Abstract
Background:
The decision of ligation at the origin of the inferior mesenteric artery (IMA) or below the origin of the left colic artery (LCA) has remained a dilemma for surgeons in colorectal cancer surgery. The available studies are controversial. The objective of this meta-analysis is to compare the predictive significance of high versus low ligation in colorectal cancer surgery.
Methods:
A literature search done using Medline, EMBASE, GoogleScholar, and references. A meta-analysis was performed to analyze the 5-year overall survival (OS) of the high and low ligation using hazard ratio (HR) and 95% confidence interval (CI). We further analyzed 2 subgroups considering the level of lymph nodes (LNs) extension. That is IMA positive (+ve) and negative (−ve) LNs. Survival differences were analyzed.
Results:
A total of 3119 patients in 5 cohorts were included in this meta-analysis. The pooled HR results showed significant OS benefit of high ligation than low ligation (HR; 0.77, 95% CI: 0.66–0.89) in the “IMA +ve” group with 33% decreased risk, while there is no statistical significance in the “IMA −ve” (HR 0.66, 95% CI: 0.30–1.46) and the “all cases” group (HR 0.69, 95% CI: 0.41–1.15).
Conclusion:
The pooled data showed high ligation of IMA has a better survival benefit for the patients with IMA positive LNs. It signifies high ligation should be recommended for the advanced cases or with the suspected high risk of IMA lymphatic metastasis. The limited number of articles demands future high-powered, well-designed randomized controlled trials (RCTs) for the further reliable conclusion.
Keywords: colorectal cancer, high ligation, IMA ligation, mesenteric lymph nodes resection, prognosis, survival benefit
1. Introduction
Colorectal cancer (CRC) is a major public health problem. The 3rd most commonly diagnosed cancer in males and the 2nd in females worldwide. It is the 4th cause of cancer death globally and over 9% of all cancer incidences with an estimated 1.4 million cases occurring in 2012.[1] Currently, the radical procedure of surgery is considered as the most prognostic treatment for CRC. The lymphatic clearance of the inferior mesenteric artery (IMA) has been widely accepted by surgeons based upon the extension of lymphatic metastasis. The ligation at the origin of IMA (high ligation) may be the rational choice to achieve the complete lymphatic clearance.[2,3] However, the high ligation may compromise the blood supply due to the sacrifice of the left colic artery (LCA), which may increase the risk of anastomotic leakage.[4] Besides this, the high ligation is associated with functional impairment of the urogenital system due to increased risk of hypogastric plexus injury. These 2 aspects could impact the survival of patients.[5] The lymphatic metastasis around IMA depends upon extension of primary tumor, and its prognostic significance remained unclear. The ligation below the origin of LCA (low ligation) could become another choice since it preserves the LCA and minimizes the risk of hypogastric plexus damage. However, the limited length of preserved LCA may hinder the tension-free long transplant in the coloanal anastomosis. Therefore, increase the risk of anastomotic leakage regardless of the intact blood supply. Moreover, the incomplete lymphatic clearance may decrease the survival while increasing the possibility of metastasis and cancer reoccurrences.
This meta-analysis aimed to evaluate the predictive significance of high ligation versus low ligation for the radical operative procedure of colorectal cancer. The lack of evidence for the prognostic significance of lymph nodes (LNs) clearance around the IMA as well as the decision of low or high ligation remained a challenge for surgeons. It may provide possible evidence for making the decision to surgeons. The primary endpoint is 5-year of overall survival (OS).
2. Material and methods
2.1. Literature search strategy
We systematically searched PubMed, Embase, and Google Scholar. Strategy based on combinations of the following search terms: “Rectal” or “sigmoidal” or “left colon” or “Colorectal” and “cancer” or “malignancy” or “neoplasm” or “tumor” and “high ligation” or “high tie” or “IMA ligation” or “flush ligation” or “apical lymph node resection” and “low ligation” or “low tie” and “mesenteric lymph node resection” And “prognosis” and “survival benefit”. The last search was performed on June 24, for additional potentially eligible studies. The references of review articles were also examined and collected.
