Abstract
There has been an increase in deaths from cardiovascular diseases following breast cancer therapy. Evidence has shown that this outcome is, in part, associated with cardiotoxicity induced by the chemotherapeutic drugs and the increase in oxidative stress. The aim of this study was to evaluate the effects of chemotherapy and hormone therapy with tamoxifen on the biomarkers of cardiac injury and oxidative stress in women with breast cancer.
Thirty women were followed-up for 1 year and were divided into 3 groups according to the treatment protocol: women treated only with tamoxifen and clinical follow up for 12 months (Tam, n = 10); women treated only with chemotherapy for 6 months with clinical follow up for an additional 6-month period (Chemo, n = 10); and women who received chemotherapy for 6 months followed by a 6-month period only with tamoxifen therapy and clinical follow up (Chemo + Tam, n = 10). Analysis of the blood levels of cardiac troponin I (cTnI), advanced oxidation protein products (AOPP) and the activity of the plasmatic isoform of the antioxidant enzyme glutathione peroxidase (GPx) was performed before treatment (T0) and at 6 (T6) and 12 (T12) months after treatment.
The Chemo group showed higher levels of cTnI (0.065 ± 0.006 ng/mL, P < .05) and AOPP (4.99 ± 0.84 μmol/L, P < .05) and reduced GPx activity (24.4 ± 1.1 nM/min/mL, P < .05) at T12 than the Tam group (cTnI: 0.031 ± 0.001 ng/mL; AOPP: 1.40 ± 0.10 μmol/L; GPx: 28.0 ± 0.7 nM/min/mL) and Chemo + Tam group (cTnI: 0.037 ± 0.002 ng/mL; AOPP: 2.53 ± 0.30 μmol/L; GPx: 29.5 ± 1.0 nM/min/mL).
These data support the hypothesis that long-term oxidative stress after chemotherapy may have an impact on cardiovascular diseases and that tamoxifen has cardioprotective effects.
Keywords: biomarkers, breast cancer, cardiac injury, chemotherapy, oxidative stress, tamoxifen
1. Introduction
In the last decade, there has been a large increase in patients’ life expectancy with great advances in the diagnosis and treatment of breast cancer. However, the long-term competitive risk of death by other causes is also increasing as women are getting older.[1] Consistent with this trend, the global disease-free survival has been the most important therapeutic outcome, but the assessment of the main causes of death after cancer treatment in this group of patients suggests that deaths are more related to the treatment than to cancer itself.[2]
The researchers speculate that the rates of cardiotoxicity cancer-related therapeutics amount to approximately 30%, and their effects may manifest several decades after the completion of treatment, conferring to the cardiac toxicity the title of the second most common cause of morbidity and mortality among survivors of the disease.[3] In fact, it was recently observed that cardiovascular disease (CVD) in cancer survivors significantly reduces the 8-year overall survival from 81% to 60% compared with cancer survivors without CVD.[4]
In this context, studies have shown that chemotherapeutic drugs used in the treatment of breast cancer can generate several adverse effects to the cardiovascular system, including ventricular dysfunction, cardiomyopathy and heart failure caused by anthracyclines.[5] Although the underlying mechanisms of these phenomena are not completely understood, it is believed that the molecular basis of drug toxicity are related to the augmented production of reactive oxygen species and consequent damage to the DNA, protein, and membrane lipids of healthy cells, thus contributing to the development of, or aggravating, preexisting CVD.[6,7]
The imbalance between the ROS generating system and the antioxidant system can lead to a condition termed oxidative stress, which is potentially harmful to tissues and cells.[8]
Oxidative stress is an important factor related to the injury in cardiac cells in the presence of chemotherapeutic agents, particularly anthracyclines, changing the cellular microenvironment, inducing disturbances in the contractile structures and apoptosis of the cardiomyocytes.[9–12]
The increase in protein oxidation due to oxidative stress represents an early indication of ROS-mediated tissue damage.[13] Therefore, in our study the analysis of protein oxidation was used as a biomarker to detect the early oxidative modifications and cytotoxic damages induced by the chemotherapy. On the other hand, the concentration of ROS is regulated mainly by the enzymatic antioxidant system, such as the glutathione peroxidase (GPx).[8]
Currently, the detection of chemotherapy-induced cardiotoxicity is carried out by a systematic evaluation of the left ventricular ejection fraction (LVEF) by transthoracic echocardiography.[14] However, there is growing interest in research with a focus on biomarkers that can predict more accurately and more rapidly cardiovascular damages before their onset, mainly because the echocardiography records cannot exclude the possibility of long-term cardiac damage in patients with normal LVEF.[15,16]
Thus, the aim of this study was to evaluate the effects of chemotherapy and hormone therapy with tamoxifen on biomarkers of cardiac injury and oxidative stress in women with breast cancer.
