Abstract
Rationale:
Malignant ascites (MA) is one of the poor prognostic factors for advanced pancreatic cancer and can bring about serious symptoms. The improvement of quality of life for patients is priority. However, there is no standard method for the treatment for pancreatic cancer-mediated MA. Apatinib is a novel and highly selective tyrosine kinase inhibitor targeting vascular endothelial growth factor receptor-2. There are no reports of concurrent apatinib with gemcitabine in patients with pancreatic cancer-mediated MA.
Patient concerns:
Herein, we presented a 64-year-old man patient who visited hospital due to abdominal pain for 1 month.
Diagnoses:
He was initially diagnosed with pancreatic cancer and his first symptom was MA.
Interventions:
After failing in tube drainage and gemcitabine therapy, the patient received gemcitabine combined apatinib orally and after administrated 1 month, the MA was evaluated as nearly clear response according to the RECIST 1.1 standard, and without further need of paracentesis. The CEA and CA199 reached the lowest level after administrating for 2.5 months during the treatment process.
Outcomes:
10.5 months following apatinib administration, the patient achieved a progression-free survival for more than 11 months. Hypertension (grade IV), hand-foot syndrome (grade I) and proteinuria (grade II) were observed.
Lessons:
It indicated that apatinib concurrent gemcitabine may be a superior choice for pancreatic cancer-mediated MA. Further clinical trials required to confirm its efficacy and safety.
Keywords: apatinib, gemcitabine, malignant ascites, pancreatic cancer
1. Introduction
Malignant ascites (MA) is a common manifestation of advanced pancreatic cancer and presents with a poor prognosis, with a median overall survival (OS) of 1 to 3 months.[1,2] One of the highest priorities is the active management of coeliac effusion for improving the patients’ quality of life. The current mode of therapy for patients with MA primarily depends on local treatment, such as tube drainage, and intrapleural administration of chemotherapeutic agents such as platinum-containing regimen.[3] These methods generally only relieve symptoms temporarily, and MA would ultimately recur soon.[4] In addition, repeated paracentesis is associated with iterative hospital admissions, which could lead to a number of problems, including infection, soreness, protein loss, and hypovolemia.
The mechanism of MA has not been fully elucidated while the function of neoangiogenesis and vascular permeability receive more attention. Apatinib (Hengrui Pharmaceutical Co., Ltd, Lianyungang, People's Republic of China), a novel small molecular tyrosine kinase inhibitor (TKI) targeting vascular endothelial growth factor receptor (VEGFR) 2, was approved in China in 2014 and admitted as a subsequent line treatment for advanced gastric cancer.[5,6] However, there is no report focusing on its efficacy and safety in patients with MA, Herein, we report a case with MA in advanced pancreatic cancer who was treated with apatinib and achieved a progression-free survival (PFS) of more than 11 months.
2. Case presentation
In March 29, 2016, a 64-year-old man suffered from abdominal pain for 1 month was admitted to our hospital. After conducting computer tomography scan (CT, Fig. 1A), there was an irregularly shaped mass for 72 × 56 mm with unclear edge of the boundary in the tail of pancreatic. Moreover, massive ascites was found in his abdominal cavity. The level of carcinoembryonic antigen (CEA) was 48.76 ng/mL (normal range: 0–5 ng/mL), and CA199 was 8078 IU/mL (normal range: 0–27 IU/mL), which went beyond the upper limit of our laboratory. Physical examination showed that consciousness was clear, with poor spirit, no touched swelling of superficial lymph nodes, and Murphy syndrome was negative, mobile dullness was positive. The patient had no history of hypertension, diabetes, heart disease, and kidney-related disease. Adenocarcinoma cell was observed in cytopathology examination by film preparation (Fig. 2) and the level of CEA and CA199 were monitored during treatment (Fig. 3).
Figure 1.