We extracted the hazard ratio (HR) from Kaplan–Meier curve from the included studies. Only those graphs were taken from an article which has provided high and low ligation curves with OS. Thus, patient's informed consent was not required. The 2nd author checked the extracted data. Disagreements were resolved through discussion. Study approved by the ethics committee of Sichuan University. The following items were collected from each study: first author's name, year of publication, a country with the study population, sample size, number of high and low ligation patients, the level of extension of +ve LNs, and type of cohort studies.
2.2. Inclusion and exclusion criteria
Studies fulfilling the following criteria were included in the meta-analysis: freshly diagnosed colorectal cancer study; association between high and low ligation with OS; the reported HR or Kaplan–Meier curve and 95% confidence interval (CI); and every included study has pathologically classified the level of LNs around IMA and diagnosed with no any tumors besides the primary origin. The following publications were excluded: study that did not report HR or Kaplan–Meier curve with 95% CI; letters, reviews, expert opinions, or case reports and nonaccessible full text; D3 resection (only mesenteric) without IMA ligation; survival with only high ligation; high ligation and low ligation with else an endpoint (leakage, disease-free survival, O2 level); no clear evidence of LN extension; and distal metastasis of LNs was excluded. Studies were identified by the search strategy by 2 independent reviewers, and a 3rd reviewer was consulted on disagreement.
2.3. Selection and characteristics of included literature search
A flowchart of the literature search is shown in Fig. 1. The initial search algorithm retrieved a total of 618 studies. After the 1st review, 91 studies related to the high and low ligations were only further evaluated. Among these studies, 86 were excluded for the following reasons: the abstracts of 5 studies unable to access full text. Three studies were letters and report. Fourteen studies resembled topic but different endpoint. (Anastomotic leakage and vascular oxygen level after high ligation). Eleven articles were review. Forty-one articles did not provide Kaplan–Meier graph or sufficient data for HR extraction and 95% CI. Seven articles are about D3 dissection (without high IMA ligation). Four articles were solely about high ligation with LN status for survival and 1 article excluded for having only disease-free survival with no OS. Thus, 5 studies having Kaplan–Meier to estimate HR, published between 1990 and 2016 were included in this meta-analysis. The characteristics of the included studies are summarized in Table 1. A total of 3119 patients were included. These Studies are taken from the USA (n = 1), Japan (n = 2), India (n = 1), and UK (n = 1).
Figure 1.

A flowchart outlining the study selection.
Table 1.
Characteristics of all identified high versus low ligation studies.

2.4. Subgroup analysis
We planned to conduct subgroup analysis. Consider the level of LN involvement along the IMA. Despite variation in classifications namely Dukes, Kirklin, Astler Coller, and TNM. The aim remained same to represent the level of LNs extension and evaluate survival benefit. To optimize variation in classification, we considered LCA as the reference line to differentiate between 2 groups of LNs extension. The presence of LN above LCA along IMA considered as IMA positive and absence of LN along as IMA negative. The OS of high and low ligation has taken from 5 selected articles and based on the Kaplan–Meier graph.
2.5. Quality assessment
The methodological quality of the study was assessed using the Newcastle-Ottawa scale by 2 reviewers. A 3rd reviewer was conferred with for the uncertainties.
2.6. Outcome measures and data analysis
We utilized the Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) to conduct the meta-analysis. To statistically evaluate the prognostic effect of high and low ligation. For OS, we extracted the HR and the associated standard error from provided Kaplan–Meier graph of the included studies. HR and their relative standard errors, P-values or CIs were not provided in the original articles directly. The HRs were approximated according to the method described by Tierney.[6] By convention, an HR < 1 suggests a better prognosis in the high ligation group compared with the low ligation group. We defined the statistical result with a P-value < .05 as significant. The interstudy heterogeneity was evaluated using the chi-square test and quantified using the I2 statistic. A fixed-effect model was used if there was no heterogeneity, whereas subgroup analysis was used if the heterogeneity was statistically significant (P < .05 and I2 > 50%). Potential of publication bias was assessed using a funnel plot and further quantified by Egge test.