2. Materials and methods
2.1. Study design
The present study was performed between August 2012 and January 2014. Eighty-one women diagnosed with breast cancer––submitted or not to total or conservative breast surgery––were evaluated during this study, among whom, 49 met the inclusion criteria. From this amount, 17 patients did not follow the collection of biological samples, and 2 patients changed their address and continued the treatment at another hospital and, therefore, were excluded from the evaluation. Thus, 30 patients were enrolled as volunteers to participate in this study. The inclusion criteria were as follows: no previous history of chemotherapy treatment and cardiomyopathy; age between 40 and 60 years; not diagnosed with diabetes and/or hypertension; not a smoker; and not under hormone replacement therapy. The disease classification and therapeutic protocol followed the references of the Clinical Practice Guideline in Oncology of the National Comprehensive Cancer Network (www.nccn.org). Figure 1 shows the division of the groups: women treated only with tamoxifen (20 mg per day) and clinical follow up for 12 months (Tam, n = 10); women treated only with chemotherapy for 6 months with clinical follow up for an additional 6-month period (Chemo, n = 10); and women who received chemotherapy for 6 months followed by a 6-month period only with tamoxifen therapy (20 mg per day) and clinical follow up (Chemo + Tam, n = 10). All volunteers read and signed an informed consent form. The ethical principles were respected according to the 466/12 Resolution of the National Health Council as well as the Helsinki declaration. This study had prior approval of the Institutional Review Board of the Center for Health Sciences, Federal University of Espírito Santo—UFES (protocol no. 115.097/CAAE: 04929212.2.0000.5060).
Figure 1.
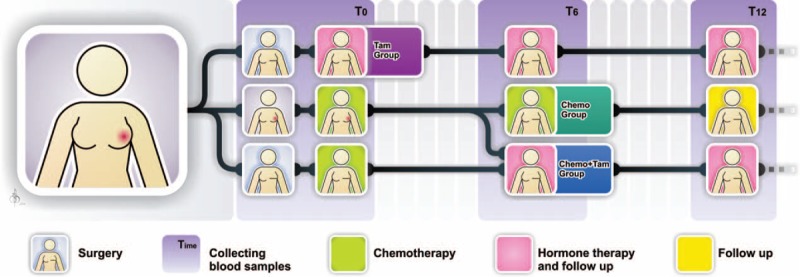
Timing of collection of blood samples and characterization of groups according to the treatment protocol. T0 = before treatment, T6 = 6 months after beginning treatment, T12 = 12 months after beginning treatment, Tam = women treated exclusively with tamoxifen, Chemo = women treated exclusively with chemotherapy, Chemo + Tam = women treated with tamoxifen after chemotherapy.
2.2. Biochemical analysis
Peripheral blood samples were collected from each patient upon research enrollment (baseline) (T0), and at 6 (T6) and 12 months (T12) after the first collection. The blood samples were obtained through conventional techniques of peripheral venipuncture preferably in the antecubital fossa using Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ). In total, 8 mL of blood were distributed in tubes with and without EDTA for plasma and serum separation, respectively. The samples were centrifuged at 3000 rpm for 10 minutes at 4°C, were aliquoted in microcentrifuge tubes (2 mL) and were frozen at −80°C.
2.3. Serum troponin I analysis
To evaluate the cardiomyocyte damage the serum concentration of cTnI was performed. A specific kit of the cardiac troponin I (cTnI) test system (Monobind, Inc., Lake Forest, CA) was used, and the reading was performed at 450 nm in a spectrophotometer (Synergy HT, BioTek Instruments, Inc., Winooski, VT). The concentration was given in nanograms per milliliter (ng/mL). The concentration range of the method was 0.1 to 50 ng/mL, and its analytical sensitivity was 0.030 ng/mL.
2.4. Advanced oxidation protein products
The advanced oxidation protein products (AOPP) were measured in microplates by spectrophotometry (Synergy HT, BioTek Instruments, Inc.) to access the oxidative stress during and after the treatment. The reaction was calibrated with chloramine-T (0–100 μM) (Vetec, Rio de Janeiro, Brazil) in the presence of potassium iodine (KI) and acetic acid at 340 nm. Plasma was diluted (1:5) in phosphate-buffered saline (PBS) followed by the addition of 10 μL of potassium iodine (1.16 M) and 20 μL of ultrapure glacial acetic acid. The optical density was read immediately at 340 nm along with the control wells containing 200 μL of PBS, 10 μL of KI, and 20 μL of acetic acid. All samples were analyzed in triplicate. The AOPP concentrations were expressed as μM of chloramine-T/mg of protein. For the determination of total proteins, the plasma was previously diluted (1:100) in distilled water and dosed by the Bradford method.[17]
2.5. Glutathione peroxidase activity
The effects of chemotherapy and of the treatments on the plasmatic activity of the antioxidant enzyme GPx were evaluated. An enzyme-coupled assay (Glutathione Peroxidase Assay Kit, Cayman Chemical Company, Ann Arbor, MI), consisting of an indirect measure of the coupling reaction with glutathione reductase (GR). NADPH oxidation was detected at an absorbance of 340 nm. The activity was measured in a microplate spectrophotometer (Synergy HT, BioTek Instruments, Inc.) of a final volume of 170 μL in each well with the following composition: 100 μL of assay buffer, 50 μL of a mixture of cosubstrates (NADPH, GSH, and GR lyophilized) and 20 μL of plasma, in triplicate. The reaction was started after the addition of plasma to the prewarmed reagent buffer (30°C) in the plate reader and was evaluated by spectrophotometry at 340 nm for 5 minutes. The data between the 1st and 4th minutes were analyzed (linear portion of the curve). The plasmatic GPx activity was equivalent to a reduction in the absorbance of 0.02 to 0.135 per minute and was described as nM/min/mL. The coefficient of variation reported by the manufacturer was 7.2%.
2.6. Statistical analyses
The data were expressed as the means ± standard error of the mean. The baseline values were analyzed by 1-way analysis of variance (ANOVA), and the treatment outcomes at the different times were analyzed by repeated-measures analysis of variance (RM-ANOVA), followed by Tukey posttest for multiple comparisons. The values were considered significant at P < .05.
