Abstract
Coeliac disease (CD) is an autoimmune disorder of the small bowel associated with increased risk of additional autoimmune diseases (ADs).
To investigate the prevalence of ADs in a population of adult coeliac patients.
This was a retrospective case–control study. Data from coeliac patients and controls referred to a tertiary center between 2013 and 2016 were collected. The frequency of ADs and the unadjusted and adjusted odds ratios (ORs) for age, gender, disease duration, and body mass index with their 95% confidence intervals (CIs) were evaluated.
Two hundred fifty-five patients with CD (median age 37.1 years; 206 women) were matched with 250 controls. ADs were more frequent (35.3%) in coeliac patients than in controls (15.2%). Adjusted ORs for the presence of only 1, at least 1, and more than 1 AD were 3.13 (95% CI 1.81–5.42, P < .0001), 3.31 (95% CI 2.00–5.46, P < .0001), and 3.93 (95% CI 1.49–10.36, P = .006), respectively. Hashimoto thyroiditis was the most prevalent AD (24.3% vs. 10%) OR = 2.55 (95% CI 1.39–4.70, P < .0001), followed by psoriasis (4.3% vs. 1.6%), type 1 diabetes (2.7% vs. 0.4%), and Sjögren syndrome (2.4% vs. 0.4%).
These findings suggest a need for a careful surveillance of autoimmune status, especially for Hashimoto thyroiditis in patients with celiac disease.
Keywords: autoimmunity, bowel, comorbidity, immunology, poly-pathology, screening, surveillance
1. Introduction
Coeliac disease (CD) is a chronic autoimmune disorder primarily affecting the small bowel and induced by the ingestion of gluten in genetically susceptible individuals.[1] Knowledge about CD has dramatically increased in the past decades. Availability of more accurate noninvasive diagnostic tools prompted a robust research on its epidemiology.[2,3] According to a recent report, the estimated CD prevalence in the Italian population was nearly 1%.[4] However, regional variations in the prevalence have been recorded. For example, Meloni et al[5] observed a prevalence of 1.43% in children from Northern Sardinia. Given its high prevalence, CD is no longer defined a rare but a chronic disease. Compared to the past, when CD was mainly characterized by diarrhea, osteomalacia, and greater childhood mortality.[6] Nowadays, especially in resource-rich countries, clinical manifestation of CD may be more heterogeneous, including typical gastroenterological symptoms as well as a myriad of extra-intestinal disorders.[7] In this scenario, it could be more difficult to suspect the disease. Patients with CD on a gluten-rich diet may have an increased risk of developing lymphoproliferative disease, gastrointestinal (GI) cancer, and additional autoimmune disorders such as type 1 diabetes mellitus and Hashimoto thyroiditis, a variety of micronutrient deficiencies and metabolic changes, osteoporosis, anemia, infertility, miscarriage, pregnancy complications, and more.[8–13] On the other hand, the occurrence of CD is more likely in patients with type 1 diabetes, thyroiditis, and psoriasis, suggesting a shared immune-related pathogenesis.[14] Indeed, current guidelines suggest to consider a screening for autoimmune disorders (in particular type 1 diabetes and Hashimoto thyroiditis) in subjects with CD.[15,16]
Sardinia is a geographic area with an increased prevalence of autoimmune diseases (ADs), including diabetes, autoimmune thyroiditis, and CD.[17] In a study involving a pediatric population from Sardinia, a strong association of autoimmune thyroiditis with CD was previously observed.[18] However, studies on adult patients are lacking.
The aim of this study was to evaluate the prevalence of chronic ADs in adult patients with CD compared with age- and gender-matched controls without CD from the same geographic area.
2. Methods
2.1. Study design
This was a retrospective single-center case–control study. Consecutive patients with a previous or a new diagnosis of CD (cases) were compared with age- and gender-matched consecutive patients with functional dyspepsia and/or functional GI symptoms (controls), for the presence of ADs. Information including age, gender, CD duration (in the case of a previous diagnosis), body mass index (BMI), and comorbidities were collected from charts of cases and controls attending the GI service at the Clinica Medica, from January 2013 to December 2016. For patients who underwent multiple visits within the study period, only information from the last visit were included.