2.7. Meta-analysis results
The pooled results have 3 different groups showed in Figs. 2–4. The OS of high ligation with LN dissection revealed OS benefit for 5 years in every studies group. Figure 2 has shown an overall 5-year survival benefit in “all cases group”. A fixed-effects model was used for analysis. Statically no heterogenicity (I2 = 0%, P = .55) exist. The pooled data (HR of 0.69, 95%CI: 0.41–1.15) showed patients with high ligation were expected to reduce the risk of 31%. However, the result of meta-analysis statistically insignificant.
Figure 2.

Overall 5-year survival of high versus low ligation of inferior mesenteric artery (IMA) in “all cases” group.
Figure 4.

Overall 5-year survival of high versus low ligation in the “lymph node-positive cases” at the base of inferior mesenteric artery (IMA).
Figure 3 shows the overall 5-year survival of high and low ligation in “IMA negative LN group”. The obvious statistical heterogeneity observed in the analysis (I2 = 82%, P = .02). A random-effects model was used for analysis. The pooled data (HR of 66, 95% CI: 30–1.46) has shown IMA negative LN group has favored high ligation and decreased in percentage of the risk yet statically insignificant.
Figure 3.
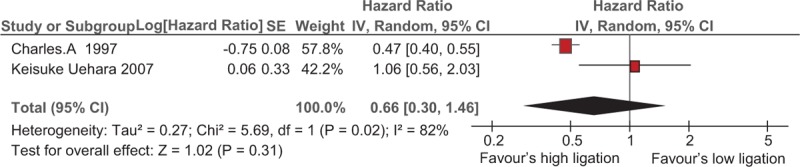
Overall 5-year survival of high versus low ligation in “lymph node negative cases” at the base of inferior mesenteric artery (IMA).
Lastly in Fig. 4, in 4 included studies, a significant decrease in mortality rate has seen in the high ligation group with IMA positive cases, compared to the low ligation group. A very low heterogeneity exists (I2 = 31%, P = .23). A fixed-effect model was used for analysis. The pooled data (HR of 0.77, 95%CI: 0.66–0.89) showed statically significant result and patients with IMA positive expected to have decreased 33% risk with the statically significant meta-analysis result.
2.8. Sensitivity analysis
The number of included studies as well as the lack of randomized control trials (RCTs) in this meta-analysis decrease importance to pool sensitivity analysis.
2.9. Publication bias
A publication bias estimate was not used to evaluate the reliability of these meta-analysis results. There were fewer numbers of included studies. It is dating back since the 19th centuries to recent years. The standards of pathology reporting might have improved over the time period. The software used for plotting Kaplan–Meier graph to pool HR might be slightly varied with individual patient data. Even though publication bias may exist somewhat it is still a supportive result for high ligation in advanced colorectal cancer surgery.
3. Discussion
This meta-analysis is investigating the association of high ligation with lymphatic resection and oncological benefit on survival. The anastomotic leakage is one of the complications of high ligation, which is debated most dominantly. However, the benefits of high ligation at different levels of IMA and LN resection need to be evaluated distinctly at the oncological aspect of survival. We found high ligation has a better oncological survival benefit for the patients with IMA positive LNs as well as gives adequate length and tension-free anastomosis. High ligation is equally important to low coloanal anastomosis and J-pouch surgery.
In this included 5 studies, Slanetz and Grimson[7] and Charan[8] have shown the results in which the high ligation associated with more extensive resection of mesentery and its lymphatic drainage. It also has clinical significance to reduce local tumor recurrence and increases the oncological survival rate. Surtees et al[9] and Uehara et al[10] did not show any statistical difference to support high ligation other than tumor staging. Yasuda et al[11] neither confirmed nor denied high ligation. The pooled data of this meta-analysis showed high ligation reduced risk by 31%, 34%, and 33% when compared to low ligation in “all cases” group, “IMA-ve” group, and “IMA +ve” group, respectively. However, the statistical significance of survival advantage achieved only with high ligation of IMA positive LNs. The results demonstrate high ligation with lymphatic resection of IMA has much better OS than low ligation. The prognosis has compared with high versus low ligation in 4 articles of “IMA +ve LN group.” Yasuda et al[11] and Surtees et al[9] found no significant differences, while Charles et al[9]and Uehara et al[10] favored survival benefit by high ligation. The data of these 4 studies of subgroup demonstrated a substantial OS improvement with high ligation than low ligation. It clearly showed high ligation carries a beneficial oncological outcome whenever it has lymphatic metastasis. Collectively, these outcomes support high ligation and should be preferably suitable for the prognostic dilemma of the oncological patient instead low ligation concerning anastomotic leakage.
