Abstract
Rationale:
Aggressive pituitary adenomas and pituitary carcinomas are rare and demand multiple treatment strategies. Temozolomide, an orally active alkylating chemotherapeutic agent, has recently been recommended as a salvage medication for refractory pituitary adenomas or carcinomas.
Patient concerns:
A 17-year-old male presenting with aggressive prolactinoma that continued to progress despite surgery, gamma knife, and dopamine agonists.
Diagnoses:
The diagnosis of refractory aggressive prolactinoma was made on the basis of clinical findings and the lack of efficacy of conventional treatment.
Interventions:
The patient received the most frequently recommended regimen of temozolomide treatment for 22 cycles.
Outcomes:
Temozolomide resulted in a remarkable shrinkage of tumor mass and inhibition of prolactin secretion and this patient's clinical condition improved progressively.
Lessons:
Temozolomide can be used as a salvage treatment to refractory pituitary tumors and o(6)-methylguanine-DNA methyltransferase (MGMT) status is a significant predictor to the effectiveness of temozolomide based on the existing literature.
Keywords: aggressive prolactinoma, efficacy, pituitary tumor, temozolomide, treatment
1. Introduction
Pituitary adenomas are the second most common tumors of central nervous system that occupy a proportion of 14% among intracranial neoplasm and the overall prevalence of pituitary adenomas is approximately 1/1500 persons.[1,2] Pituitary tumors are usually benign and sensitive to conventional therapies, including surgery, radiotherapy, and medication such as dopamine agonists or somatostatin analogues.[3] Nevertheless, some pituitary tumors demonstrate aggressive behavior, characterized by accelerated growth, large size, high recurrence rate, and persistent growth despite repeated treatment attempts.[4] Pituitary carcinomas are characterized by invasion of adjacent structures and rapid proliferation and defined by the presence of craniospinal and/or systemic metastases and account no more than 0.1% to 0.2% of all pituitary tumors and average survival time is less than 4 years.[5,6] These tumors bring clinical challenges for endocrinologists and neurosurgeons due to their locally invasive nature and rapid growth and are largely unresponsive to current combined treatment strategies.
Temozolomide is an alkylating agent first used to treat glioblastoma in cases resistant to standard therapy in 1992.[7] Later, the use of temozolomide became the first-line treatment against glioblastoma and expanded to astrocytomas, gliomas, advanced melanomas, and neuroendocrine tumors.[8,9] At physiological pH, temozolomide rapidly converted into active 5-(3-methyl-triazeno)-imidazole-4-carboxamide responsible for DNA lesions by binding methyl groups at O6-guanine leading to DNA mismatch and cell apoptosis.[10] O(6)-methylguanine-DNA methyltransferase (MGMT), which removes methyl groups from O6-guanine, can counteract the effect of temozolomide leading to failure of treatment.[11] The first uses of temozolomide in pituitary tumors were reported in 2006.[12,13] Since then, many cases or case series of treatment with temozolomide in aggressive pituitary adenomas or pituitary carcinomas have been reported, yet, there is not an accordant conclusion. Here, we present the case of a young man with aggressive prolactinoma that was resistant to conventional therapy treated with temozolomide. The literature regarding to the effectiveness of the usage of temozolomide in aggressive pituitary adenomas or carcinomas that resist to concurrent conventional therapy is also reviewed.
2. Presentation of the case
The authors report a 17-year-old male who was admitted to hospital with complaints of headache and progressive visual disturbance in October 2008. Ophthalmologic evaluation detected an impaired vision and vision field. Laboratory examination showed an evaluated prolactin (PRL) level (PRL >2000 ng/mL, reference value 4.6–21.4 ng/mL). Magnetic resonance imaging (MRI) revealed a pituitary macroadenoma with suprasellar extension and invasion of right carvernous sinus (Fig. 1A). On the basis of the symptoms and clinical features, the diagnosis of prolactinoma was made and this patient was treated with gamma knife stereotactic radiosurgery in another hospital in November 2008. This patient experienced a recovery of eyesight and alleviation of headache after radiosurgery. Nevertheless, the PRL level remained high so bromocriptine was initiated.
Figure 1.
