maximal pain) up to severe (6.8/10) at the worst moment of the last 7 days before, corresponding to a score of 6.5 of 10 on the MIDAS (Table 2). The MIDAS revealed on average 40.1% (37.7/92) days with headache within the last 3 months. Of the 51 patients, 33 (65%) reported <45 days with headache in the last 3 months. The number of days with impaired function owing to headache (sum of the items 1–5) averaged to a value of 59.5 (of 276), which corresponds to 21.6%. Mean pain on the SF-36 (last 4 weeks) was 40.3 (100 = no pain, which would correspond to 6.0 on the VAS) and is significantly higher than expected from the normative data for the general population (mean: 58.1, P < .001).
Table 2.
Outcomes for medication overuse headache patients after an inpatient pain program (n = 51).
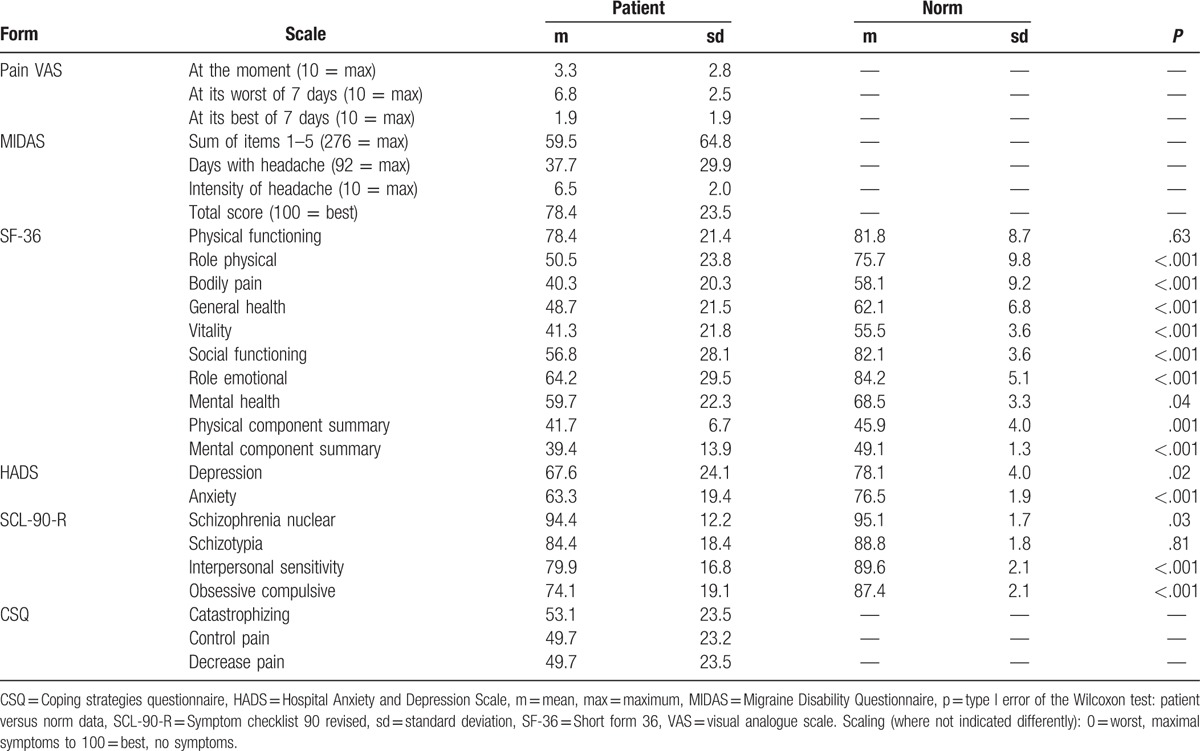
Physical functioning was not affected on the SF-36 when compared to the norm (means: 78.4 vs. 81.8, P = .66; 100 = best). All other scales of the SF-36 showed highly significant (P ≤ .001) impairments with levels far from the expected scores, for example, general health 48.7 versus 62.1 and social functioning 56.8 versus 82.1. The only exception is SF-36 mental health: 59.7 versus 68.5 = norm, P = .04.