3. Results
3.1. Characteristics of patients
Thirty women with a mean age of 53 ± 1.04 years, diagnosed with breast cancer were followed up during 1 year. Most of them were in the postmenopausal period (56.7%) and without significant differences in their body mass index (BMI) during the follow-up period. Invasive ductal cancer was the most prevalent histopathologic classification (77%) detected mainly in the left breast (63%). Partial mastectomy was the surgical procedure adopted in most cases (57%), and the chemotherapeutic adjuvant treatment with anthracyclines and cyclophosphamide predominated with an average variability of 7 and 6 cycles for the Chemo and Chemo + Tam groups, respectively. Thirty sessions of radiotherapy were also prescribed for 53.3% of the patients. During the study period, there was no death. Other clinical data are detailed in Table 1.
Table 1.
Data of the patients, disease, and treatment.
3.2. Biomarkers of cardiac injury and oxidative stress
3.2.1. Cardiac troponin I
No differences were detected in cardiac injury between the groups at T0 concerning the serum concentrations of cTnI (Tam, 0.037 ± 0.003 ng/mL; Chemo, 0.037 ± 0.001 ng/mL; Chemo + Tam, 0.041 ± 0.002 ng/mL). However, only the Chemo group showed significantly increased levels of cTnI at T12 (0.065 ± 0.006 ng/mL, P < .05) compared with the Tam (0.031 ± 0.001 ng/mL), and Chemo + Tam groups (0.037 ± 0.002 ng/mL). In fact, increased levels of cTnI were detected in the Chemo group at T6 (0.045 ± 0.003 ng/mL, P < .05) compared with that at T0 (0.037 ± 0.001 ng/mL), but this was not different from the other groups for the same period (T6: Tam, 0.035 ± 0.003 ng/mL; Chemo + Tam, 0.040 ± 0.002 ng/mL) (Fig. 2).
Figure 2.
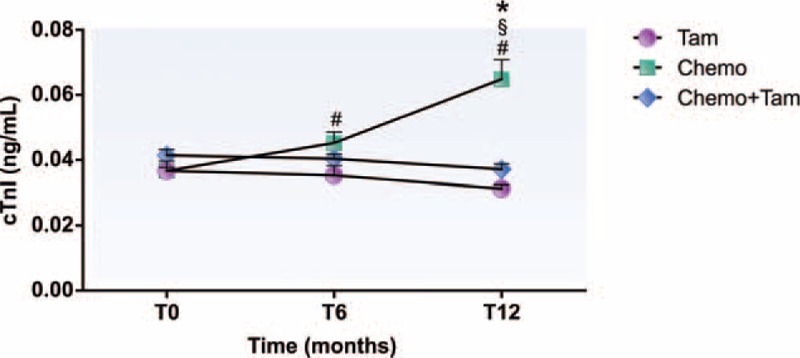
Cardiac troponin I serum concentration of different groups during the 12-month follow up. #P < .05 compared with T0; §P < .05 compared with T6; ∗P < .05 compared with Tam and Chemo + Tam groups. cTnI = cardiac troponin I, T0 = before treatment, T6 = 6 months after beginning treatment, T12 = 12 months after beginning treatment, Tam = women treated exclusively with tamoxifen, Chemo = women treated exclusively with chemotherapy, Chemo + Tam = women treated with tamoxifen after chemotherapy.
3.2.2. Advanced oxidation protein products
Regarding the oxidative stress analysis, Fig. 3 shows that only the Chemo group exhibited AOPP concentrations significantly higher at T12 (4.99 ± 0.84 μmol/L, P < .05) than those at T0 (2.13 ± 0.19 μmol/L) and T6 (3.03 ± 0.69 μmol/L) and those observed in the other groups for the same period (T12, Tam: 1.40 ± 0.10 μmol/L; T12, Chemo + Tam: 2.53 ± 0.30 μmol/L). Nevertheless, it is worth noting that the Chemo + Tam group showed levels of AOPP significantly higher (2.53 ± 0.30 μmol/L, P < .05) than those of the Tam group (1.40 ± 0.10 μmol/L).
Figure 3.
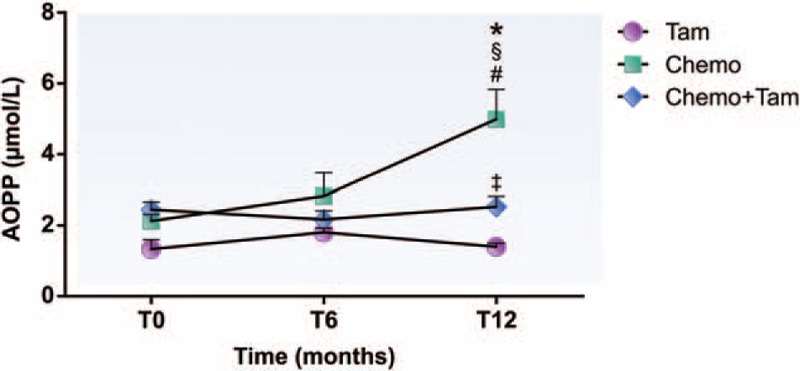
Advanced oxidation protein products plasma concentration of different groups during the 12-month follow up. #P < .05 compared with T0; §P < .05 compared to T6; ∗P < .05 compared with Tam and Chemo + Tam groups; ‡P < .05 compared with the group Tam T12. AOPP = advanced oxidation protein products, T0 = before treatment, T6 = 6 months after beginning treatment, T12 = 12 months after beginning treatment, Tam = women treated exclusively with tamoxifen, Chemo = women treated exclusively with chemotherapy, Chemo + Tam = women treated with tamoxifen after chemotherapy.