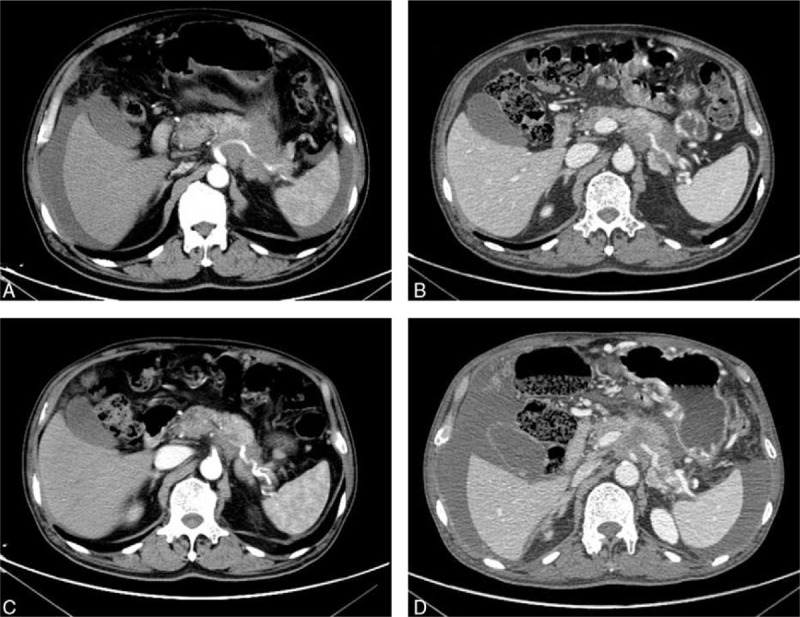
Abdominal CT scans before and after apatinib therapy. (A) CT scans before apatinib therapy showed that there was an irregularly shaped mass of 72 × 56 mm with unclear edge of the boundary in the tail of pancreatic, and massive ascites was found in the abdominal cavity; (B, C) the CT showed the MA disappeared; and (D) the CT demonstrated that the patient progression on April 14, 2017. CT = computer tomography scan, MA = malignant ascites.
Figure 2.
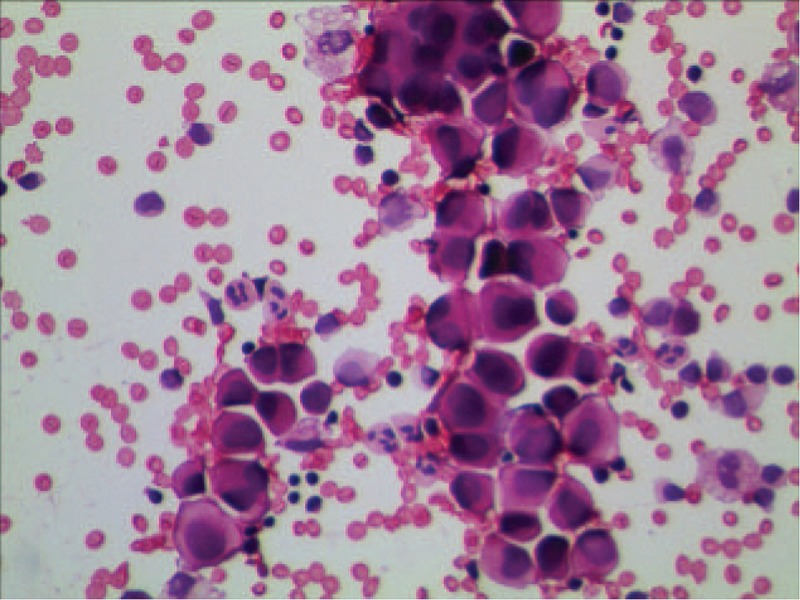
Cytopathology examination of MA found adenocarcinoma cell by film preparation. MA = malignant ascites.
Figure 3.
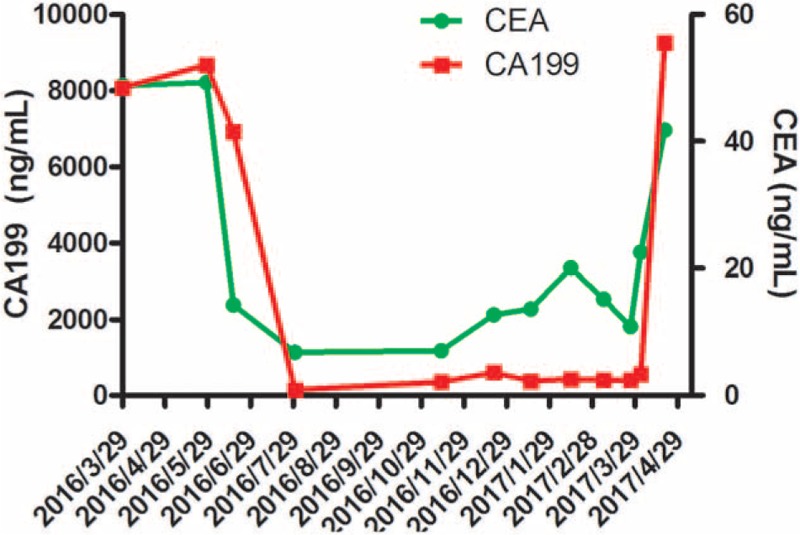
The change of serum CEA and CA199 level. CEA = carcinoembryonic antigen, CA19-9 = carbohydrate antigen 19-9.
