Abstract
Background:
Splenosis is a benign and relatively uncommon condition caused by trauma or splenectomy or other procedures involving splenic tissue. It is usually asymptomatic, and often diagnosed accidentally, especially misdiagnosed as malignant tumor.
Methods:
A 54-year-old man with prior history of chronic hepatitis B virus infection and underwent splenectomy for traumatic splenic rupture following a traffic accident 23 years previously was admitted to our hospital and found a hepatic mass in the right upper quadrant during an imaging examination. The diagnosis of his was not clear and finally he agreed to receive a surgical treatment.
Results:
During the operation, we found a mass in the right posterior lobe of the liver and a hard nodule on the right side of the diaphragm, both were completely resected, and postoperative histopathologic examination revealed that all excised tissues were proved to have histological structure typical for the spleen.
Conclusions:
The occurrence of intrahepatic splenosis is rare with only few cases previously reported in the literature. It is a benign disease and sometimes difficult to distinguish from diseases of the liver. The need for positive surgical resection of splenosis is still controversial.
Keywords: case report, diagnosis, hepatocellular carcinoma, intrahepatic splenosis
1. Introduction
Splenosis is one type of ectopic splenic tissue (the other being accessory spleen), it refers to the phenomena of heterotopic autotransplantation and implantation of splenic tissue caused by trauma, surgery (e.g., splenectomy) or other procedures involving splenic tissue.[1] Although the incidence of splenosis after trauma or splenectomy is 44% to 76%,[2] splenosis found in clinical practice is relatively rare because most patients do not have obvious clinical manifestations. Mainly, it is only detected by accidental discovery during a physical examination or an imaging examination. In addition, because there are various sites of splenosis and variable shapes and sizes, it is often misdiagnosed by clinicians, especially when it is misdiagnosed as a malignant tumor and is surgically removed, which greatly increases the burden on patients with regard to their physical, psychological, and economic status. Therefore, increasing awareness of clinicians about heterotopic spleens after splenectomy and spleen trauma has become increasingly important. This case report describes the differential diagnosis of an intrahepatic mass in a patient with chronic hepatitis B and a history of splenectomy following abdominal trauma, who had no significant liver function and multiple tumor marker abnormalities. The mass was ultimately characterized as intrahepatic splenosis. In addition, 10 cases (Table 1) of intrahepatic splenosis with computed tomography (CT) or magnetic resonance imaging (MRI) were collected and reviewed here and some characteristics were found in recent years.
Table 1.
Review of the literatures about intrahepatic splenosis.
2. Case report
A 54-year-old man who had pain and discomfort in the right upper quadrant for 10 days was referred to our hospital for further treatment of a hepatic mass, which was discovered incidentally 1 week previously when the patient visited the local hospital for a physical examination. He had a history of chronic hepatitis B virus (HBV) infection, had not taken antiviral drugs for approximately 15 years, and had undergone splenectomy for traumatic splenic rupture (without concomitant liver injury) in 1994.
The physical examination revealed a paramedian incision of epigastrium and a sequela of surgical cicatrix of approximately 15 cm but no other positive signs. The patient's laboratory findings were within normal ranges with total bilirubin of 17.4 μmol/L, alanine aminotransferase of 16 U/L, aspartate aminotransferase of 19 U/L, alpha fetal protein (AFP) of 5.8 μg/L, des-γ-carboxy prothrombin (DCP) of 3.2 μg/L, carcinoembryonic antigen of 3.6 μg/L, carbohydrate antigen 19-9 of 8.2 U/mL, and carbohydrate antigen 125 of 10.6 U/mL. However, the serological markers of HBV infection were positive for hepatitis B surface antigen, hepatitis B e antibody, and hepatitis B core antibody. The HBV-DNA was 5.5 × 102 IU/mL. An abdominal ultrasound showed a round-like equal echo area approximately 3.0 × 2.8 cm between the liver and kidney (Fig. 1), and the boundary of this area was identified, which suggested the presence of a space-occupying lesion between the liver and kidney (the properties of which had not yet been determined, and it might have been derived from the liver). Nonhepato-specific contrast mediums of iodinated contrast agent and gadolinium diethylenetriaminepentaacetic acid were, respectively, applied to dynamic contrast-enhanced CT (Fig. 2) and MRI scans (Fig. 3) suggested the presence of an accessory spleen and that the nodular hepatocellular carcinoma (HCC) originated from the right posterior lobe of the liver. Because the lesion on the diaphragm was very small, we could not find it on imaging. It was clinically diagnosed as “a space-occupying lesion of the right liver: HCC.” Since the current diagnosis was unclear, an operation was undertaken for both diagnosis and treatment.