3.2.3. Glutathione peroxidase activity
As seen in Fig. 4, the analysis of plasmatic GPx antioxidant enzyme activity showed no differences between the groups at T0 (Tam: 27.6 ± 0.3 nM/min/mL; Chemo: 25.3 ± 1.5 nM/min/mL; Chemo + Tam: 25.5 ± 0.9 nM/min/mL). Nevertheless, the Chemo + Tam group showed an increase in GPx activity at T6 (28.4 ± 1.3 nM/min/mL, P < .05) that persisted until T12 (29.5 ± 1.0 nM/min/mL, P < .05) compared with that at T0. On the other hand, the Chemo group showed a significant reduction in GPx activity at T12 (24.4 ± 1.1 nM/min/mL, P < .05) compared with that at T6 (27.4 ± 1.0 nM/min/mL) and to the other groups at T12 (Tam: 28.0 ± 0.7 nM/min/mL, P < .05; Chemo + Tam 29.5 ± 1.0 nM/min/mL, P < .05). No alterations during the study period were observed in the Tam group (T0: 27.6 ± 0.3 nM/min/mL; T6: 27.3 ± 0.2 nM/min/mL; T12: 28.0 ± 0.7 nM/min/mL).
Figure 4.
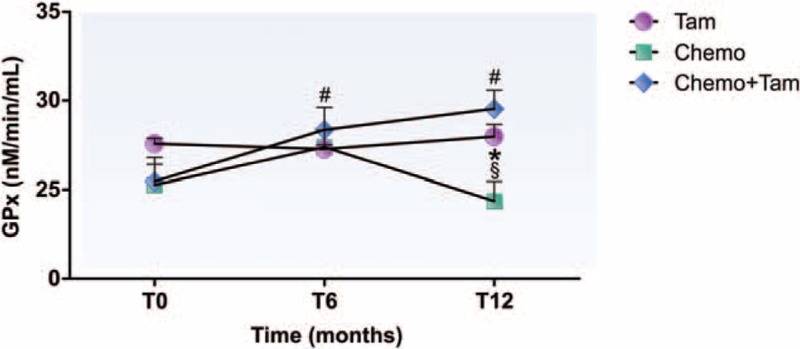
Glutathione peroxidase activity in plasma of different groups during the 12-month follow up. #P < .05 compared with T0; §P < .05 compared with T6; ∗P < .05 compared with Tam and Chemo + Tam groups. GPx = glutathione peroxidase, T0 = before treatment, T6 = 6 months after beginning treatment, T12 = 12 months after beginning treatment, Tam = women treated exclusively with tamoxifen, Chemo = women treated exclusively with chemotherapy, Chemo + Tam = women treated with tamoxifen after chemotherapy.
4. Discussion
The present study demonstrated that women submitted to chemotherapy had increased levels of circulating cTnI and AOPP and reduced plasmatic GPx enzyme activity, mainly 6 months after the end of the treatment. However, the women submitted to hormone therapy with tamoxifen maintained their levels of cTnI and AOPP at a similar level to that of baseline, and they also showed an increase in plasmatic GPx activity. These findings provide additional evidence about the increased cardiovascular risk caused by chemotherapy.
Although the determination of cardiovascular risk in patients exposed to chemotherapeutic drugs is of a complex nature, several studies have proposed that increased oxidative stress upon chemotherapy with anthracyclines is primarily responsible for damage to cardiomyocytes.[9–12] An example of this is the evidence that doxorubicin induces an increase in reactive oxygen species, such as the production of mitochondrial superoxide anion, peroxyl, and alkoxyl mediated by iron oxidation.[18] Moreover, we also observed that women treated only with chemotherapy presented plasma concentrations of cTnI greater than those of women treated only with tamoxifen. However, it is important to note that the cTnI concentrations detected in all groups were within the normal range for healthy subjects. Interestingly, Cardinale et al[9] noted that a small portion of patients whose cTnI concentrations were normal also developed transient ventricular dysfunction, characterized by reduced LVEF. In previous research from our laboratory, we also observed subclinical changes in the proBNP levels and LVEF outcomes in patients who received only chemotherapy for breast cancer. In this case, the data were significantly higher than those for patients receiving tamoxifen.[19] These findings encourage the possibility that such subclinical changes are predictive of cardiac damage in the future.
The correlation between chemotherapy-associated cardiovascular damage and circulating levels of cTnI has been observed in clinical studies. The increase in cTnI concentrations during chemotherapeutic treatment with anthracyclines and 1 month after the end of treatment found that 63 of 703 patients were more likely to have deleterious cardiac events.[20] Hence, the use of anthracyclines was identified as a risk factor for cardiotoxicity after treatment, revealing progressive damage of cardiomyocytes along with an increase of cTnI levels, even in patients with normal LVEF.[21] Sawaya et al investigated 81 patients with breast cancer treated with chemotherapy for 15 months. In 32% of these patients, cTnI could predict subsequent heart damage, such as the reduction in systolic longitudinal myocardial strain and consequent reduction in LVEF. However, there is a lack of studies investigating the effects of hormone therapy with tamoxifen on cTnI levels.[22]
Unlike the group treated only with chemotherapy, there were no changes in the concentrations of cTnI in patients treated with tamoxifen alone or tamoxifen plus chemotherapy. Nonexposure to chemotherapy may explain, in part, the stability of cTnI levels in the group treated only with tamoxifen. Thus, we believe that the stability of cTnI in the women treated with Chemo + Tam occurs due to the beginning of hormone therapy with tamoxifen by some volunteers who had finished chemotherapy sessions in a period earlier than 6 months of sample collection.
