Abstract
Little is known about association between ABO blood groups and tumor markers in gastric cancer (GC) patients. The aim of the present study was to assess the prognostic value of ABO blood groups in GC patients with different levels of preoperative serum carcinoembryonic antigen (CEA).
From September 2008 to April 2015, a total of 3234 GC patients who received radical gastrectomy were retrospectively analyzed. The clinicopathological characteristics including ABO blood groups and preoperative CEA were recorded. The prognostic value of ABO blood groups within different levels of serum CEA was analyzed.
Overall, the ratio of male to female patients was 3.5:1; the median age was 57.4 years (range 20–87). The median overall survival (OS) for GC patients with blood type A, B, AB, and O were 52.6, 52.8, 53.8, and 53.6 months, respectively. There was no significant difference for the survival of patients among the 4 groups (P = .736). Also, no significant difference was found among the OS of the 4 blood types with negative (P = .875) and positive (P = .131) preoperative serum CEA. Further, we found that the OS of patients with positive preoperative serum CEA and blood type AB was significantly higher than that with blood type non-AB (P = .026). For patients with positive preoperative serum CEA, multivariate analysis showed that ABO blood groups were an independent prognostic factor.
Blood type AB was a favorable prognostic factor for GC patients with positive preoperative serum CEA.
Keywords: ABO blood groups, CEA, gastric cancer, prognosis
1. Introduction
Gastric cancer (GC) is the 4th most common malignancy around the world, and ranks the 2nd of cancer-related death in developing countries.[1] Surgery still occupies the dominating treatment for patients with resectable GC. Tumor markers produced by tumor tissue itself usually are circulating molecules and that have a casual association with malignant diseases.[2] Tumor markers are often used in the early detection of various tumors and monitoring tumor occurrence and progression, among which carcinoembryonic antigen (CEA) is the most commonly used alternative for GC.[3]
The ABO blood group system, which was first discovered by Karl Landsteiner in 1900, is the most common means to classify the type of blood groups and is most widely used in clinical practice. The prognostic impact of the ABO blood groups on survival was investigated in a series of malignancies. However, the results varied. With regard to GC, our previous study revealed that ABO blood groups were not associated with the outcomes of GC patients. However, blood type AB was a favorable prognostic factor among patients who smoked and patients with stage III tumors.[4]
As mentioned above, both blood groups and serum CEA could predict the prognosis of GC, but the connection between them remains unknown. Thus, the aim of our present study was to investigate the prognostic value of ABO blood groups in GC patients with different levels of CEA.
2. Patients and methods
2.1. Patients
This study was performed in the Xijing Hospital of Digestive Diseases affiliated to the Fourth Military Medical University. From September 2008 to April 2015, 3234 GC patients were enrolled in the present study. Inclusion criteria: diagnosed as GC pathologically; treated with gastrectomy with D2 lymphadenectomy, with complete follow-up data. Exclusion criteria: accompanied with other malignancy; with neoadjuvant chemotherapy. This study was approved by the Ethics Committee of Xijing Hospital, and written informed consent was obtained from all patients before surgery.
2.2. Clinicopathological data
Serum CEA was determined within 7 days before surgery. The serum CEA was detected using the Roche electrochemiluminescence immunoassay analyzer at the clinical laboratory of Xijing Hospital. The cut-off values for serum CEA were 5 ng/mL, according to the manufacturer's instructions.[5] Levels beyond the cut-off value were considered as positive.
All patients were treated with proximal, distal, or total gastrectomy with D2 lymphadenectomy. D2 lymphadenectomy is defined as removal of the perigastric lymph nodes and second tier of lymph nodes in the extra perigastric areas, which generally fall along branches of the celiac axis including the left gastric, splenic, common hepatic, and proper hepatic arteries.[6,7] The surgical procedure was based on the recommendations of the Japanese Gastric Cancer Treatment Guidelines.[8] Preoperative data including gender, age, smoking history, ABO blood types, tumor location, serum CEA were recorded. The tumor size, differentiation status, tumor depth, and lymph node metastasis were collected according to the pathological examination. Patients were followed every 3 months by enhanced chest and abdominal computed tomography and gastroscopy. The last follow-up time was September 2015.