Figure 1.
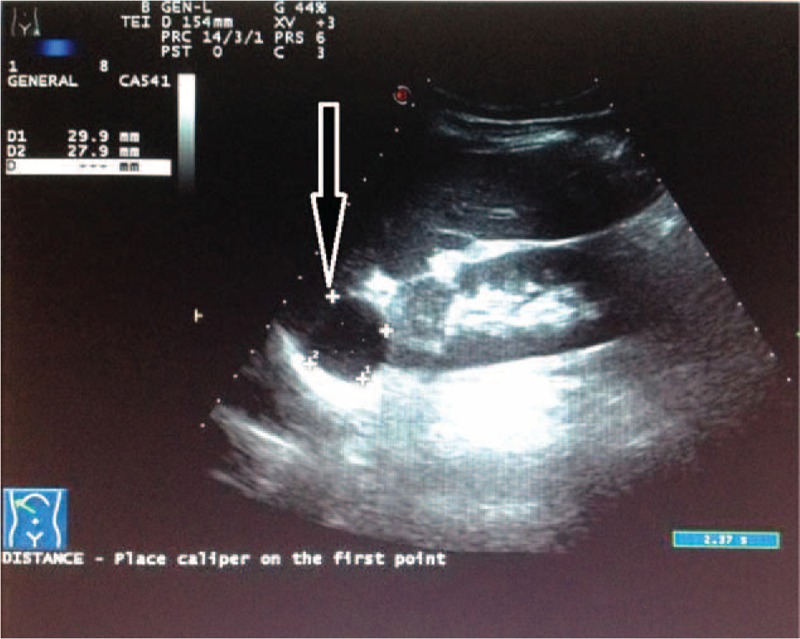
Abdominal ultrasound of intrahepatic splenosis (the arrow). It showed a round-like equal echo area approximately 3.0 × 2.8 cm between the liver and kidney.
Figure 2.
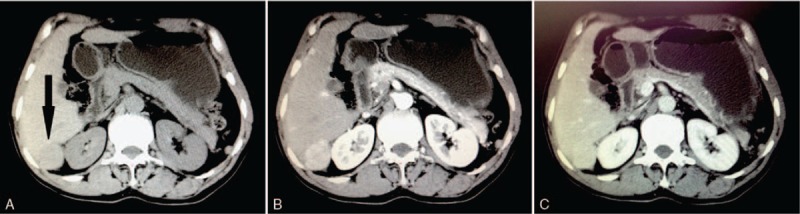
Contrast-enhanced computed tomography scan of intrahepatic splenosis (black arrow). It revealed a round solid hypodense mass (A) approximately 3.9 × 3.6 cm in the right lobe of the liver with strong homogeneous enhancement in the arterial phase (B); the lesion was hypodense during the portal phase (C).
Figure 3.
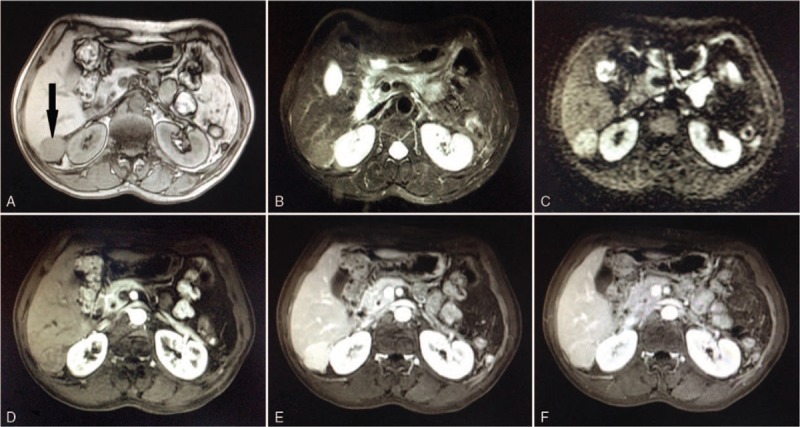
Magnetic resonance imaging scan of intrahepatic splenosis (black arrow). It showed a slight hypointense signal on T1WI (A) and high signal intensity on T2WI (B) as well as on DWI (C), an enhanced scan suggested uneven enhancement (E), and the signal decreased during the delay phase (F). DWI = diffusion weighted imaging, T1WI = T1 weighted imaging, T2WI = T2 weighted imaging.