It is important to note that although anthracyclines are indicated as one of the main chemotherapeutic treatments responsible for cardiovascular risk, they do not constitute alone the treatments presented in this study. Other chemotherapeutics associated with anthracyclines also exhibit cardiotoxic potentials that may have influenced our results. Cyclophosphamide (also present in all drug combinations described in our methodology) is recognized for contributing to the increase in oxidative stress during its metabolism by the citrocom P450,[23] which can be virtually correlated with cardiotoxicity. However, according to the clinical trial of De Azambuja et al,[24] adverse effects on LVEF and the heart rate of patients receiving cyclophosphamide-based chemotherapy were lower than those observed in the anthracycline-treated group, as well as no changes in cTnI levels were detected in both groups 18 years after the end of treatment.
Recent data on acute treatment with paclitaxel have indicated a disruption in the cholesterol synthesis pathway, with a reduction in plasma levels of high-density lipoproteins and an increase in hydroperoxide levels, and an increase in the concentration of C-reactive protein and creatine kinase.[25] Similar results were observed in previous studies of our laboratory after a year of observation of patients who also used paclitaxel associated with the therapeutic regimen.[26]
Initially, the cardiotoxicity of docetaxel has been described by its effect on increasing the density of microtubules, leaving them more stable and preventing the division of tumor cells. However, this effect on cardiac cells would potentiate a contractile dysfunction in hypertrophy.[27] In a clinical evaluation of the administration of a dose of docetaxel (52.36 mg/m2) in women with breast cancer and skin metastasis, it was suggested that docetaxel induced acute left ventricular diastolic dysfunction and a transient increase in serum brain natriuretic peptide (BNP) without increasing both preload or afterload.[28] If these results are projected over the years following treatment, we cannot rule out their contribution to the development of diseases such as heart failure, especially in patients with ventricular dysfunction.
Most studies investigating the cardiovascular effects of tamoxifen have shown cardioprotective effects of hormone therapy by modulation of the lipid profile and inflammatory markers.[29–31] However, none of these studies highlighted the impact of tamoxifen after chemotherapy and its effects on the cardiovascular system. In another study from our laboratory, we demonstrated that women treated with tamoxifen after chemotherapy had higher concentrations of apolipoprotein A in relation to apolipoprotein B and reduced plasma levels of C-reactive protein, all important cardiovascular risk biomarkers.[26] These findings support the hypothesis that the cardioprotective effects of tamoxifen against damage caused by chemotherapy on the cardiovascular system may be a result of long-term mechanisms. Cardiotoxicity resulting from anthracycline-based antineoplastic treatments is an important parameter in the therapeutic prognosis that has driven experimental and clinical investigations to understand its mechanisms and establish appropriate management.
This is the first study assessing AOPP as a biomarker of oxidative stress sensitive to different treatment protocols for breast cancer. This biomarker is directly involved in the death of cardiomyocytes in vitro and in cardiac lesions in vivo.[32] In this regard, we observed that women undergoing chemotherapy alone or combined with tamoxifen showed higher plasma concentrations of AOPP at the end of the study than those treated only with tamoxifen. However, the women treated only with chemotherapy showed AOPP concentrations even higher than those who incorporated the tamoxifen in to the treatment. We believe that these results can be attributed, in part, to the antioxidant effects of tamoxifen, which act as a scavenger of reactive oxygen species to reduce the protein oxidation triggered by chemotherapy. In this context, Ek et al[33] reported that tamoxifen could reduce the levels of lipid peroxidation, malondialdehyde, and creatine kinase activity in animals subjected to ischemia and the reperfusion protocol. These animals also showed increased concentrations of glutathione and glutathione peroxidase.[33]
Therefore, it is important to note that the significant increase in the AOPP levels among the patients treated exclusively with chemotherapy was detected only at the final evaluation. In fact, chemotherapy was the only stressful condition for which all the volunteers engaged in this study were exposed because they did not present any previous cardiac and metabolic alterations. On the other hand, at the end of the evaluation period, it seems that the residual oxidative effects of the chemotherapy have overlapped with the antioxidant system capacity.
Although alterations in the AOPP levels were not detected at the sixth month, we observed the indications of cardiac injury (rise in circulating cTnI), which can be a consequence of the oxidative stress caused by treatment based only on chemotherapy. At this initial stage, anthracyclines may activate other pathways (MAPK/ERK), which causes the increase in the expression of proapoptotic factors, such as p53, BAX, and BAK.[18] Moreover, its interference on the iron metabolism (Fe3+)[34] and in the cardiac synthesis of ankyrin repeat protein increases reactive oxygen species production and compromises the myocyte survival and cellular transcription, respectively.[35] In a prospective study, an increase in proapoptotic proteins (sFas and sTRAIL) was detected 1 year after the end of the chemotherapy treatment for breast cancer, evidencing the chronic residual effects of this treatment.[36] However, the authors did not find a correlation between these biomarkers levels with the predictive risk of cardiac dysfunction or with the subclinical alterations in the left ventricle ejection fraction and NT-proBNP levels (a biomarker of heart failure), also detected in the study.