Currently, in the early stage of cancer with low risk of lymphatic metastasis or in the advanced stage with a high risk of IMA lymphatic metastasis, application of high or low ligation solely depends upon the practitioner. In both of the cases, surgeon's opinion varies. The controversy on the choice of high or low ligation of IMA has focused on the anastomotic leakage and oncological outcomes, these 2 have their own aspects.[12] High ligation ensures the IMA lymphatic clearance even though debated for increasing the risk of anastomotic leakage as it jeopardized blood supply of LCA.[13,14] Low ligation preserves the LCA and ensured blood supply of marginal artery while leading to incomplete lymphatic resection of IMA.[15] As far concerning anastomosis leakage, a basic study recommends sigmoid colon is not only suitable for anastomosis due to its natural course of insufficient vascular supply, however the marginal artery delivers sufficient vascular supply to the transverse and descending colon. Thus, sigmoid colon is sacrificed and there should be no uncertainty in performing a high ligation.[16] Therefore, the oncological prognostic significance of IMA lymphatic clearance dominates the choice of surgeons over the risk of anastomotic leakage.
The sentinel lymph nodes (SLNs) mapping may have a significant advantage in prognostic evaluation, diagnosis, and therapeutic management of colorectal cancer.[17] The lodging of SLNs can be along mesocolic or periaortic stations through its vascular or lymphatic channels. The level of ligation could play a significant role in the harvesting of SLNs in the histopathological staging. In high ligation procedure, we perform a complete resection of LCA and vein along with its mesocolic fat. It indicates more possibilities of detecting SLNs which may skip in low ligation. High ligation also ensures more radical fat resection around the aorta with the root of IMA, IMV along mesocolic fat. Consequently, more metastases can be obtained by possible extensive resection and may predominantly associated with histopathological tumor staging Chen et al[18] reported, the IMA LN metastases were 0% (pT1), 1.0%(pT2), 2.6% (pT3), and 4.3% (pT4) by of TNM staging. Kanemitsu et al[19] study showed the 8.3% (99 of 1188) incidence of metastasis to the origin of LCA. Nodal metastasis occurred more commonly in patients with pT3 and pT4 lower rectal cancer. The incidence of metastasis at the root of IMA about 1.7% (20 of 1188). This represented how residual metastatic LN could be usually forgotten in low ligation. Some surgeons still claim of no evidence that high ligation may increase the prognosis and prefer to apply low ligation even in advanced cancer cases. However, our findings do not support for low ligation in advanced or IMA positive cases.
Reviewing articles on anastomosis leakage some studies have concluded, high ligation has no undisputable proof of increased survival. Although the usage of IMA high ligation plays an important role in the improvement of LN retrieval, the precision of tumor staging, and to avoid tension in low pelvic anastomoses.[12,15,16] Dworak[20] reported the high ligation results 41% to 86% decrease in sigmoid blood supply around for 5 days. The sacrifice of LCA lead to the poor blood supply and it is one of the most important risk factors of anastomotic leakage. However, an RCT has shown the level of IMA ligation in patients with rectal cancer did not show any difference in anastomotic leakage.[21] In addition, the local recurrence of cancer, hand sewn versus stapled anastomoses, intraoperative blood pressure, nutritional status of the patient including many other factors subsequently leads to anastomotic leakage and can reduce survival.[22–24] Therefore, the accessible studies on the anatomical concern of leakage are controversial and somehow favor the high ligation on oncological perspective. In fact, the supply of blood could be satisfied once if colonic marginal arch well maintained. Furthermore, a meta-analysis showed high ligation reduced 13% of 5-year OS compared to low ligation. However, study neither used HR nor analyzed the prognostic significance of IMA lymphatic metastasis.[18] Our study confirms these findings and further defines the pure nature of the LN association, we extracted the HR from Kaplan–Meier graph to pool data and further compared the prognosis of high and low ligation with the status of the level of lymphatic metastasis along IMA. This ensured the reliability of our results to the best extent.