2.3. Statistical analysis
SPSS 22.0 for Windows (SPSS Inc., Chicago, IL) was employed for data analysis. Overall survival (OS) time was calculated from the date of surgery to the date of death or the last follow-up. The Kaplan–Meier method was used to estimate the median OS time. Discrete variables were analyzed by Chi-squared test or Fisher exact test. Significant risk factors identified by univariate analysis were further assessed by multivariate analysis using the logistic regression analysis. A P value of .05 was used as the threshold for statistical significance.
3. Results
The clinicopathological characteristics were summarized in Table 1. There were 2514 males (77.7%) and 720 females (22.3%), with the median age being 57.4 years (range 20–87). The positive rate of serum CEA was 19.0%. Nine hundred eighty patients (30.3%) were blood type A, 935 cases (28.9%) were blood type B, 331 cases (10.2%) were blood type AB, and 988 cases (30.6%) were blood type O. Nine hundred ninety-two tumors (30.7%) exceeded 5 cm in diameter. Three hundred fifty-nine tumors (11.1%) were well differentiated, 814 tumors (25.2%) were moderately, 1882 tumors (58.2%) were poorly, and 179 tumors (5.5%) were signet ring cell or mucinous. With respect to tumor depth, 617 tumors (19.1%) were stage T1, 495 tumors (15.3%) were stage T2, 1165 tumors (36.0%) were stage T3, and 957 (29.6%) tumors were stage T4.
Table 1.
Clinicopathological characteristics of 3234 gastric cancer patients.
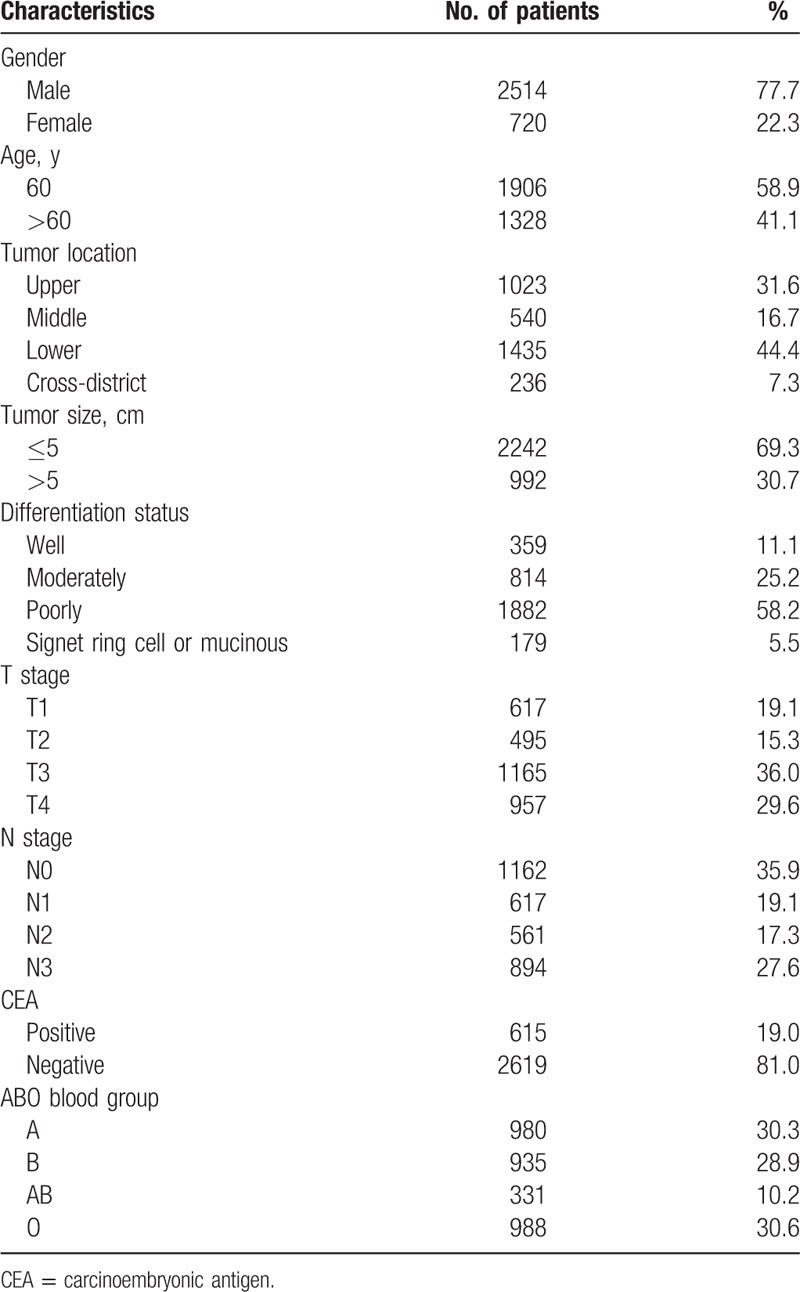
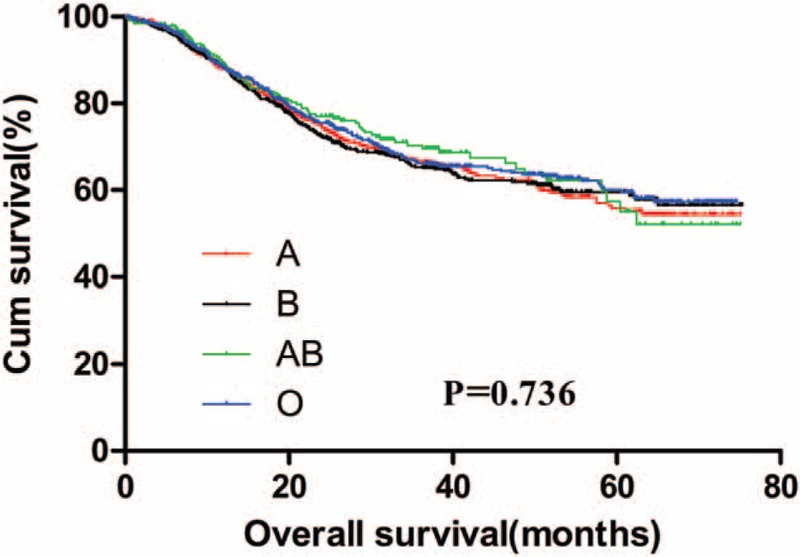
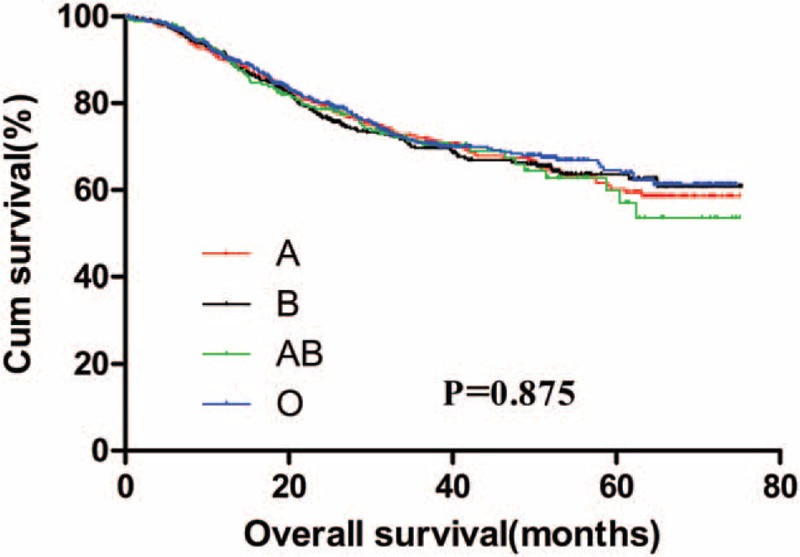
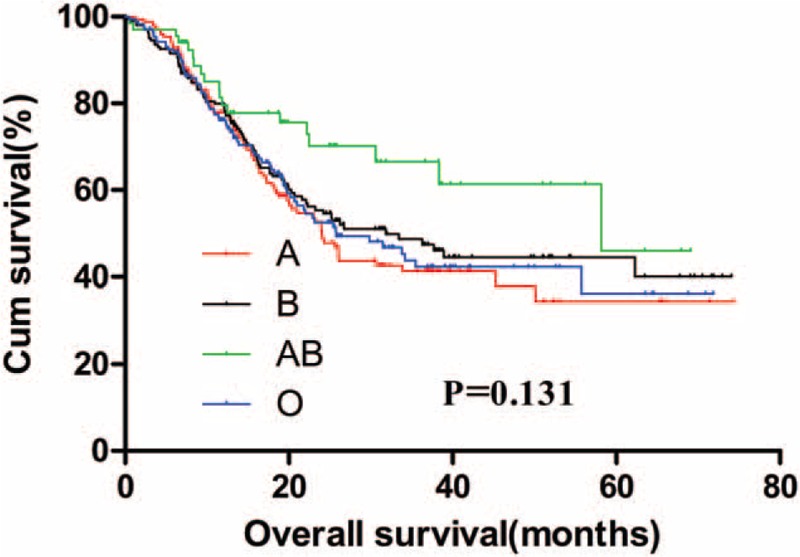
The 5-year OS for GC patients with the 4 ABO blood groups were analyzed. The median OS for patients with blood type A, B, AB, and O were 52.6, 52.8, 53.8, and 53.6 months, respectively. There was no significant difference for the survival of patients among the 4 groups (Fig. 1, P = .736). The OS of the 4 blood groups according to different levels of serum CEA were analyzed. No significant difference was found between the OS of the 4 blood types of GC patients with negative (Fig. 2, P = .875) and positive (Fig. 3, P = .131) preoperative serum CEA. The median OS time for patients with negative preoperative serum CEA with blood type A, B, AB, and O were 55.6, 55.7, 54.7, and 56.4 months, respectively. The median OS time for patients with positive preoperative serum CEA with blood type A, B, AB, and O were 24.0, 31.8, 58.2, and 25.7 months, respectively. However, we noticed that the blood type AB seemed to have a relatively higher OS than blood type non-AB (A, B, and O) in GC patients with positive preoperative serum CEA. Thus, patients with positive preoperative serum CEA were divided into 2 groups, patients with blood type AB and patients with blood type non-AB. There was a significant difference between the OS of the 2 groups (Fig. 4, P = .026). The median OS time for the 2 groups was 58.2 and 25.7 months, respectively.
Figure 1.
Overall survival for gastric cancer patients stratified by ABO blood groups.
Figure 2.
Overall survival for gastric cancer patients with negative preoperative serum carcinoembryonic antigen stratified by ABO blood groups.
Figure 3.
Overall survival for gastric cancer patients with positive preoperative serum carcinoembryonic antigen stratified by ABO blood groups.
Figure 4.