2.2. Setting
The Clinica Medica (University of Sassari, Italy) is a teaching hospital and its tertiary referral center provides care for outpatients with GI problems, including most adult cases of CD from Northern Sardinia. The Sardinians are a well-documented genetically homogeneous population.[19]
2.3. Inclusion criteria
Information from charts of consecutive patients with a new or previous diagnosis of CD (cases) were collected. The diagnosis of CD was established on the basis of a combination of clinical features, biochemical testing, serology markers, and histopathological alterations of duodenal mucosa according to current international guidelines.[15,16] All CD patients were on GFD.
Patients with functional dyspeptic and GI symptoms referred to our service by physicians and/or specialists and negative for CD (based on serology and/or genetic profile) were used as the control group. They were sequentially selected by a retrospective revision of medical charts of patients attending our service within the study period and matched for gender and age with cases. According to clinical practice, patients with functional dyspepsia were tested for Helicobacter pylori infection and eventually treated.
2.4. Exclusion Criteria
Cases and controls with GI alarm signs and/or symptoms, ova and parasites, and/or other intestinal infections in the stool, inflammatory bowel disease (only in controls), drug-induced enteropathy, were excluded from the study. An additional exclusion criterion was age younger than 18 years.
2.5. Diagnosis of autoimmune disorders
When an autoimmune disorder was suspected in cases and controls on the basis of clinical history, physical examination, and laboratory tests, the patient was referred to the appropriate specialist. Only patients (cases and controls) with a written report from a specialist confirming the diagnosis were considered positive and included for the analysis.
2.6. Ethical consideration
An Institutional Review Board approval was obtained from the local “Comitato Etico dell’Azienda Ospedaliero-Universitaria di Sassari” (Prot. 1892/13).
2.7. Statistical analysis
Cases and controls were stratified into 2 subgroups based on the presence or absence of ADs and expressed as absolute numbers and percentages. Unadjusted odds ratios (ORs) with their 95% confidence intervals (CIs) were calculated to estimate the strength of associations with age, gender, disease duration, and BMI. Thereafter, a logistic regression analysis was carried out to calculate adjusted ORs for