Corroborating our data, Perik et al[36] reported that a chemotherapy regimen led to an increase in the plasmatic concentration of apoptotic proteins in breast cancer for a period of 6 years after treatment began.[37] Vera-Ramirez et al[38] also noted that chemotherapy with anthracyclines and/or taxanes produce a certain level of systemic oxidative stress, which is maintained throughout the treatment and correlates with higher levels of oxidized proteins and DNA damage. This effect could have a negative impact on the clinical outcomes of women with breast cancer, although women undergoing hormone therapy had greater disease-free survival.
We also found that women who had made use of tamoxifen alone or after chemotherapy maintained stable levels of AOPP. In part, it can be speculated that the prescribed hormone therapy after chemotherapy reduced oxidative stress in the months following the assessment, thus reducing the deleterious effects of chemotherapy. Recently, experimental studies have shown that the use of tamoxifen improves the antioxidant response, endothelial dysfunction, and inflammation. Indeed, tamoxifen increases the enzyme activity of aldehyde dehydrogenase which is responsible for the protection against the toxic effects of aldehydes, such as lipid peroxidation.[39] Moreover, tamoxifen improves the endothelium-dependent vasorelaxation, increasing the expression of the endothelial nitric oxide synthase and prevents the release of proinflammatory cytokines.[40]
Another important result found in this study was the reduced activity of the plasmatic isoform of GPx enzyme in women undergoing chemotherapy. Also, the increased concentration of this enzyme in the group treated with tamoxifen after chemotherapy suggests that chemotherapy may have an impact on this enzyme. These data corroborate the hypothesis that tamoxifen has estrogen agonist effects on the antioxidant system. Population studies have observed that women in the fertile period have higher GPx plasmatic activity and levels than men, likely because of the action of estrogens.[41,42]
Indeed, it was reported that estrogen has a regulatory function in the expression of plasmatic GPx.[43] In cell culture, estrogen could increase the expression of antioxidant enzymes such as SOD and GPx mediated by the intracellular signaling pathways ERK, MAPK, and the nuclear transcription factor kappa B.[44] Similarly, phytoestrogens derived from Agaricus blazei Murril mushroom can stimulate the synthesis of plasmatic GPx, therefore preventing the vascular oxidative damage of atherosclerosis.[45] The study by Ek et al[33] showed that animals treated with tamoxifen had higher plasmatic concentrations of glutathione and GPx after a protocol of ischemia and reperfusion compared with the control group. Given the above evidence and data found in our study, we believe that hormone therapy may maintain the antioxidant activity of GPx in women treated with tamoxifen after chemotherapy.
Although there was an increase in GPx activity in women treated with chemotherapy and tamoxifen at the sixth-month follow up (when compared to baseline), it was not different from the findings of women treated only with chemotherapy for the same period. However, we believe that physiological defense mechanisms were triggered in both groups, differing only in the following 6 months. This fact can be evidenced by the observation of patients undergoing chemotherapy and parenteral nutritional supplementation after bone marrow transplant. These patients also showed a progressive increase in GPx activity correlated with physiological antioxidant defense.[46] Brigelius-Flohé and Maiorino[47] described that there is an upregulation of the plasmatic GPx isoform resulting from oxidative stress during the inflammatory process, as a protective response mechanism.
We also observed that chemotherapy alone led to a significant decline in GPx activity between the sixth month and end of the study compared with tamoxifen alone or after chemotherapy. These results are likely associated with chronic residual effects of chemotherapy, which escalates the consensual concern about the cardiotoxic effects of anthracyclines. Mercuro et al[48] described an association between the reduction in GPx concentration, the increase in the proinflammatory cytokine interleukin-6, and echocardiographic changes in patients treated with anthracyclines. The authors raised the hypothesis that mild cardiac anomalies can occur at cumulative levels of the drug, even at low doses. Recently, the reduction in GPx activity was correlated inversely and linearly with mortality from cardiovascular diseases (including coronary heart disease, arteriosclerotic diseases, and stroke) in subjects with low concentrations of high-density lipoprotein, independently of conventional risk factors.[49]
A potential limitation of our study is the fact that it was a single-center study and the total number of patients became limited. This occurred mainly because some patients did not meet our inclusion criteria and a portion of the patients who met the criteria gave up following the study for personal reasons.
The therapeutic outcome of disease-free patients is by far the most desired in the treatment of breast cancer. However, evidence has questioned the impact of chemotherapy on the damage of other organs and systems, which in turn, has driven research towards the early identification of risks. Indeed, we have demonstrated in the present study an important relationship between the antineoplastic treatments over the biomarkers of oxidative stress and cardiac injury, where it was possible to observe that women with breast cancer only submitted to chemotherapy showed an increase in oxidative stress associated with a subclinical increase of cTnI and a decrease in GPx activity, unlike women who are treated with tamoxifen after the end of chemotherapy.
Based on these findings, our study concludes that there is evidence of health-related changes in chemotherapy treatment, over a period of 6 months after its completion. However, although the biomarkers are within the spectrum of levels clinically accepted, they are significant and heterogeneous compared with the other therapeutic modalities evaluated. In addition, tamoxifen hormone therapy was demonstrated to not only maintain the levels of cardiac injury and oxidative stress biomarkers but also enhance one of the antioxidant defense mechanisms in women who have been prescribed their use after chemotherapy. Therefore, new studies are needed to investigate a direct correlation with cardiac function and to evaluate the changes that may have developed in the years ahead of the end of the treatments, as well as to analyze the survival rates in the different treatment groups.