Consistently, HADS depression and anxiety were much higher than expected from the normative levels: 67.6 versus 78.1, P = .02 and 63.3 versus 76.5, P < .001. The same was true for the obsessive/compulsive scale (74.1 vs. 87.4) and, somewhat less, for the interpersonal sensitivity scale (79.9 vs. 89.6) of the SCL-90-R. In contrast, patients were not more schizotypal (84.4 vs. 88.8, P = .81), but were less “schizophrenic” (schizophrenia nuclear: 94.4 vs. 95.1) than expected. In the SCL-90-R schizophrenia nuclear, the median of the patients was 100, whereas the mean norm was 95.3, which led to a significant difference (P = .03) in favor of the patients.
Coping with pain showed moderate levels in the middle range of the scale on the CSQ (catastrophizing: 53.1). This corresponds to the 37% replies of “much better” and 31% “slightly better” on the transition item (Table 1).
4. Discussion
This cross-sectional pilot study quantified health and HRQoL in patients with history of MOH after detoxification and a subsequent headache-specific inpatient rehabilitation program with a comprehensive set of generic and condition-specific questionnaires. The study did not aim to quantify treatment effects, but to provide data about patients’ state of health and appropriateness of the assessment as base for planning future prospective studies. Where possible, empirical data were compared to normative values obtained by general population surveys. The hypothesis that deficits persisted in some health dimensions after withdrawal and subsequent rehabilitation was confirmed and psychopathological symptoms persisted. In our sample, MOH was associated with female sex, middle age (40–50 years), and relatively low education level. These characteristics are consistent with many other European studies.[6,18,47–51]
Substantial burden of headache was measured by the NRS, the MIDAS, and the SF-36—the score of SF-36 bodily pain showed statistically significant difference from the norm. The same was true for all other scales of the SF-36, except for the SF-36 physical functioning. The number of days with headache and with consequent limitations at work and in daily life on the MIDAS showed levels compatible with significant burden. Comparison to norms is not possible because of lack of data. Affective symptoms as well as obsessive and compulsive symptoms and interpersonal sensitivity were significantly increased, whereas schizotypal or schizophrenic symptoms were not. Finally, ability to cope with pain was moderate and comparable to other chronic pain patients.[26]
The pattern of our empirical SF-36 scores compared with that of the norm scores was similar to that of previous studies: the highest differences were in role physical, bodily pain, social functioning, and the lowest were in physical functioning. This pattern was consistent with a population-based sample in Spain (n = 74 MOH).[6] However, in that study, the smallest difference in SF-36 physical functioning still reached significance, whereas it did not in our data. A previously conducted survey by the same study group (n = 22 MOH) found SF-36 scores, which were much lower than those of our patients for physical functioning (59.2 vs. 78.4 in our study) and mental health (48.2 vs. 59.7 in our study), whereas the other scales were comparable.[52]
To the best of our knowledge, no further study exists, using either the SF-36 or the SF-12 to compare MOH (after detoxification and headache specific rehabilitation) with healthy controls, whereas several studies of chronic daily headache sufferers have been published. One review of comparative studies concluded that 3 of 5 studies having used SF-36 or SF-12 suggested that MOH patients have worse HRQoL than those with chronic headache without medication overuse.[53] However, the reviewed data presented mean differences between different studies in bar plots and did not provide the exact figures.[53] For this reason, it is important to know—besides the previous diagnosis apart from MOH—whether the patients had a history of MOH or not. The primary diagnosis of most patients in this study was migraine (92%, Table 1).