The patient received tube drainage 1 time every half month, combined gemcitabine of 1000 mg/m2 on day 1, 8, and 15 intravenously. After 1.5 months for 3 times tube drainage, the MA was still reappeared soon, the level of CEA and CA199 was not significantly decreased and the vascular endothelial growth factor A (VEGFA) level in MA assessed by ELISA was 1980 ng/mL, which was significant higher than that of benign ascites.[7] Then, the patient received gemcitabine intravenously combined apatinib since May 14, 2016 until he suffered intolerable adverse events. After treating with apatinib for 1 month, the MA was evaluated as nearly clear response. There was no further need for paracentesis during his treatment course, and the primary pancreatic cancer was evaluated as partial response by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 standard revealed by CT scanning (Fig. 1B, C). The CEA and CA199 were also decreased sharply and reached the lowest level during the treatment process (6.82 ng/mL, 149 IU/mL, respectively) after treating with apatinib for 2.5 months. Until April 14, 2017, the patient came back to our hospital for reexamination due to his abdominal pain, the CT demonstrated progression (Fig. 1D), indicated that the patient received a PFS of more than 11 months. Then the patient received best supportive care and died on June 13, 2017. The treatment schedule is as follows (Fig. 4).
Figure 4.
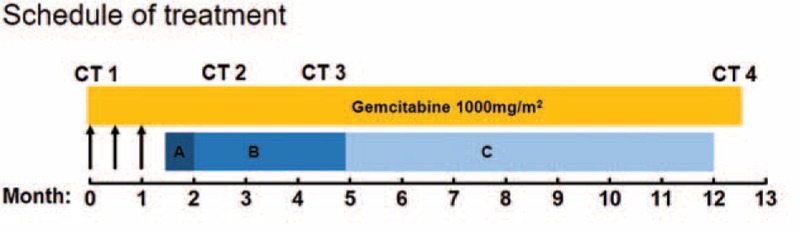
The treatment schedule of the patient. The arrowhead meant tube drainage. (A) Apatinib 850 mg; (B) apatinib 500 mg; and (C) apatinib 250 mg. CT = computer tomography scan.
After administration with apatinib for half a month, hypertension (grade IV) and hand-foot syndrome (grade I) appeared, so the dose of apatinib was adjusted from 850 to 500 mg. Three months later, the dose was changed to 250 mg because of proteinuria (grade II), and then no other obvious hematological and nonhematological toxicity was observed. On March 29, 2017, 24-hour urine protein quantity was 3.3 g, herein, the administration of apatinib was terminated. Toxicity was evaluated and graded according to the NCI-CTC for adverse events, version 3.0.
The study was approved by the Hospital Ethics Committee of the Affiliated Lianyungang Hospital of Xuzhou Medical University and the patient provided written informed consent.
3. Discussion
Pancreatic cancer is a kind of worldwide intractable tumor and MA is one of common complications. Ascites may result in a poor quality of life due to symptoms of abdominal distention, pain, nausea, vomiting, anorexia, dyspnea, limb edema, insomnia, and fatigue. Current data strongly suggested that VEGF and vessel hyperpermeability may act as 2 critical mediators in the pathogenesis of MA.[8,9] Cancer cells may produce VEGF through autocrine signaling.[10] It has been suggested that K-ras gene mutation was observed in more than 90% of pancreatic cancers, which could upregulate the secretion of VEGF and metalloproteinase.[11] VEGF is a family of endothelial growth factors which includes VEGF-A-B-C-D-E and placental growth factor PlGF. VEGF made effects through binding to their cognate VEGF receptors, including VEGFR1 (flt1), vascular endothelial growth factor receptor 2 (VEGFR2) (flk1 in the mouse, KDR in the human), and VEGFR3 (flt4), among which VEGFA and VEGFR2 mediate the leading role in promoting tumor angiogenesis. Increased permeability as a result of the signal transduction cascade downstream of the VEGF receptors is mediated by several mechanisms including induction of endothelial fenestrations, loss of junctional integrity, and the formation of transcellular gaps.[9] The permeability of the vasculature is a property of the capillary wall to obstruct movement of fluid or the migration of tumor cells. It is a primary contributor to the formation of MA and increased tumor cells in MA. Elevated levels of VEGF produced by tumor cells, thus forming a positive feedback loop (summary in Fig. 5). Preclinical evidence for the role of VEGF/VEGFR2 in MA formation led to the hypothesis that its blockade might benefit patients with MA in advanced cancer. Some phase II clinical trials have preliminary showed beneficial palliative effects in patients with MA.[12–14]
Figure 5.