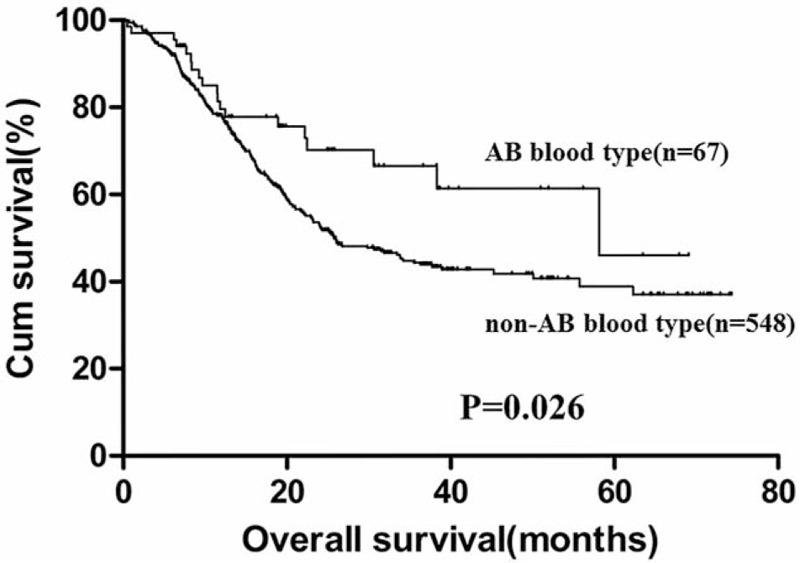
Overall survival for gastric cancer patients with positive preoperative serum carcinoembryonic antigen grouped by AB and non-AB blood groups.
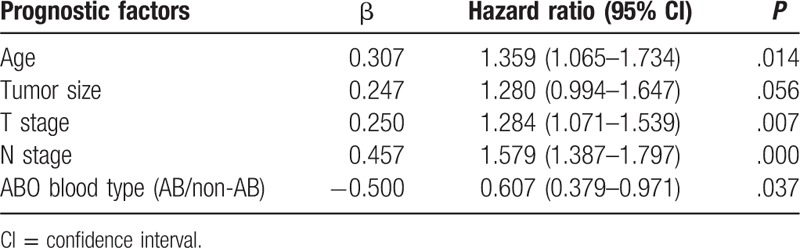
The univariate analysis of prognostic factors for GC patients with positive preoperative serum CEA was shown in Table 2. The results showed that age, tumor size, tumor depth, lymph node metastasis, and ABO blood groups were significant prognostic factors. Further, multivariate analysis showed that age, tumor depth, lymph node metastasis, and ABO blood groups were independent prognostic factors (Table 3).
Table 2.
Univariate analysis of prognostic factors for gastric cancer patients with positive preoperative serum carcinoembryonic antigen.
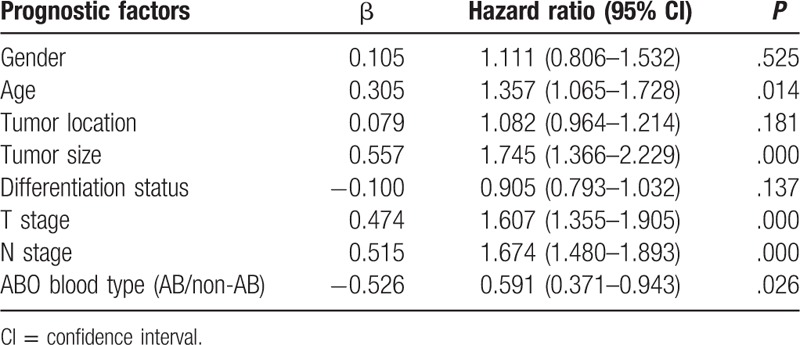
Table 3.
Multivariate analysis of prognostic factors for gastric cancer patients with positive preoperative serum carcinoembryonic antigen.