The SF-36 PCS and MCS scores of this present study were comparable to a study conducted at the 6-month follow-up after an inpatient withdrawal therapy for MOH: the mean PCS at follow-up was 43.2 versus 41.7 in our study, the MCS 42.2 versus 39.4, respectively.[54] At the 6-month follow-up, both scores showed significant improvement when compared to baseline.[54]
In a study about the effectiveness of an intensive multidisciplinary headache treatment program lasting 96 hours, pretreatment PCS was 37.9 and MCS was 39.0.[18] Post-treatment SF-36 were not reported. However, patients significantly improved in the depression and in the number of days with headache, but not on pain intensity.
On the MIDAS, MOH-related disability continuously decreased during the follow-up period after an inpatient withdrawal treatment from an average of 70.8 (MIDAS sum of items 1–5) before treatment to 34.1 one year later and to 17.0 at 5 years.[55] These post-treatment levels were lower than ours at 59.5. In contrast, our data are comparable to the baseline MIDAS score of 59.9 of a multicenter, multinational study.[56] Those cases improved to a score of 25.7 at the 6-month follow-up. This means that the posttreatment scores of our patients indicated much greater disability because of headache than in the 2 aforementioned studies. Based on the observed improvements on the MIDAS score as reported in 4 treatment studies, it may be hypothesized that our sample was a selection of highly disabled patients admitted to intensive inpatient rehabilitation.[55–58]
Scores for schizophrenia, and equally schizotypia, were significantly lower, but scores for interpersonal sensitivity and obsessive compulsive (symptoms) were significantly higher, and scores for depression and anxiety were higher than expected from the norm. Schizophrenia nuclear is a milder version of the core diagnostic symptoms of psychosis, which is thought to be a biologically based perception disorder.[43] Schizotypal signs are characterized by generalized distrust, odd interpersonal beliefs, and paranoid ideation, which lead to a distorted perception of the environment, influenced by psychosocial conditions.[43] The 2 symptom dimensions show relatively high overlap on the psychopathological level, but coincide only partly.[43] They are expression of psychotic symptoms with different levels of severity and persistence on the continuum of the psychotic phenotype.[59] This is consistent with previous studies. MOH was associated with obsessive-compulsive disorders; in one study, the prevalence of the latter was 28%.[60] Psychiatric comorbidities are predictors for prognosis and response to treatment.[60–62] A small study showed a significantly increased risk of suffering from overall mood disorders (odds ratio, OR = 4.5), anxiety (OR = 5.0) and disorders associated with the use of psychoactive substances other than analgesics (OR = 7.6) in MOH sufferers when compared to patients with migraine only (n = 41 in both groups).[63] The HADS anxiety score of 63.3 in our study is comparable to a 3-month follow-up score of 66.7[62] and a 6-month follow-up score of 66.2,[56] whereas the depression score in our study (67.6) is lower when compared to the aforementioned studies (81.0 at 3-month follow-up; 80.4 at 6-month follow-up).
In this cross-sectional pilot study, the combination of generic and condition-specific assessments provided comprehensive but also disease-specific measurements of health and HRQoL. This is the strength of the study. The questionnaire set gave moderate respondent and administrative burden and the single instruments turned out to be appropriate for MOH. Furthermore, the instruments were internationally used, validated, and standardized, and allowed comparison of empirical with normative data for some instruments. Even after completion of the therapies, deficits on various health dimensions could still be measured as compared to normative data. This means that the instruments used did not reveal ceiling effects, which may have reduced validity of the measurements. Further strengths were a compact cohort with representative sociodemographic characteristics and a standardized intervention throughout the whole period of treatment.
The cross-sectional design of this study does not allow the evaluation of health changes or evaluation of the therapeutic effects of the rehabilitation program. Furthermore, this study does not allow a conclusion about the compliance of the patients to the recommended treatment and instructions after the rehabilitation program. However, the study aimed to provide basic data on the state of health and quality of life after specific treatment for MOH in a sample of severely affected patients. A further limitation is that the time interval between the completion of the headache program and the postal survey varied between 0.5 and 2.5 years. The sample size of 51 out of a patient population of 106 with confirmed MOH diagnosis is limited and cannot exclude a selection bias, for example, towards patients with better health status, which is an inherent problem in every voluntary survey.