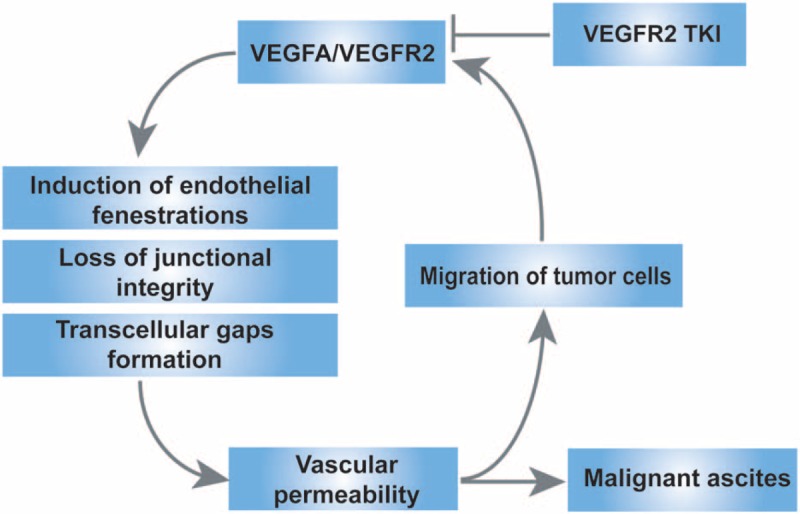
Mechanism of action of VEGFA/VEGFR2 pathway on the formation of MA. TKI = tyrosine kinase inhibitor, VEGFA = vascular endothelial growth factor A, VEGFR2 = vascular endothelial growth factor receptor 2.
Apatinib is the latest inhibitor of VEGFR2 targeting the intracellular ATP-binding site of the receptor, which could inhibit VEGF-stimulated endothelial cell migration and proliferation, decrease tumor microvascular density, and promote apoptosis.[10,15] There were several practice case reports and clinical trials proved the effects and safety of apatinib. Results from a recent published phase III randomized trial demonstrated that both OS and PFS were prolonged in patients with advanced gastric or gastroesophageal junction adenocarcinoma, who failed in 2 or more prior lines of chemotherapy while taking apatinib 850 mg once daily than those taking placebo (median OS 6.5 vs 4.7 months, and median PFS 2.6 vs 1.8 months).[6] Recently, apatinib also showed satisfactory efficacy in triple-negative[16] or nontriple-negative breast cancer,[17] and osteosarcoma.[18] This is the 1st report that apatinib applied to treat pancreatic cancer-mediated MA. According to the instruction of apatinib, we first prescribed him with apatinib of 850 mg daily. Besides, hypertension and proteinuria were observed, leading to dosage adjustment.
There is no generally accepted evidence-based guidelines for the treatment of MA. Treatment modalities including abdominal cavity drainage, intrapleural taxanes, bleomycin, cytarabine, anthracyclines, platinum agents, and etoposide.[3] Gemcitabine is a standard 1st-line treatment for advanced pancreatic cancer for many years.[19] However, the addition of bevacizumab to gemcitabine did not improve survival.[20] In this case, apatinib concurrent gemcitabine successfully controlled MA in patient with advanced pancreatic cancer, achieving a PFS of more than 11 months. It indicated that apatinib may have a synergistic effect on the gemcitabine chemotherapy. Apatinib is a TKI targeting VEGFR2, which abrogated the interaction of VEGF with VEGFR and directly inhibited angiogenesis. In addition, apatinib could prevent multidrug resistance conferred by ABCB1 and ABCG2 proteins.[21,22] It illuminated that apatinib might cooperate superiorly with chemotherapy for treating pancreatic cancer and managing MA. Moreover, apatinib is an oral preparation, the patient has a good compliance and it can decrease the plague by numerous hospitalizations and repeated paracentesis.