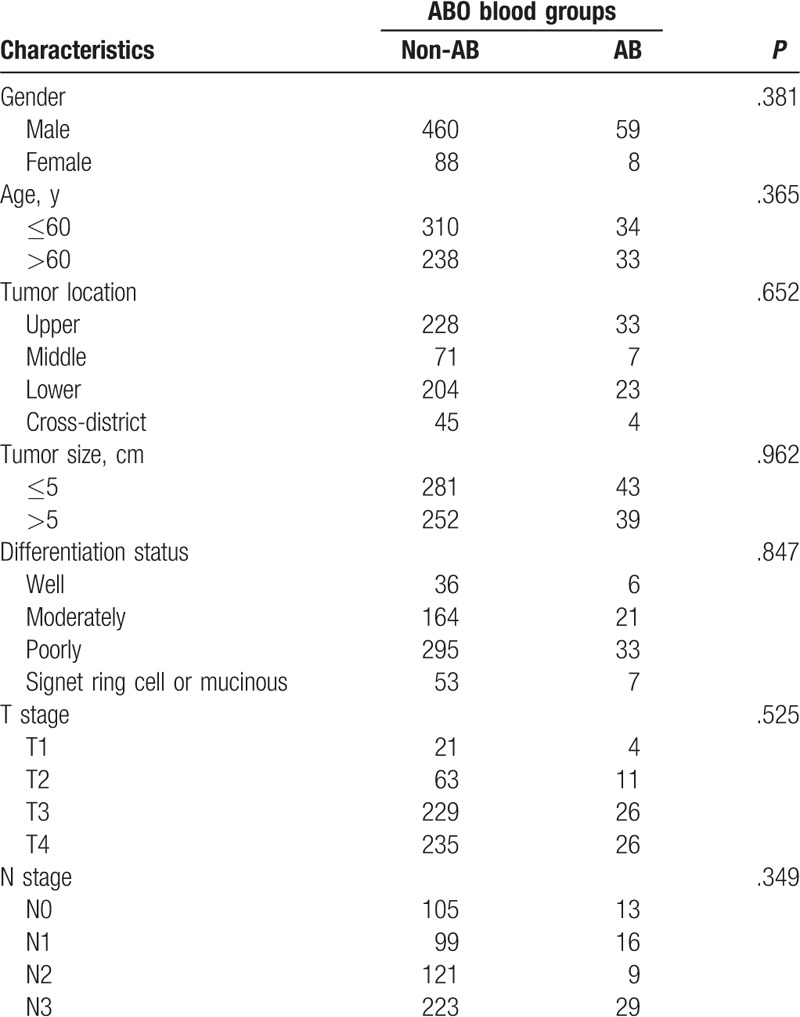
The clinicopathological characteristics of GC patients with positive preoperative serum CEA between blood type AB and non-AB group were compared and summarized in Table 4. The results showed that the clinicopathological characteristics were comparable between the 2 groups (all P > .05).
Table 4.
Clinicopathological characteristics of gastric cancer patients with positive preoperative serum carcinoembryonic antigen grouped by ABO blood groups.
4. Discussion
Since been discovered a century ago, ABO blood system is one of the most widely used blood types worldwide. It is defined by the carbohydrates expressed on the surface of erythrocyte. The antigenic structure of ABO blood groups is determined by ABO gene which located on chromosome 9q34.[9] Modified ABO blood antigens are also found to be expressed on many epithelial cells, including urogenital, skin cells, and gastrointestinal cells.[10] Since the mid-1900s, the relationship between tumor and ABO blood groups has been widely investigated. Beyond defining ABO blood types, glycoconjugates were found to be important mediators of immune surveillance, intercellular adhesion, and membrane signaling, which are both contribute to the malignant progression and spread of malignant cells.[11] These may explain the associations between ABO blood types and tumor.