This study is the first that presents a comprehensive and simultaneously specific assessment of health and quality of life of MOH patients after detoxification and inpatient rehabilitation. Moderate to high levels of pain and self-reported disability towing to headache were observed, whereas physical function on the SF-36 was not different from the expected level of the norm. This may be because of high expectations of functionality and corresponds to low self-reported coping with pain, relatively high catastrophizing, depression, and anxiety. The data of this cross-sectional pilot study sampled over 2 years can be taken as valid estimate of the outcome of the pain program in our clinic. Generalizability to other pain programs should be done with care because comparable data with standardized instruments are lacking from other programs. Future longitudinal data will provide quantification of treatment effects and refine insight into processes of treatment, individual developments, and need specific to MOH during the course of treatment and recovery.
Acknowledgments
The authors thank Joy Buchanan for the English language editing and all patients for participating in the study.
Footnotes
Abbreviations: CSQ = coping strategies questionnaire, ES = effect size, HADS = Hospital Anxiety and Depression Scale, m = Mean, max = maximum, MCS = mental component summary, MIDAS = Migraine Disability Assessment Score, MOH = medication overuse headache, NRS = numeric rating scale, NSAIDs = non-steroidal anti-inflammatory drugs, p = type I error of the Wilcoxon test, PCS = physical component summary, PROM = patient-reported outcome measure, SCL-90-R = Symptom Checklist 90 revised, sd = standard deviation, SF-36 = short form 36, VAS = visual analogue scale, ZKP = Zurzach headache program (Zurzacher Kopfschmerz Programm).
The authors report no conflicts of interest.
References
- [1].Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- [2].Stovner L, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 2007;27:193–210. [DOI] [PubMed] [Google Scholar]
- [3].Goadsby PJ. Goadsby PJ, Silverstein SD, Dodick DW. Medication Overuse Headache. Chronic Daily Headache for Clinicians. Har/Cdr. Hamilton, Ontario; Lewiston, NY: B C Decker Inc; 2005. 117–25. [Google Scholar]
- [4].Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol 2012;19:703–11. [DOI] [PubMed] [Google Scholar]
- [5].Fritsche G, Nitsch C, Pietrowsky R, et al. Psychological descriptors of excessive use of analgesic medication [Article in German]. Schmerz Berl Ger 2000;14:217–25. [DOI] [PubMed] [Google Scholar]
- [6].Colás R, Muñoz P, Temprano R, et al. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology 2004;62:1338–42. [DOI] [PubMed] [Google Scholar]
- [7].Bendtsen L, Jensen R, Munksgaard S, et al. Disability caused by medication-overuse headache can be considerably reduced by detoxification. Results from multinational COMOESTAS study. J Headache Pain 2013;14:227. [DOI] [PubMed] [Google Scholar]
- [8].Hagen K, Albretsen C, Vilming ST, et al. A 4-year follow-up of patients with medication-overuse headache previously included in a randomized multicentre study. J Headache Pain 2011;12:315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Olesen J. Detoxification for medication overuse headache is the primary task. Cephalalgia 2012;32:420–2. [DOI] [PubMed] [Google Scholar]
- [10].Shah AM, Bendtsen L, Zeeberg P, et al. Reduction of medication costs after detoxification for medication-overuse headache. Headache 2013;53:665–72. [DOI] [PubMed] [Google Scholar]