During the operation, the mass was found to be partially embedded in the right posterior lobe of the liver. The intrahepatic mass was completely resected, and a section of the mass specimen that was approximately 3.0 × 3.0 cm appeared dark red, with a border surrounded by an incomplete capsule, which adhered tightly to the posterior peritoneal tissues. We also found a hard nodule approximately 0.8 × 0.7 cm on the right side of the diaphragm and resected it along with the intrahepatic mass. The postoperative surgical specimens revealed that the liver specimen was approximately 12.1 × 4.6 × 4.0 cm in size (Fig. 4A), and from the cut surfaces of the liver specimen, we could observe a dark red mass specimen of approximately 3.1 × 2.7 cm. The posterior peritoneal tissues and the diaphragmatic muscle tissue that were removed during the operation were approximately 0.9 × 0.6 cm and 0.7 × 0.6 cm, respectively. The pathological sections revealed that the intrahepatic mass was similar to splenic tissue (Fig. 4B). It was rich in spleen trabeculae and splenic sinuses. Furthermore, the suspected metastatic nodule on the diaphragm was also histologically proved to be splenosis. This study protocol was approved by the Biomedical Ethics Committee of the Eastern Hepatobiliary Surgery Hospital.
Figure 4.
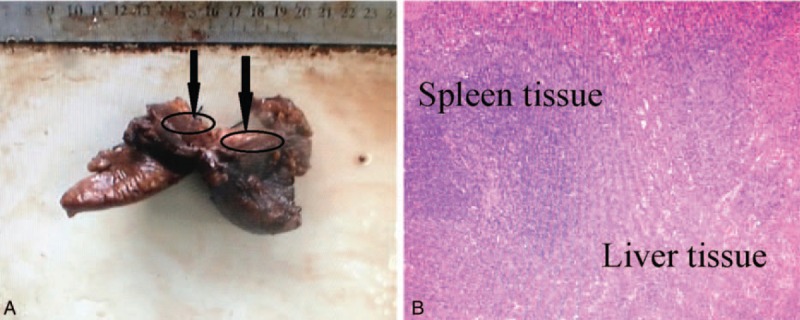
Histopathologic findings. (A) View of the surgical specimen, showing that it was 12.1 × 4.6 × 4.0 cm in size, and the red mass specimen of approximately 3.1 × 2.7 cm (black arrow); (B) histopathologic examination showed abundant spleen trabeculae and splenic sinuses that confirmed the diagnosis of splenosis, with no evidence of malignancy (hematoxylin and eosin, ×100).
The patient recovered well and was followed for 18 months without evidence of recurrence or progression.
3. Discussion
Splenosis occurs most commonly in young people, and 70% of patients are younger than 20 years of age at the time of the splenic injury. It also occurs more often in men than in women, especially in people at greater risk of spleen or abdominal injury. Splenosis can occur in various compartments of the body, such as the enterocoelia, pelvic cavity, pleural cavity, pericardium, subcutaneous tissue, and even the brain. The average time interval of splenosis that has been found after traumatic rupture of the spleen and splenectomy is 16 years (range: 5 months to 32 years),[11] and even up to 60 years has been recorded.[12] Most patients do not have obvious clinical manifestations or they only have slight symptoms. Moreover, the majority of cases only become evident 5 to 10 years after the trauma.[11] To a certain extent, the occurrence and development of ectopic spleen cultivation is a gradual process.
The spleen is the biggest peripheral immune organ in the human body that could protect the body from infection. Some data have shown that patients with ectopic splenic planting or an accessory spleen preserved after splenectomy have a better prognosis than those patients with a ruptured spleen that has been completely resected. This result suggests that residual accessory spleens after trauma and splenectomy or ectopic planted splenic tissues have similar functions as normal splenic tissues, can partially or fully play the role of the spleen in immune clearance of pathogens, and have certain benefits for immune function to compensate for the lack of a spleen.[13,14] Meanwhile, considering the fact that death caused by ectopic spleen is extremely rare, we do not advocate aggressive surgical intervention at this time. Therefore, the current view of most medical scholars is that when splenosis is diagnosed, surgical removal is recommended only in the case of symptomatic complicated splenosis and patients with hematological disease for whom splenectomy is beneficial.[15]
Thus far, splenic tissue ectopically planted in the liver has rarely been observed, and it is easy to clinically misdiagnose this as liver cancer and have patients undergo surgical resection, in which case the splenic tissue is often found intraoperatively and confirmed by subsequent surgical pathology. Therefore, combined with the clinical manifestations of patients, the auxiliary examination results, and the special history of splenectomy or traumatic rupture of the spleen, clinicians should be alert to the possibility of an ectopic spleen, and if this diagnosis can be confirmed before surgery, it may be possible to avoid surgery. B-mode ultrasonography, CT, and MRI can only play a role determining the location of the lesion and do not provide significant help for judging the nature of the lesion. Currently, there are more effective methods with higher diagnostic specificity and sensitivity; that is, B-mode ultrasound-guided fine needle aspiration cytology and 99mTc-heat denaturation of red blood cells (99mTc-DRBC) imaging.