all cofactors, using a backward stepwise elimination procedure with a P value of a covariate to leave the model set at <0.10. In addition, ORs adjusted for age, gender, disease duration, and BMI were calculated. All the statistical analyses were performed using SPSS Statistical Package (version 16.0, Chicago, IL) and the results were considered significant when P values were <.05.
3. Results
A total of 505 patients’ charts were collected, 255 with CD (49 males and 206 females) and 250 patients as a control group (50 males and 200 females). Demographic and anthropometric parameters were similar in both groups (Table 1).
Table 1.
Demographic and anthropometric characteristics of enrolled patients.
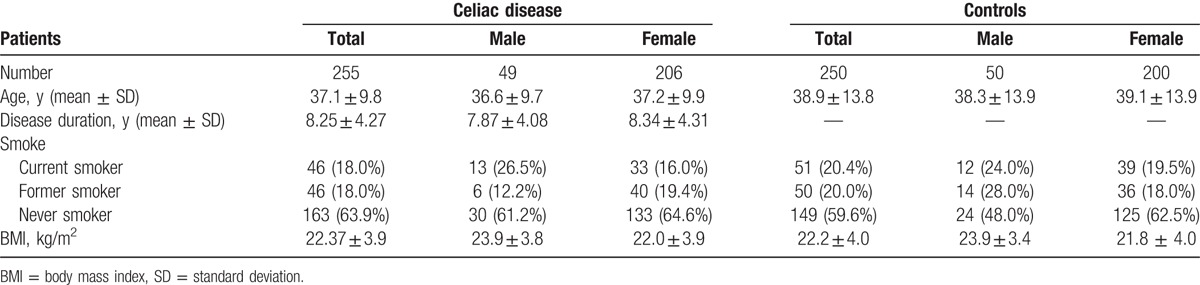
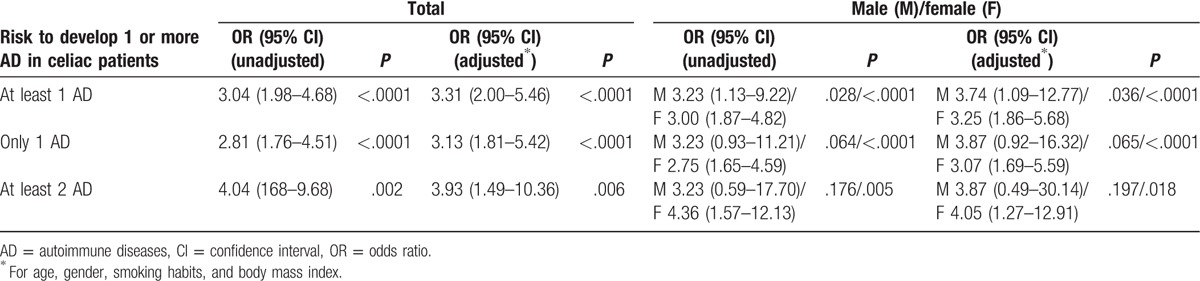
Table 2 shows the prevalence of none, 1, 2, or more ADs in both studied groups, respectively. Overall, ADs were more frequent (35.3%) in cases compared with control (15.2%). Among patients with CD and other ADs, the majority were females (75/131, 36.4% vs. 15/49, 30.6%). Interestingly, CD patients with only 1 additional autoimmune disorder were 26.7% compared with 12.4% of controls. The OR to have at least 1 AD in CD patients was 3.31 (95% CI 2.00–5.46, P < .0001) after adjusting for age, gender, disease duration, and BMI. The OR was even higher for the presence of at least 2 ADs (OR 3.93; 95% CI 1.49–10.36, P = .006). Overall, the risk was increased in both genders (Table 3).
Table 2.
Prevalence of single or multiple autoimmune diseases in coeliac disease group and control group.
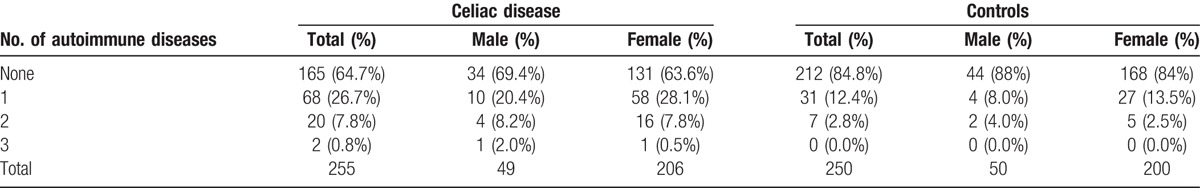
Table 3.
Logistic regression demonstrated increased risk for developing autoimmune disease in coeliac patients.
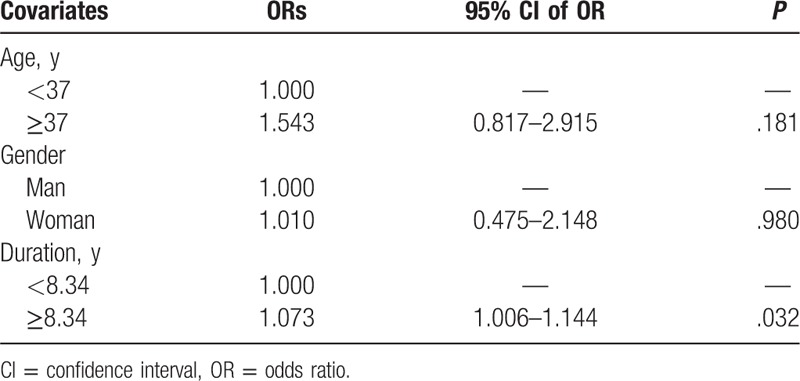
More specifically, Hashimoto thyroiditis was the most prevalent AD among coeliac patients (24.3%) and the frequency was similar in both genders (20.4% males and 25.2% females). In the control group, thyroiditis was less common (10%; 2% males and 12% females) compared with CD patients (OR 2.55; 95% CI 1.39–4.70, P < .0001). Psoriasis was observed in 4.3% of coeliac patients and in 1.6% of controls (P = .274), followed by type 1 diabetes and Sjögren syndrome (Table 4). There was a trend for an increased risk to have other chronic ADs; however, the small sample size did not allow further analysis (Table 4). The logistic regression analysis revealed that disease duration was a significant predictor (P = .032), of additional ADs in CD patients (Table 5).