- [11].Tassorelli C, Jensen R, Allena M, et al. A consensus protocol for the management of medication-overuse headache: Evaluation in a multicentric, multinational study. Cephalalgia 2014;34:645–55. [DOI] [PubMed] [Google Scholar]
- [12].Rossi P, Faroni JV, Tassorelli C, et al. Advice alone versus structured detoxification programmes for complicated medication overuse headache (MOH): a prospective, randomized, open-label trial. J Headache Pain 2013;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rossi P, Jensen R, Nappi G, et al. COMOESTAS Consortium. A narrative review on the management of medication overuse headache: the steep road from experience to evidence. J Headache Pain 2009;10:407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Diener HC. Leitlinie für Diagnostik und Therapie in der Neurologie: Kopfschmerz bei Übergebrauch von Schmerz- und Migränemitteln [Deutsche Gesellschaft für Neurologie Web site]. October 29, 2015. Available at: http://www.dgn.org/leitlinien/2286-ll-57-2012-kopfschmerz-bei-uebergebrauch-von-schmerz-und-migraenemitteln. Accessed March 11, 2016. [Google Scholar]
- [15].Katsarava Z, Muessig M, Dzagnidze A, et al. Medication overuse headache: rates and predictors for relapse in a 4-year prospective study. Cephalalgia 2005;25:12–5. [DOI] [PubMed] [Google Scholar]
- [16].Gaul C, Visscher CM, Bhola R, et al. Team players against headache: multidisciplinary treatment of primary headaches and medication overuse headache. J Headache Pain 2011;12:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gunreben-Stempfle B, Griessinger N, Lang E, et al. Effectiveness of an intensive multidisciplinary headache treatment program. Headache 2009;49:990–1000. [DOI] [PubMed] [Google Scholar]
- [18].Katsarava Z, Obermann M. Medication-overuse headache. Curr Opin Neurol 2013;26:276–81. [DOI] [PubMed] [Google Scholar]
- [19].Rabe K, Pageler L, Gaul C, et al. Prednisone for the treatment of withdrawal headache in patients with medication overuse headache: a randomized, double-blind, placebo-controlled study. Cephalalgia 2013;33:202–7. [DOI] [PubMed] [Google Scholar]
- [20].Pageler L, Katsarava Z, Diener HC, et al. Prednisone vs. placebo in withdrawal therapy following medication overuse headache. Cephalalgia 2008;28:152–6. [DOI] [PubMed] [Google Scholar]
- [21].Andrée C. Therapieempfehlungen. [Schweizerische Kopfwehgesellschaft Web site] 9. revidierte Auflage 2014. Available at: http://www.headache.ch/Therapieempfehlungen. Accessed April 24, 2016. [Google Scholar]
- [22].Angst F, Aeschlimann A, Steiner W, et al. Responsiveness of the WOMAC osteoarthritis index as compared with the SF-36 in patients with osteoarthritis of the legs undergoing a comprehensive rehabilitation intervention. Ann Rheum Dis 2001;60:834–40. [PMC free article] [PubMed] [Google Scholar]
- [23].Angst F, Brioschi R, Main CJ, et al. Interdisciplinary rehabilitation in fibromyalgia and chronic back pain: a prospective outcome study. J Pain 2006;7:807–15. [DOI] [PubMed] [Google Scholar]
- [24].Angst F, Verra ML, Lehmann S, et al. Responsiveness of five condition-specific and generic outcome assessment instruments for chronic pain. BMC Med Res Methodol 2008;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].de C, Williams AC, Davies HT, et al. Simple pain rating scales hide complex idiosyncratic meanings. Pain 2000;85:457–63. [DOI] [PubMed] [Google Scholar]
- [26].Bullinger M, Kirchberger I. Fragebogen zum Gesundheitszustand: SF-36; Handanweisung. Göttingen, Germany: Hogrefe: Verlag für Psychologie; 1998. [Google Scholar]
- [27].Ware JE, Snow K K, Kosinski M, et al. SF-36 health survey: manual and interpretation guide. Boston: New England Medical Center Hospital, Health Institute; 1993. [Google Scholar]