[8,12] Given that needle aspiration cytology for invasive manipulation does not completely rule out the possibility of malignancy before surgery, we advocate the use of the latter. To date, the mechanism of peritoneal splenosis has still not been very clear; however, the most common view is that intrahepatic splenosis is mainly caused by the following: seeding of injured splenic tissue fragments or cells and splenic pulp or splenic erythrocytic progenitor cell growth caused by hematogenous migration.[5,16] It has been noted that intrahepatic ectopic splenic tissue is mainly composed of red pulp containing a large number of red blood cells. In addition, the formation of red pulp is associated with the proliferation and differentiation of splenic erythrocytic progenitor cells. Therefore, we infer that the occurrence of intrahepatic splenosis is a complexly biological process that may have a certain relationship with the following 3 factors: local hypoxia of the liver caused by the aging of hepatocytes and pathological changes of liver; increases in the reactivity and sensitivity of splenic erythrocytic progenitor cells in the condition of hypoxia stress; and splenic erythrocytic progenitor cells migrating in a variety of ways from the spleen to the liver. Erythroid progenitor cells are primarily derived from bone marrow and the spleen; however, the reflection response is quite different in the case of hypoxia stress. Erythrogenesis in bone marrow is a homeostatic process, but erythrogenesis in the spleen will rise rapidly during stress. The molecular biological mechanism triggering the proliferation/differentiation of the erythrocytic progenitor cells has been elucidated. In the case of acute anemia, the proliferate/differentiate of the erythrocytic progenitor cells in the adult spleen is mainly mediated by BMP4 and Madh5, which may also be the main molecular mechanism for the formation of ectopic splenic tissues in the liver that are induced by a variety of factors.[17] Intrahepatic splenosis cannot be defined as a disease but should be considered an early sign of liver function recession or a compensatory response of the body to the lack of a spleen.[18]
In this case study, we noticed that many tumor markers were negative. As the most commonly used indicator of HCC screening, AFP plays an important role in the diagnosis, the evaluation of clinical efficacy, and the monitoring of tumor relapse. However, the AFP level may be normal in 35% to 45% of HCC patients, indicating the diagnostic limits of single HCC biomarker and nearly 40% of HCC patients may be missed.[19] Moreover, DCP was well documented to be another promising marker for the diagnosis of HCC, which was reported to have a higher diagnostic sensitivity and specificity than AFP in patients with HCC ≤ 3 cm, particularly for patients with well-differentiated HCC. It was found that serum DCP was not correlate with AFP levels, and had a sensitivity ranging from 48% to 62%, a specificity of 81% to 98%, and a diagnostic accuracy of 59% to 84% for the detection of HCC.[20] Studies had also shown that 30% of HCC patients with AFP negative were DCP positive, and combined assays of DCP and AFP increased the sensitivity of HCC diagnosis in more than 85% of patients, suggesting that the synthetic analysis of multiple tumor markers may reduce missed diagnosis and misdiagnosis and complement imaging examination for early HCC detection.[20,21] In addition, the detection of other HCC markers, such as AFP-L3, GPC-3, and Midkine, may also improve the HCC diagnosis performance.[22]
Intrahepatic splenosis is sometimes difficult to distinguish from diseases of the liver such as HCC, hepatic hemangioma, hepatic adenoma, and focal nodular hyperplasia, especially in patients with liver cirrhosis, aberrant tumor marker level, and background of hepatitis virus infection. This case is in accord with “quick in and quick out”—the characteristic imaging findings of malignant tumors. However, if doctors pay more attention to the special history of traumatic rupture of the spleen and splenectomy, the diagnosis may be made before surgery, unnecessary diagnostic procedures or inappropriate treatment may be avoided. This case should warn clinicians to take this type of rare case seriously and employ more analysis and judgment.
Acknowledgment
The authors thank all nurses and staff of our department for their precious help and cooperation.
Footnotes
Abbreviations: AFP = alpha fetal protein, CT = computed tomography, DCP = des-γ-carboxy prothrombin, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, MRI = magnetic resonance imaging.
W-CW and X-FL have contributed equally to this work.