Table 4.
Prevalence of autoimmune disease in coeliac patients group and control group.
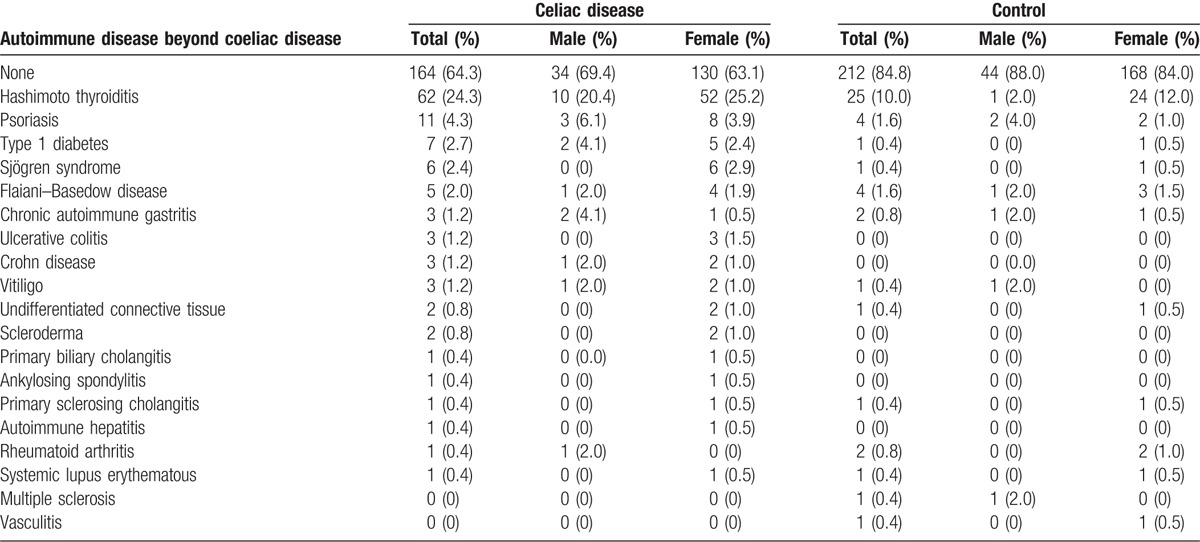
Table 5.
Multiple logistic regression analysis for the risk of additional autoimmunity in patients with coeliac disease.
4. Discussion
In this study, patients with CD on GFD showed a higher prevalence of additional ADs, confirming previously reported results. Demirezer Bolat et al in a recent study on 145 coeliac patients found a prevalence of poly-autoimmunity in 33.1% of the cohort studied. More specifically, Hashimoto thyroiditis was the most prevalent (24.1%), followed by type 1 diabetes (5.5%) and psoriasis (2.8%).[20] These data are substantially similar to our findings. However, in this study, the absence of a healthy control group did not allow to evaluate the relative risk and the regional differences in the prevalence of ADs.
In the past decades, similar data about the prevalence of ADs in CD have been reported in the Italian population. Interestingly, the researchers evaluated whether the duration of exposure to gluten-containing diet is a risk factor for the occurrence of ADs. However, the results of such investigations were not univocal.[21–23]
On the contrary, in our study, it was considered the duration of CD (or in other words the exposure to GFD). The logistic regression analysis showed that disease duration was a significant predictor of further ADs suggesting that the increased risk of autoimmunity may be mainly linked to a genetic predisposition in these subjects.