Regardless of the above major related issues, available research for the anatomical consideration preferred the high ligation. A significant benefit of the high ligation is definitely the resection of the IMA at its origin, which allows to gain extra length and facilitate tension-free anastomosis.[25] Ghavami et al[26] reported, the precise mobilization of the splenic flexure significantly reduces, the anastomotic tension and in most cases allow the preservation of LCA. However, practically it is very difficult to achieve the additional length in low coloanal anastomosis or even in colonic J-pouch surgery unless ligation of IMA. LCA is comparatively shorter and less feasible to the low coloanal anastomosis. The advantages of additional length usually support using the descending colon rather than the sigmoid when performing an anastomosis. Not just the sigmoid colon generates fairly high pressure but additionally because it could consequently lead to relatively poor function and more importantly, the marginal artery may be minimal or absent in the sigmoid which is prone to ischemia if used for anastomosis. Hence in colonic implant anastomosis will almost always need a high ligation. However, this is for technical rather than cancer-specific reason which also does support high ligation.[27]
The limitation of this meta-analysis could be less number of paper and non-RCT studies, which is taken in between decades of the time interval. A long-time interval might have brought a significant change in identifying the level of LNs grading and management of colorectal cancer in terms of surgical technique (TME, D3 laparoscopy, robotic). There is a significant shift from open to minimally invasive and robotic techniques. The moral and ethical concern should also be drowned on this topic. Our meta-analysis consisting of a comparative meta-analysis of nonrandomized studies may be an excellent tool for identifying reasons for heterogeneity and inconsistency of the studies analyzed. Thus, well-designed future RCT studies with properly staging of LN are required.
4. Conclusion
This meta-analysis showed high ligation has an OS benefit in the colorectal cancer surgery with the group of IMA positive LNs. High ligation should be recommended for the suspected advanced cases or with the high risk of IMA positive metastatic LNs. This study statically does not signify high ligation other than IMA positive groups, thus low ligation could be applicable for the early stage of limited intramural cancer. The less number of articles demand future high-powered, well-designed RCTs for the further conclusion. An unambiguous consensus remains to be achieved for routine high ligation.
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, IMA = inferior mesenteric artery, LCA = left colic artery, LN = lymph node, OS = overall survival, RCT = randomized control trial, SLN = sentinel lymph node.
Funding/support: This study was granted by the Natural Science Foundation of China (No. 81472304).
The authors have no conflicts of interest to disclose.
References
- [1].Favoriti P, Carbone G, Greco M, et al. Worldwide burden of colorectal cancer: a review. Updates Surg 2016;68:7–11. [DOI] [PubMed] [Google Scholar]
- [2].Steup WH, Moriya Y, van de Velde CJ. Patterns of lymphatic spread in rectal cancer. A topographical analysis on lymph node metastases. Eur J Cancer 2002;38:n911–8. [DOI] [PubMed] [Google Scholar]
- [3].Yi JW, Lee TG, Lee HS, et al. Apical-node metastasis in sigmoid colon or rectal cancer: is it a factor that indicates a poor prognosis after high ligation? Int J Colorectal Dis 2012;27:81–7. [DOI] [PubMed] [Google Scholar]
- [4].Buunen M, Lange MM, Ditzel M, et al. Level of arterial ligation in total mesorectal excision (TME): an anatomical study. Int J Colorectal Dis 2009;24:1317–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moszkowicz D, Alsaid B, Bessede T, et al. Where does pelvic nerve injury occur during rectal surgery for cancer? Colorectal Dis 2011;13:1326–34. [DOI] [PubMed] [Google Scholar]
- [6].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Slanetz CA, Jr, Grimson R. Effect of high and intermediate ligation on survival and recurrence rates following curative resection of colorectal cancer. Dis Colon Rectum 1997;40:1205–18. discussion 1218–1219. [DOI] [PubMed] [Google Scholar]