The correlation between ABO blood groups and GC was first discovered in 1953.[12] Since then a lot of investigations have discussed the prognostic value of ABO blood group in a variety of malignancies, and some of them have found significant findings.[13–16] However, the results were controversial even for the same kind of tumor. Qiu et al[17] analyzed 474 GC patients who accepted chemical therapy or surgery, and found that ABO blood groups were not a prognostic factor. However, in a retrospective analysis of 1412 cases of GC who underwent radical gastrectomy, Xu et al[18] demonstrated that blood type AB is a favorable prognostic factor. Our previous study indicated that among patients with stage III and patients who ever smoked, blood type AB had a better prognosis than the non-AB blood group. However, the correlation between ABO blood groups and serum CEA has not been discussed. For the first time, the present study explored the prognostic value of ABO blood type among GC patients with different levels of preoperative serum CEA. We found that AB blood type was independently correlated with favorable survival.
Recently, some studies have shown an association between ABO genotype and serum markers including tumor necrosis factor-α, E-selectin, and P-selectin.[19] This may support the possibility that ABO blood group alleles may influence the systemic inflammatory response and immune cell recruitment, and thereby influence the initiation, development, and outcomes of GC. Especially, a significant correlation has been found between soluble intercellular adhesion molecule-1 (sICAM-1) and single-nucleotide polymorphisms at the ABO locus.[20] sICAM-1 is a glycoprotein with an extracellular region that has an immunoglobulin-like structure and it belongs to the immunoglobulin superfamily.[21] It has a strong effect on cancer-related inflammation and the metastatic capability of tumor cells by cell adhesion functions. Positive relation between the high concentration of sICAM-1 and aggressive biological features has also been reported in GC patients.[22] This may explain the association between ABO blood groups and survival of GC patients to some extent.
A convincing research comprised of Chinese patients have reported that blood type AB is a favorable prognostic factor for patients with colon cancer.[15] Gastric adenocarcinomas are stratified into 2 major histological types: diffuse and intestinal by Lauren in 1965.[23] The intestinal-type GC derives from intestinal metaplasia mucosa and presents with well-differentiated adenocarcinoma structure, especially its histopathologic morphology is analogous to colon cancer. Park et al[24] have observed that serum CEA shows a higher positive rate in the intestinal type of the Lauren classification than in the diffuse type. Therefore, it was possible that the intestinal-type GC accounted for larger proportion than other types in the patients with preoperative serum CEA elevated. Accordingly, patients with blood type AB have a favorable survival than patients with blood type non-AB in the cohort of GC patients with positive preoperative serum CEA. However, it is but one of many possible and rational hypotheses.
There are several limitations in our present study. First, it was a single center retrospective analysis; multicenter studies are needed to further verify the findings in our present study. Second, the sample size of blood type AB patients was not large enough, which will result in bias during analysis. The last, the Lauren classification of GC was not identified by our pathology department; therefore, most patients lack of pathological data to support our hypothesis.
5. Conclusion
In summary, our results suggested that patients with blood type AB were more likely to have a better survival than those with blood type non-AB for GC patients with positive preoperative serum CEA.
Footnotes
Abbreviations: CEA = carcinoembryonic antigen, GC = gastric cancer, OS = overall survival, sICAM-1 = soluble intercellular adhesion molecule-1.
SX, FF, and LS have contributed equally to this work.
This study was supported in part by grants from the National Natural Scientific Foundation of China (No. 31100643 and 31570907).
The authors have no conflicts of interest to disclose.