An association between CD and chronic ADs was also reported in pediatric populations. Guariso et al suggested an increased co-occurrence of autoimmunity in children affected by CD compared with healthy controls (27% vs. 1%).[8] Moreover, in 2 additional studies on children and adolescents, findings similar to our results were recently observed.[9,10] Assa et al in a large cohort of 2,001,353 Jewish Israeli adolescents (of whom 10,565 with CD) reported an increased risk of ADs, especially type 1 diabetes (OR 5.5), inflammatory bowel diseases (OR 3.8), arthritis (OR 2.4), thyroid diseases (OR 1.8), and psoriatic skin disorders (OR 1.6).[9] Canova et al[10] provided further evidence for an increased risk of occurrence of hypothyroidism and type 1 diabetes in CD patients. Moreover, the risk to develop type 1 diabetes was doubled before the age of 20 years in a Swedish coeliac population.[24]
The increased risk of Hashimoto thyroiditis (OR 2.55; P < .0001) in coeliac patients was the most relevant result observed in our study. This association was previously demonstrated in other prospective studies. For instance, Fallahi et al[25] reported an increased prevalence of CD in an Italian cohort of subjects affected by Hashimoto thyroiditis. The authors suggested that in each of the 2 diseases the risk to develop the other one is significantly increased, hence supporting the hypothesis of a genetic predisposition.
Several studies reported an association between CD and ADs. The pathophysiological basis of this association may lie in sharing multiple genetic loci such as HLA-DR3, HLA-DQ2, and others that are involved in the development of AD.[26,27] Overall, the prevalence of AD among the coeliac patients investigated in this study does not diverge from the range reported in other published studies, except for small discrepancies that may be explained by differences in age, disease duration, and study design.[8–10,20,21]
The main strength of our study is the genetic homogeneity of the population investigated. Migratory flows were rare and the intermarriages frequent in Sardinia holding gene pool variability of the population at minimum.[19] On the other hand, the Sardinian population is characterized by a particularly high prevalence of ADs, providing valuable information not easily retrievable in more heterogeneous ethnic groups. In addition, in the last decade, there were not many studies on this topic in Italy and, more importantly, not all were case–control studies. Nonetheless, our study has also some weakness, such as the retrospective nature of the design, and the limited sample size that did not allow a meaningful estimate of the risk of the rarest ADs.
5. Conclusion
In conclusion, our findings provide evidence for increased prevalence of chronic ADs, and more specifically Hashimoto thyroiditis was the most prevalent disorder among females and males, in a population of Sardinian coeliac patients compared with a control group from the same area. In addition, a trend for poly-autoimmunity was observed. These results underline the need of an accurate screening for ADs in celiac patients, especially in those who already have 1 AD beside celiac disease.
Footnotes
Abbreviations: AD = autoimmune disease, BMI = body mass index, CD = coeliac disease, CI = confidence interval, GFD = gluten-free diet, GI = gastrointestinal, OR = odds ratio.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Di Sabatino A, Corazza GR. Coeliac disease. Lancet 2009;373:1480–93. [DOI] [PubMed] [Google Scholar]
- [2].Ianiro G, Bibbo S, Pecere S, et al. Current technologies for the endoscopic assessment of duodenal villous pattern in celiac disease. Comput Biol Med 2015;65:308–14. [DOI] [PubMed] [Google Scholar]
- [3].Ianiro G, Bibbo S, Bruno G, et al. Prior misdiagnosis of celiac disease is common among patients referred to a tertiary care center: a prospective cohort study. Clin Transl Gastroenterol 2016;7:e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tommasini A, Not T, Kiren V, et al. Mass screening for coeliac disease using antihuman transglutaminase antibody assay. Arch Dis Child 2004;89:512–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Meloni G, Dore A, Fanciulli G, et al. Subclinical coeliac disease in schoolchildren from northern Sardinia. Lancet 1999;353:37. [DOI] [PubMed] [Google Scholar]