Conception of the study: W-CW and X-FZ; design of the study: X-FZ, L-HS, and Z-LY; acquisition of data: W-CW, X-FL, YW, J-YM, and Z-LY; analysis and interpretation of data: W-CW and X-FZ; drafting of the article: W-CW and X-FL; and article revision: W-CW, X-FL, and X-FZ.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Jereb S, Trotovsek B, Skrbinc B. Hepatic splenosis mimicking liver metastases in a patient with history of childhood immatureteratoma. Radiol Oncol 2016;50:212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].He ZL, Xu X, Ke QH, et al. Successful diagnosis of intrahepatic splenosis mimicking hepatic tumor. Kaohsiung J Med Sci 2016;32:224–5. [DOI] [PubMed] [Google Scholar]
- [3].Toktaş O, Yavuz A, İliklerden Ü, et al. Intrahepatic splenosis after splenectomy performed for idiopathic thrombocytopenic purpura. Ulus Cerrahi Derg 2015;31:247–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li T, Yang XY, Tang ZY. Intrahepatic and intraperitoneal splenosis mimicking hepatocellular carcinoma with abdominal wall metastasis in a patient with hepatitis C cirrhotic liver. Surgery 2015;157:954–6. [DOI] [PubMed] [Google Scholar]
- [5].Liu C, Liu J, Wang FY. Intrahepatic splenosis mimicking liver cancer: report of a case and review of literature. Int J Clin Exp Pathol 2015;8:1031–5. [PMC free article] [PubMed] [Google Scholar]
- [6].Sato N, Abe T, Suzuki N, et al. Intrahepatic splenosis in a chronic hepatitis C patient with no history of splenic trauma mimicking hepatocellular carcinoma. Am J Case Rep 2014;15:416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tinoco González J, Suárez Artacho G, Ramallo Solís IM, et al. Intrahepatic splenosis as a differential diagnosis in focal liver lesions. Cir Esp 2014;92:690–1. [DOI] [PubMed] [Google Scholar]
- [8].Kang KC, Cho GS, Chung GA, et al. Intrahepatic splenosis mimicking liver metastasis in a patient with gastric cancer. J Gastric Cancer 2011;11:64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Menth M, Herrmann K, Haug A, et al. Intra-hepatic splenosis as an unexpected cause of a focal liver lesion in a patient with hepatitis C and liver cirrhosis: a case report. Cases J 2009;2:8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Abu Hilal M, Harb A, Zeidan B, et al. Hepatic splenosis mimicking HCC in a patient with hepatitis C liver cirrhosis and mildly raised alpha feto protein; the important role of explorative laparoscopy. World J Surg Oncol 2009;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rickert CH, Maasjosthusmann U, Probst-Cousin S, et al. A unique case of cerebral spleen. Am J Surg Pathol 1998;22:894–6. [DOI] [PubMed] [Google Scholar]
- [12].Baldari G, Sindoni A, Belletti A, et al. (99m)Tc disphosphonate uptake due to splenosis:incidental finding 60 years after splenectomy. Clin Nucl Med 2015;40:533–5. [DOI] [PubMed] [Google Scholar]
- [13].Liu Y, Ji B, Wang G, et al. Abdominal multiple splenosis mimicking liver and colon tumors: a case report and review of the literature. Int J Med Sci 2012;9:174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Leemans R, Manson W, Snijder JA, et al. Immune response capacity after human splenic autotransplantation: restoration of response to individual pneumococcal vaccine subtypes. Ann Surg 1999;229:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ksiądzyna D. A case report of abdominal splenosis—a practical minireview for a gastroenterologist. J Gastrointestin Liver Dis 2011;20:321–4. [PubMed] [Google Scholar]
- [16].Chinzei R, Tanaka Y, Shimizu-Saito K, et al. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology 2002;36:22–9. [DOI] [PubMed] [Google Scholar]
- [17].Lenox LE, Perry JM, Paulson RF. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood 2005;105:741–8. [DOI] [PubMed] [Google Scholar]
- [18].Kwok CM, Chen YT, Lin HT, et al. Portal vein entrance of splenic erythrocytic progenitor cells and local hypoxia of liver, two events cause intrahepatic splenosis. Med Hypotheses 2006;67:1330–2. [DOI] [PubMed] [Google Scholar]
- [19].Wang CS, Lin CL, Lee HC, et al. Usefulness of serum des-gamma-carboxy prothrombin in detection of hepatocellular carcinoma. World J Gastroenterol 2005;11:6115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bertino G, Ardiri AM, Calvagno GS, et al. Prognostic and diagnostic value of des-γ-carboxy prothrombin in liver cancer. Drug News Perspect 2010;23:498–508. [DOI] [PubMed] [Google Scholar]
- [21].Park SJ, Jang JY, Jeong SW, et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lou J, Zhang L, Lv S, et al. Biomarkers for hepatocellular carcinoma. Biomark Cancer 2017;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