References
- [1].Greenlee RT, Murray T, Bolden S, et al. Cancer statistics, 2000. CA Cancer J Clin 2000;50:7–33. [DOI] [PubMed] [Google Scholar]
- [2].Sikaroodi M, Galachiantz Y, Baranova A. Tumor markers: the potential of “omics” approach. Curr Mol Med 2010;10:249–57. [DOI] [PubMed] [Google Scholar]
- [3].Zhou YC, Zhao HJ, Shen LZ. Preoperative serum CEA and CA19-9 in gastric cancer—a single tertiary hospital study of 1,075 cases. Asian Pac J Cancer Prev 2015;16:2685–91. [DOI] [PubMed] [Google Scholar]
- [4].Xiao S, Feng F, Sun L, et al. Study on prognosis relationship between ABO blood groups of patients with gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2015;18:1011–5. [PubMed] [Google Scholar]
- [5].Feng F, Sun L, Liu Z, et al. Prognostic values of normal preoperative serum cancer markers for gastric cancer. Oncotarget 2016;7:58459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439–49. [DOI] [PubMed] [Google Scholar]
- [7].Kim HI, Hur H, Kim YN, et al. Standardization of D2 lymphadenectomy and surgical quality control (KLASS-02-QC): a prospective, observational, multicenter study [NCT01283893]. BMC Cancer 2014;14:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2010 (ver.3). Gastric Cancer 2011;14:113–23. [DOI] [PubMed] [Google Scholar]
- [9].Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O their changes associated with human cancer. Biochim Biophys Acta 1999;1473:247–66. [DOI] [PubMed] [Google Scholar]
- [10].Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv Exp Med Biol 2001;491:369–402. [DOI] [PubMed] [Google Scholar]
- [11].Nakao M, Matsuo K, Ito H, et al. ABO genotype and the risk of gastric cancer, atrophic gastritis, and Helicobacter pylori infection. Cancer Epidemiol Biomarkers Prev 2011;20:1665–72. [DOI] [PubMed] [Google Scholar]
- [12].Liumbruno GM, Franchini M. Beyond immunohaematology: the role of the ABO blood group in human diseases. Blood Transfus 2013;11:491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ouyang PY, Su Z, Mao YP, et al. Prognostic value of ABO blood group in southern Chinese patients with established nasopharyngeal carcinoma. Br J Cancer 2013;109:2462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Klatte T, Xylinas E, Rieken M, et al. Impact of ABO blood type on outcomes in patients with primary nonmuscle invasive bladder cancer. J Urol 2014;191:1238–43. [DOI] [PubMed] [Google Scholar]
- [15].Cao X, Wen ZS, Sun YJ, et al. Prognostic value of ABO blood group in patients with surgically resected colon cancer. Br J Cancer 2014;111:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kaffenberger SD, Morgan TM, Stratton KL, et al. ABO blood group is a predictor of survival in patients undergoing surgery for renal cell carcinoma. Bju Int 2012;110:E641–6. [DOI] [PubMed] [Google Scholar]
- [17].Qiu M, Zhang D, Ruan D, et al. A relationship between ABO blood groups and clinicopathologic characteristics of patients with gastric adenocarcinoma in China. Med Oncol 2011;28:268–73. [DOI] [PubMed] [Google Scholar]
- [18].Xu YQ, Jiang TW, Cui YH, et al. Prognostic value of ABO blood group in patients with gastric cancer. J Surg Res 2016;201:188–95. [DOI] [PubMed] [Google Scholar]
- [19].Sun P, Chen C, Zhang F, et al. The ABO blood group predicts survival in esophageal squamous cell carcinoma in patients who ever smoked: a retrospective study from China. Tumor Biol 2014;35:7201–8. [DOI] [PubMed] [Google Scholar]
- [20].Pare G, Chasman DI, Kellogg M, et al. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet 2008;4:e1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dong Z, Fu S, Xu X, et al. Leptin-mediated regulation of ICAM-1 is Rho/ROCK dependent and enhances gastric cancer cell migration. Br J Cancer 2014;110:1801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Velikova G, Banks RE, Gearing A, et al. Serum concentrations of soluble adhesion molecules in patients with colorectal cancer. Br J Cancer 1998;77:1857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zheng H, Takahashi H, Murai Y, et al. Pathobiological characteristics of intestinal and diffuse-type gastric carcinoma in Japan: an immunostaining study on the tissue microarray. J Clin Pathol 2007;60:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Park SH, Ku KB, Chung HY, et al. Prognostic significance of serum and tissue carcinoembryonic antigen in patients with gastric adenocarcinomas. Cancer Res Treat 2008;40:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]