- [8].Charan I, Kapoor A, Singhal MK, et al. High ligation of inferior mesenteric artery in left colonic and rectal cancers: lymph node yield and survival benefit. Indian J Surg 2015;77(Suppl 3):1103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Surtees P, Ritchie JK, Phillips RK, et al. High versus low ligation of the inferior mesenteric artery in rectal cancer. Br J Surg 1990;77:618–21. [DOI] [PubMed] [Google Scholar]
- [10].Uehara K, Yamamoto S, Fujita S, et al. Impact of upward lymph node dissection on survival rates in advanced lower rectal carcinoma. Dig Sur 2007;24:375–81. [DOI] [PubMed] [Google Scholar]
- [11].Yasuda K, Kawai K, Ishihara S, et al. Level of arterial ligation in sigmoid colon and rectal cancer surgery. World J Surg Oncol 2016;14:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guraya SY. Optimum level of inferior mesenteric artery ligation for the left-sided colorectal cancer. Systematic review for high and low ligation continuum. Saudi Med J 2016;37:731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bacon HE, Dirbas F, Myers TB, et al. Extensive lymphad enectomy and high ligation of the inferior mesenteric artery for carcinoma of the left colon and rectum. Dis Colon Rectum 1958;1:457–64. discussion 464–465. [DOI] [PubMed] [Google Scholar]
- [14].Dixon CF. Anterior resection for malignant lesions of the upper part of the rectum and lower part of the sigmoid. Ann Surg 1948;128:425–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Titu LV, Tweedle E, Rooney PS. High tie of the inferior mesenteric artery in curative surgery for left colonic and rectal cancers: a systematic review. Dig Surg 2008;25:148–57. [DOI] [PubMed] [Google Scholar]
- [16].Hall NR, Finan PJ, Stephenson BM, et al. High tie of the inferior mesenteric artery in distal colorectal resections – a safe vascular procedure. Int J Colorectal Dis 1995;10:29–32. [DOI] [PubMed] [Google Scholar]
- [17].Gurzu S, Jung I, Bara T, et al. Practical value of the complex analysis of sentinel lymph nodes in colorectal carcinomas. Rom J Morphol Embryol 2011;52:593–8. [PubMed] [Google Scholar]
- [18].Chen SC, Song XM, Chen ZH, et al. Role of different ligation of the inferior mesenteric artery in sigmoid colon or rectal cancer surgery: a meta-analysis. Zhonghua Wei Chang Wai Ke Za Zhi 2010;13:674–7. [PubMed] [Google Scholar]
- [19].Kanemitsu Y, Hirai T, Komori K, et al. Survival benefit of high ligation of the inferior mesenteric artery in sigmoid colon or rectal cancer surgery. Br J Surg 2006;93:609–15. [DOI] [PubMed] [Google Scholar]
- [20].Dworak O, et al. Morphology of lymph nodes in the resected rectum of patients with rectal carcinoma. Pathol Res Pract 1991;187:1020–4. [DOI] [PubMed] [Google Scholar]
- [21].Matsuda K, Hotta T, Takifuji K, et al. Randomized clinical trial of defaecatory function after anterior resection for rectal cancer with high versus low ligation of the inferior mesenteric artery. Br J Surg 2015;102:501–8. [DOI] [PubMed] [Google Scholar]
- [22].Alves A, Panis Y, Trancart D, et al. Factors associated with clinically significant anastomotic leakage after large bowel resection: multivariate analysis of 707 patients. World J Surg 2002;26:499–502. [DOI] [PubMed] [Google Scholar]
- [23].MacRae HM, McLeod RS, et al. Handsewn vs. stapled anastomoses in colon and rectal surgery: a meta-analysis. Dis Colon Rectum 1998;41:180–9. [DOI] [PubMed] [Google Scholar]
- [24].Mirnezami A, Mirnezami R, Chandrakumaran K, et al. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 2011;253:890–9. [DOI] [PubMed] [Google Scholar]
- [25].Bacon E. Cancer of the Colon, Rectum, and Anal Canal. Rationale of lymphadenectomy concomitant with curative resection. London: JB Lippincott Company; 1964. 549–70. [Google Scholar]
- [26].Ghavami B. Doit-on préserver l’artère mésentérique inférieure lors d’une sigmoïdectomie pour maladie bénigne? J De Coelio-chirurgie 2008;65:11e3. [Google Scholar]
- [27].Philips RKS, Clark S, Specialist Surgical Practice, A companion to specialist surgical practice, Colorectal Surgery. fifth ed. Elsevier Saunders, 2014. [Google Scholar]