- [6].Byass P, Kahn K, Ivarsson A. The global burden of childhood coeliac disease: a neglected component of diarrhoeal mortality? PLoS ONE 2011;6:e22774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dore MP, Cuccu M, Pes GM, et al. Clinical pattern of celiac disease in a population residing in North Sardinia (Italy). Recenti Prog Med 2012;103:564–9. [DOI] [PubMed] [Google Scholar]
- [8].Guariso G, Conte S, Presotto F, et al. Clinical, subclinical and potential autoimmune diseases in an Italian population of children with coeliac disease. Aliment Pharmacol Ther 2007;26:1409–17. [DOI] [PubMed] [Google Scholar]
- [9].Assa A, Frenkel-Nir Y, Tzur D, et al. Large population study shows that adolescents with celiac disease have an increased risk of multiple autoimmune and nonautoimmune comorbidities. Acta Paediatr 2017;106:967–72. [DOI] [PubMed] [Google Scholar]
- [10].Canova C, Pitter G, Ludvigsson JF, et al. Celiac disease and risk of autoimmune disorders: a population-based matched birth cohort study. J Pediatr 2016;174:146.e1–52.e1. [DOI] [PubMed] [Google Scholar]
- [11].Dore MP, Pes GM, Dettori I, et al. Clinical and genetic profile of patients with seronegative coeliac disease: the natural history and response to gluten free diet. BMJ Open Gastroenterol 2017;4:e000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pes GM, Tolu F, Bazzu M, et al. Cholesterol change in coeliac patients following gluten-free diet depends on baseline levels. Dig Liver Dis 2014;46:662–3. [DOI] [PubMed] [Google Scholar]
- [13].Usai-Satta P, Oppia F, Scarpa M, et al. Delayed gastric empting does not normalize after gluten withdrawal in adult celiac disease. Scand J Gastroenterol 2016;51:923–6. [DOI] [PubMed] [Google Scholar]
- [14].Lundin KE, Wijmenga C. Coeliac disease and autoimmune disease-genetic overlap and screening. Nat Rev Gastroenterol Hepatol 2015;12:507–15. [DOI] [PubMed] [Google Scholar]
- [15].Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut 2014;63:1210–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rubio-Tapia A, Hill ID, Kelly CP, et al. American College of G. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 2013;108:656–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sardu C, Cocco E, Mereu A, et al. Population based study of 12 autoimmune diseases in Sardinia, Italy: prevalence and comorbidity. PLoS ONE 2012;7:e32487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Meloni A, Mandas C, Jores RD, et al. Prevalence of autoimmune thyroiditis in children with celiac disease and effect of gluten withdrawal. J Pediatr 2009;155:51–5. [DOI] [PubMed] [Google Scholar]
- [19].Cavalli-Sforza LL, Menozzi P, Piazza A. The History and Geography of Human Genes. Princeton, NJ: Princeton University Press; 1994. [Google Scholar]
- [20].Demirezer Bolat A, Akin FE, Tahtaci M, et al. Risk factors for polyautoimmunity among patients with celiac disease: a cross-sectional survey. Digestion 2015;92:185–91. [DOI] [PubMed] [Google Scholar]
- [21].Sategna-Guidetti C, Volta U, Ciacci C, et al. Prevalence of thyroid disorders in untreated adult celiac disease patients and effect of gluten withdrawal: an Italian multicenter study. Am J Gastroenterol 2001;96:751–7. [DOI] [PubMed] [Google Scholar]
- [22].Ventura A, Magazzu G, Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology 1999;117:297–303. [DOI] [PubMed] [Google Scholar]
- [23].Sategna Guidetti C, Solerio E, Scaglione N, et al. Duration of gluten exposure in adult coeliac disease does not correlate with the risk for autoimmune disorders. Gut 2001;49:502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ludvigsson JF, Ludvigsson J, Ekbom A, et al. Celiac disease and risk of subsequent type 1 diabetes: a general population cohort study of children and adolescents. Diabetes Care 2006;29:2483–8. [DOI] [PubMed] [Google Scholar]
- [25].Fallahi P, Ferrari SM, Ruffilli I, et al. The association of other autoimmune diseases in patients with autoimmune thyroiditis: review of the literature and report of a large series of patients. Autoimmun Rev 2016;15:1125–8. [DOI] [PubMed] [Google Scholar]
- [26].Houlston RS, Tomlinson IP, Ford D, et al. Linkage analysis of candidate regions for coeliac disease genes. Hum Mol Genet 1997;6:1335–9. [DOI] [PubMed] [Google Scholar]
- [27].Smyth DJ, Plagnol V, Walker NM, et al. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med 2008;359:2767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]