- [28].Kurth BM, Ellert U. The SF-36 questionnaire and its usefulness in population studies: results of the German Health Interview and Examination Survey 1998. Soz- Präventivmedizin 2002;47:266–77. [DOI] [PubMed] [Google Scholar]
- [29].Angst F, Pap G, Mannion AF, et al. Comprehensive assessment of clinical outcome and quality of life after total shoulder arthroplasty: usefulness and validity of subjective outcome measures. Arthritis Rheum 2004;51:819–28. [DOI] [PubMed] [Google Scholar]
- [30].Busija L, Pausenberger E, Haines TP, et al. Adult measures of general health and health-related quality of life: Medical Outcomes Study Short Form 36-Item (SF-36) and Short Form 12-Item (SF-12) Health Surveys, Nottingham Health Profile (NHP), Sickness Impact Profile (SIP), Medical Outcomes Study Short Form 6D (SF-6D), Health Utilities Index Mark 3 (HUI3), Quality of Well-Being Scale (QWB), and Assessment of Quality of Life (AQoL). Arthritis Care Res 2011;63(suppl 11):S383–412. [DOI] [PubMed] [Google Scholar]
- [31].Stewart WF, Lipton RB, Dowson AJ, et al. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology 2001;56:S20–8. [DOI] [PubMed] [Google Scholar]
- [32].Agosti R, Chrubasik JE, Kohlmann T. Der MIDAS-Fragebogen. Ars Medici 2008;16:700–1. [Google Scholar]
- [33].Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain 1983;17:33–44. [DOI] [PubMed] [Google Scholar]
- [34].Verra ML, Angst F, Lehmann S, et al. Translation, cross-cultural adaptation, reliability, and validity of the German version of the Coping Strategies Questionnaire (CSQ-D). J Pain 2006;7:327–36. [DOI] [PubMed] [Google Scholar]
- [35].Snow-Turek AL, Norris MP, Tan G. Active and passive coping strategies in chronic pain patients. Pain 1996;64:455–62. [DOI] [PubMed] [Google Scholar]
- [36].Lafittau M, Radat F, Irachabal S, et al. Headache and transformed migraine with medication overuse: what differences between disability, emotional distress and coping? [Article in French]. L’Encéphale 2006;32:231–7. [DOI] [PubMed] [Google Scholar]
- [37].Merikangas KR, Stevens DE, Angst J. Psychopathology and headache syndromes in the community. Headache 1994;34:S17–22. [DOI] [PubMed] [Google Scholar]
- [38].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- [39].Herrmann-Lingen C, Buss U, Snaith RP. HADS-D: Hospital Anxiety and Depression Scale—Deutsche Version: ein Fragebogen zur Erfassung von Angst und Depressivität in der somatischen Medizin: Testdokumentation und Handanweisung. Bern: H Huber 1995. [Google Scholar]
- [40].Hinz A, Brähler E. Normative values for the hospital anxiety and depression scale (HADS) in the general German population. J Psychosom Res 2011;71:74–8. [DOI] [PubMed] [Google Scholar]
- [41].Derogatis LR. SCL-90-R: Administration, Scoring of Procedures Manual-II for the R (evised) Version and Other Instruments of the Psychopathology Rating Scale Series. Baltimore: Clinical Psychometric Research Incorporated; 1992. [Google Scholar]
- [42].Göttingen: Hogrefe, Franke GH. Symptom-Checklist-90-Standard: SCL-90-S. 2014. [Google Scholar]
- [43].Rössler W, Riecher-Rössler A, Angst J, et al. Psychotic experiences in the general population: a twenty-year prospective community study. Schizophr Res 2007;92:1–4. [DOI] [PubMed] [Google Scholar]
- [44].Rössler W, Ajdacic-Gross V, Haker H, et al. Subclinical psychosis syndromes in the general population: results from a large-scale epidemiological survey among residents of the canton of Zurich, Switzerland. Epidemiol Psychiatr Sci 2015;24:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Franke GH. SCL-90-R. Die Symptom-Checkliste von L. R. Derogatis (Deutsche Version). Göttingen: Beltz; 1995. [Google Scholar]