Acknowledgments
We would like to thank the Santa Rita de Cassia Hospital for opening the doors of their clinics and each patient who kindly volunteered for this research.
Footnotes
Abbreviations: AOPP = advanced oxidation proteins products, Chemo = women treated with chemotherapy, Chemo + Tam = women treated with tamoxifen after chemotherapy, cTnI = cardiac troponin I, GPx = glutathione peroxidase, Tam = women treated with tamoxifen.
The authors have no conflicts of interest to disclose.
References
- [1].Du XL, Fox EE, Lai D. Competing causes of death for women with breast cancer and change over time from 1975 to 2003. Am J Clin Oncol 2008;31:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chapman JA, Meng D, Shepherd L, et al. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst 2009;100:252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fradley MG, Brown AC, Shields B, et al. Developing a comprehensive cardio-oncology program at a cancer institute: the Moffitt Cancer Center Experience. Oncol Rev 2017;11:340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Armenian SH, Xu L, Ky B, et al. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J Clin Oncol 2016;34:1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Raj S, Franco VI, Lipshultz SE. Anthracycline-induced cardiotoxicity: a review of pathophysiology, diagnosis, and treatment. Curr Treat Options Cardiovasc Med 2014;16:314–28. [DOI] [PubMed] [Google Scholar]
- [6].Chen Y, Jungsuwadee P, Vore M, et al. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv 2007;7:147–56. [DOI] [PubMed] [Google Scholar]
- [7].Zhao Y, McLaughlin D, Robison E, et al. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with doxorubicin chemotherapy. Cancer Res 2010;70:9287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kanaan GN, Harper ME. Cellular redox dysfunction in the development of cardiovascular diseases. Biochim Biophys Acta 2017;1861(11 Pt A):2822–9. [DOI] [PubMed] [Google Scholar]
- [9].Cardinale D, Sandri M, Martinoni A, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol 2000;36:517–22. [DOI] [PubMed] [Google Scholar]
- [10].Heide RS, L’Ecuyer TJ. Molecular basis of anthracycline-induced cardiotoxicity. Heart Metabol 2007;35:1–4. [DOI] [PubMed] [Google Scholar]
- [11].Verma S, Ewer MS. Is cardiotoxicity being adequately assessed in current trials of cytotoxic and targeted agents in breast cancer? Ann Oncol 2011;22:1011–8. [DOI] [PubMed] [Google Scholar]
- [12].Jiji RS, Kramer CM, Salerno M. Non-invasive imaging and monitoring cardiotoxicity of cancer therapeutic drugs. J Nucl Cardiol 2012;19:377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Klafke JZ, Porto FG, Batista R, et al. Association between hypertriglyceridemia and protein oxidation and proinflammatory markers in normocholesterolemic and hypercholesterolemic individuals. Clin Chim Acta 2015;448:50–7. [DOI] [PubMed] [Google Scholar]
- [14].Plana JC, Galderisi CM, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014;27:911–39. [DOI] [PubMed] [Google Scholar]
- [15].Cardinale D, Cipolla CM. Assessment of cardiotoxicity with cardiac biomarkers in cancer patients. Herz 2011;36:325–32. [DOI] [PubMed] [Google Scholar]
- [16].Colombo A, Sandri M, Salvatici M, et al. Cardiac complications of chemotherapy: role of biomarkers. Curr Treat Options Cardiovasc Med 2014;16:312–25. [DOI] [PubMed] [Google Scholar]
- [17].Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- [18].Geisberg CA, Sawyer DB. Mechanisms of anthracycline cardiotoxicity and strategies to decrease cardiac damage. Curr Hypertens Rep 2010;12:404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Silva FB, Romero WG, Carvalho AR, et al. Hormone therapy with tamoxifen reduces plasma levels of NT-B-type natriuretic peptide but does not change ventricular ejection fraction after chemotherapy in women with breast cancer. Braz J Med Biol Res 2015;48:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cardinale D, Sandri M, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004;109:2749–54. [DOI] [PubMed] [Google Scholar]
- [21].Ewer MS, Ewer SM. Troponin I provides insight into cardiotoxicity and the anthracycline-trastuzumab interaction. J Clin Oncol 2010;28:3901–4. [DOI] [PubMed] [Google Scholar]
- [22].Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circulation 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mansour HH, El kiki SM, Hasanb HF. Protective effect of N-acetylcysteine on cyclophosphamide-induced cardiotoxicity in rats. Environ Toxicol Pharmacol 2015;40:417–22. [DOI] [PubMed] [Google Scholar]
- [24].De Azambuja E, Ameye L, Diaz M, et al. Cardiac assessment of early breast cancer patients 18 years after treatment with cyclophosphamide-, methotrexate-, fluorouracil- or epirubicin-based chemotherapy. Eur J Cancer 2015;51:2517–24. [DOI] [PubMed] [Google Scholar]
- [25].Panis C, Binato R, Correa VJ, et al. Short infusion of paclitaxel imbalances plasmatic lipid metabolism and correlates with cardiac markers of acute damage in patients with breast cancer. Cancer Chemother Pharmacol 2017;80:469–78. [DOI] [PubMed] [Google Scholar]