4. Conclusion
Apatinib concurrent gemcitabine may provide an additional option for the treatment of pancreatic cancer-mediated MA. Further large-scale clinical trials should be performed to prove its effect and safety.
Acknowledgments
The authors thank the patient for his consent to publication of this case report.
Footnotes
Abbreviations: CT = computer tomography scan, MA = malignant ascites, OS = overall survival, PFS = progression-free survival, TKI = tyrosine kinase inhibitor, VEGFA = vascular endothelial growth factor A, VEGF = vascular endothelial growth factor, VEGFR = vascular endothelial growth factor receptor.
LL and LW contributed equally to this work.
Funding/support: This study was supported by the National Natural Science Foundation of China (Grant NO. 81472792).
The authors have no conflicts of interest to disclose.
References
- [1].Hicks AM, Chou J, Capanu M, et al. Pancreas adenocarcinoma: ascites, clinical manifestations, and management implications. Clin Colorectal Cancer 2016;15:360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Takahara N, Isayama H, Nakai Y, et al. Pancreatic cancer with malignant ascites: clinical features and outcomes. Pancreas 2015;44:380–5. [DOI] [PubMed] [Google Scholar]
- [3].Becker G, Galandi D, Blum HE. Malignant ascites: systematic review and guideline for treatment. Eur J Cancer 2006;42:589–97. [DOI] [PubMed] [Google Scholar]
- [4].Markman M. Palliation of symptomatic malignant ascites: an (often) unmet need. Oncology 2012;82:313–4. [DOI] [PubMed] [Google Scholar]
- [5].Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219–25. [DOI] [PubMed] [Google Scholar]
- [6].Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448. [DOI] [PubMed] [Google Scholar]
- [7].Zebrowski BK, Yano S, Liu W, et al. Vascular endothelial growth factor levels and induction of permeability in malignant pleural effusions. Clin Cancer Res 1999;5:3364–8. [PubMed] [Google Scholar]
- [8].Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res 2010;87:262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bradshaw M, Mansfield A, Peikert T. The role of vascular endothelial growth factor in the pathogenesis, diagnosis and treatment of malignant pleural effusion. Curr Oncol Rep 2013;15:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lin Y, Zhai E, Liao B, et al. Autocrine VEGF signaling promotes cell proliferation through a PLC-dependent pathway and modulates Apatinib treatment efficacy in gastric cancer. Oncotarget 2017;14:11990–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Buscail L, Bournet B, Dufresne M, et al. [Advance in the biology of pancreatic of cancer]. Bull Cancer 2015;102(6 Suppl 1):S53–61. [DOI] [PubMed] [Google Scholar]
- [12].Gotlieb WH, Amant F, Advani S, et al. Intravenous aflibercept for treatment of recurrent symptomatic malignant ascites in patients with advanced ovarian cancer: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Oncol 2012;13:154–62. [DOI] [PubMed] [Google Scholar]
- [13].Jordan K, Luetkens T, Gog C, et al. Intraperitoneal bevacizumab for control of malignant ascites due to advanced-stage gastrointestinal cancers: a multicentre double-blind, placebo-controlled phase II study – AIO SUP-0108. Eur J Cancer 2016;63:127–34. [DOI] [PubMed] [Google Scholar]
- [14].Mulder SF, Boers-Sonderen MJ, van der Heijden HF, et al. A phase II study of cediranib as palliative treatment in patients with symptomatic malignant ascites or pleural effusion. Target Oncol 2014;9:331–8. [DOI] [PubMed] [Google Scholar]
- [15].Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xichun H, Jian Z, Binghe X, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer 2013;22:1961–9. [DOI] [PubMed] [Google Scholar]
- [17].Hu X, Cao J, Hu W, et al. Multicenter phase II study of Apatinib in non-triple-negative metastatic breast cancer. BMC Cancer 2014;14:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhou Y, Zhang W, Tang F, et al. A case report of apatinib in treating osteosarcoma with pulmonary metastases. Medicine (Baltimore) 2017;96:e6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403–13. [DOI] [PubMed] [Google Scholar]
- [20].Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010;28:3617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tong XZ, Wang F, Liang S, et al. Apatinib (YN968D1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and ABCB1-overexpressing leukemia cells. Biochem Pharmacol 2012;83:586–97. [DOI] [PubMed] [Google Scholar]
- [22].Mi YJ, Liang YJ, Huang HB, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res 2010;70:7981. [DOI] [PMC free article] [PubMed] [Google Scholar]