- [46].Hedderich J, Sachs L. Angewandte Statistik: Methodensammlung mit R. 2015;Berlin: Springer Spektrum, 317. [Google Scholar]
- [47].Jonsson P, Hedenrud T, Linde M. Epidemiology of medication overuse headache in the general Swedish population. Cephalalgia 2011;31:1015–22. [DOI] [PubMed] [Google Scholar]
- [48].Straube A, Pfaffenrath V, Ladwig K-H, et al. Prevalence of chronic migraine and medication overuse headache in Germany–the German DMKG headache study. Cephalalgia 2010;30:207–13. [DOI] [PubMed] [Google Scholar]
- [49].Cheung V, Amoozegar F, Dilli E. Medication overuse headache. Curr Neurol Neurosci Rep 2015;15:509. [DOI] [PubMed] [Google Scholar]
- [50].Evers S, Marziniak M. Clinical features, pathophysiology, and treatment of medication-overuse headache. Lancet Neurol 2010;9:391–401. [DOI] [PubMed] [Google Scholar]
- [51].Da Silva AN, Lake AE. Clinical aspects of medication overuse headaches. Headache 2014;54:211–7. [DOI] [PubMed] [Google Scholar]
- [52].Guitera V, Muñoz P, Castillo J, et al. Quality of life in chronic daily headache: a study in a general population. Neurology 2002;58:1062–5. [DOI] [PubMed] [Google Scholar]
- [53].Lantéri-Minet M, Duru G, Mudge M, et al. Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: a systematic review. Cephalalgia 2011;31:837–50. [DOI] [PubMed] [Google Scholar]
- [54].Zebenholzer K, Thamer M, Wöber C. Quality of life, depression, and anxiety 6 months after inpatient withdrawal in patients with medication overuse headache: an observational study. Clin J Pain 2012;28:284–90. [DOI] [PubMed] [Google Scholar]
- [55].Andrasik F, Grazzi L, Usai S, et al. Disability in chronic migraine with medication overuse: treatment effects through 5 years. Cephalalgia 2010;30:610–4. [DOI] [PubMed] [Google Scholar]
- [56].Bendtsen L, Munksgaard S, Tassorelli C, et al. Disability, anxiety and depression associated with medication-overuse headache can be considerably reduced by detoxification and prophylactic treatment. Results from a multicentre, multinational study (COMOESTAS project). Cephalalgia 2014;34:426–33. [DOI] [PubMed] [Google Scholar]
- [57].Kristoffersen ES, Straand J, Vetvik KG, et al. Brief intervention by general practitioners for medication-overuse headache, follow-up after 6 months: a pragmatic cluster-randomised controlled trial. J Neurol 2016;263:344–53. [DOI] [PubMed] [Google Scholar]
- [58].Munksgaard SB, Bendtsen L, Jensen RH. Treatment-resistant medication overuse headache can be cured. Headache 2012;52:1120–9. [DOI] [PubMed] [Google Scholar]
- [59].Rössler W, Ajdacic-Gross V, Rodgers S, et al. Childhood trauma as a risk factor for the onset of subclinical psychotic experiences: Exploring the mediating effect of stress sensitivity in a cross-sectional epidemiological community study. Schizophr Res 2016;172:46–53. [DOI] [PubMed] [Google Scholar]
- [60].Curone M, Tullo V, Savino M, et al. Outcome of patients with chronic migraine with medication overuse and depression after duloxetine: influence of coexisting obsessive compulsive disorder. Neurol Sci 2013;34:S175–7. [DOI] [PubMed] [Google Scholar]
- [61].Munksgaard SB, Jensen RH. Medication overuse headache. Headache 2014;54:1251–7. [DOI] [PubMed] [Google Scholar]
- [62].Kristoffersen ES, Straand J, Russell MB, et al. Disability, anxiety and depression in patients with medication-overuse headache in primary care - the BIMOH study. Eur J Neurol 2016;23:28–35. [DOI] [PubMed] [Google Scholar]
- [63].Radat F, Creac’h C, Swendsen JD, et al. Psychiatric comorbidity in the evolution from migraine to medication overuse headache. Cephalalgia 2005;25:519–22. [DOI] [PubMed] [Google Scholar]