- [26].Romero WG, Silva FB, Borgo MV, et al. Tamoxifen alters the plasma concentration of molecules associated with cardiovascular risk in women with breast cancer undergoing chemotherapy. Oncologist 2012;17:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sato H, Nagai T, Kuppuswamy D, et al. Microtubule stabilization in pressure overload cardiac hypertrophy. J Cell Biol 1997;139:963–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shimoyama M, Murata Y, Sumi K, et al. Docetaxel induced cardiotoxicity. Heart 2001;86:217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cushman M, Constantino JP, Tracy RP, et al. Tamoxifen and cardiac risk factors in healthy women: suggestion of an anti-inflammatory effect. Arterioscler Throm Vasc Biol 2001;21:255–61. [DOI] [PubMed] [Google Scholar]
- [30].Hayes DF, Skaar TC, Rae J, et al. Estrogen receptor genotypes, menopausal status, and the effects of tamoxifen on lipid levels: revised and updated results. Clin Pharmacol Ther 2010;88:626–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Maurea N, Coppola C, Ragone G, et al. Women survive breast cancer but fall victim to heart failure: the shadows and lights of targeted therapy. J Cardiovasc Med 2010;11:861–8. [DOI] [PubMed] [Google Scholar]
- [32].Valente AJ, Yoshida T, Clark RA, et al. Advanced oxidation protein products induce cardiomyocyte death via Nox2/Rac1/superoxide-dependent TRAF3IP2/JNK signaling. Free Radic Biol Med 2013;60:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ek RO, Yildiz Y, Cecen S, et al. Effects of tamoxifen on myocardial ischemia-reperfusion injury model in ovariectomized rats. Mol Cell Biochem 2008;308:227–35. [DOI] [PubMed] [Google Scholar]
- [34].Horenstein MS, Vander Heide RS, L’Ecuyer TJ. Molecular basis of anthracycline-induced cardiotoxicity and its prevention. Mol Genet Metab 2000;71:436–44. [DOI] [PubMed] [Google Scholar]
- [35].Zhang N, Xie XJ, Wang JA. Multifunctional protein: cardiac ankyrin repeat protein. J Zhejiang Univ Sci B 2016;17:333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Perik P, Vries EGE, Boomsma F, et al. The relation between soluble apoptotic proteins and subclinical cardiotoxicity in adjuvant-treated breast cancer patients. Anticancer Res 2006;26:3803–12. [PubMed] [Google Scholar]
- [37].Perik PJ, Van Der Graaf WTA, De Vries EGE, et al. Circulating apoptotic proteins are increased in long-term disease-free breast cancer survivors. Acta Oncol 2006;45:175–83. [DOI] [PubMed] [Google Scholar]
- [38].Vera-Ramirez L, Sanchez-Rovira P, Ramirez-Tortosa MC, et al. Does chemotherapy-induced oxidative stress improve the survival rates of breast cancer patients? Antioxid Redox Signal 2011;15:903–9. [DOI] [PubMed] [Google Scholar]
- [39].Belmont-Díaz JA, Calleja-Castañeda LF, Yoval-Sánchez B, et al. Tamoxifen, an anticancer drug, is an activator of human aldehyde dehydrogenase 1A1. Proteins 2015;83:105–16. [DOI] [PubMed] [Google Scholar]
- [40].Lamas AZ, Caliman IF, Dalpiaz PLM, et al. Comparative effects of estrogen, raloxifene and tamoxifen on endothelial dysfunction, inflammatory markers and oxidative stress in ovariectomized rats. Life Sci 2015;124:101–9. [DOI] [PubMed] [Google Scholar]
- [41].Rush JWE, Sandiford SD. Plasma glutathione peroxidase in healthy young adults: influence of gender and physical activity. Clin Biochem 2003;36:345–51. [DOI] [PubMed] [Google Scholar]
- [42].Ho SP, Chan-Yeung M, Chow K, et al. Antioxidant enzyme activities in healthy Chinese adults: influence of age, gender and smoking. Respirology 2005;10:305–9. [DOI] [PubMed] [Google Scholar]
- [43].O’Lone R, Knorr K, Jaffe IZ, et al. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol 2007;21:1281–96. [DOI] [PubMed] [Google Scholar]
- [44].Vina J, Gambini J, Lopez-Grueso R, et al. Females live longer than males: role of oxidative stress. Curr Pharm Des 2011;17:3959–65. [DOI] [PubMed] [Google Scholar]
- [45].Dong S, Furutania Y, Suto Y, et al. Estrogen-like activity and dual roles in cell signaling of an Agaricus blazei Murrill mycelia-dikaryon extract. Microbiol Res 2012;167:231–7. [DOI] [PubMed] [Google Scholar]
- [46].Jonas CR, Puckett AB, Jones DP, et al. Plasma antioxidant status after high-dose chemotherapy: a randomized trial of parenteral nutrition in bone marrow transplantation patients. Am J Clin Nutr 2000;72:181–9. [DOI] [PubMed] [Google Scholar]
- [47].Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta 2013;1830:3289–303. [DOI] [PubMed] [Google Scholar]
- [48].Mercuro G, Cadeddu C, Piras A, et al. Early epirubicin-induced myocardial dysfunction revealed by serial tissue Doppler echocardiography: correlation with inflammatory and oxidative stress markers. Oncologist 2007;12:1124–33. [DOI] [PubMed] [Google Scholar]
- [49].Buijsse B, Lee DH, Steffen L, et al. Low serum glutathione peroxidase activity is associated with increased cardiovascular mortality in individuals with low HDLc's. PLoS ONE 2012;7:e38901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