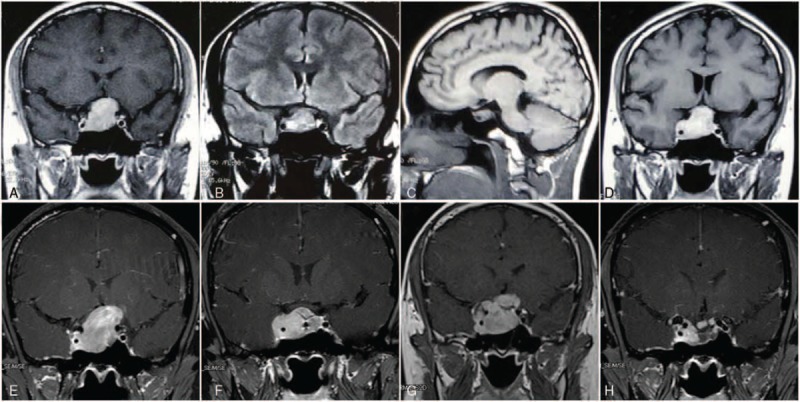
(A) Contrast-enhanced coronal T-1 weighted MRI showed a sellar and suprasellar tumor. (B) Residual tumor 5 months after gamma knife therapy. (C) Sagittal image showed the apparent shrinkage of tumor and the decompressed of optic nerve 7 months after the initiation of cabergoline. (D) Regrowth of tumor mass 26 months after cabergoline. (E) Preoperative MRI demonstrated a 3.5 cm × 3.1 cm × 3.7 cm tumor mass compressing pituitary stalk and optic chiasma. (F) MRI indicated a residual tumor 5 months after operation. (G) MRI indicated that the residual tumor expanded again after second course of gamma knife. (H) Sellar tumor remarkable reduction with cystic degeneration after 22 months of temozolomide treatment.
Six months after radiotherapy, MRI revealed a residual tumor in sellar region (Fig. 1B) and then he referred to our hospital in June 2009 because of the persistent evaluated PRL level despite maximal doses of bromocriptine. Cabergoline was suggested (1 mg every week) as a result of bromocriptine resistance and PRL was suppressed to a normal level. Seven months after the initiation of cabergoline, MRI found an obvious shrinkage of remanent tumor and the decompressed of optic nerve (Fig. 1C). However, after a 26-month period of remission, the patient developed headache and impaired vision again due to a regrowth of the tumor (Fig. 1D), and then we increased cabergoline to a tolerable dosage (2 mg every week). Nevertheless, there was no obvious amelioration of the symptoms and the patient developed an enlarged temporal hemianopsia in his left eye.
Afterwards, the patient went through another MRI examination in July 2012 indicated an enlargement of tumor mass with invasion of right carvernous sinus that compressed the pituitary stalk and optic chiasma (Fig. 1E). Therefore, the patient underwent his first operation (craniotomy) and the tumor was found to be relatively hard during surgery. Immunohistochemistry demonstrated immunopositive for PRL and immunonegative for thyroid-stimulating hormone (TSH), growth hormone (GH), adrenocorticotrophic hormone (ACTH), and follicle-stimulating hormone (FSH), which was consistent with the diagnosis of prolactin adenoma. The proliferative maker Ki-67 labeling index was 10∼20%. A followed gamma knife radiosurgery was given 5 months after surgery in January 2013 because of MRI indicated a residual tumor after operation (Fig. 1F).
Prolactin level was on a persistent evaluated stage even when the patient was continuously taking dopamine agonist after gamma knife therapy and another MRI revealed the residual tumor expanded again (Fig. 1G). Because of the lack of efficacy of dopamine agonist, surgery, and radiotherapy, the patient received the most frequently recommended regimen of temozolomide treatment (total 300 mg/day), administrated 5 days every 28 days for 22 months, after we reviewed the literature of similar cases that showed a positive response. The therapy is approved by the ethics committee of West China Hospital. This patient's clinical condition improved progressively and PRL concentration had fallen from 6491 to 773.4 ng/mL (reference value 4.6–21.4 ng/mL) in the end of the treatment (Fig. 2). MRI also detected a remarkable reduction of tumor volume with cystic degeneration inside the mass (Fig. 1 h). He only experienced mild nausea, fatigue, and no serious hematologic complications such as neutropenia and thrombocytopenia were noted. By the time we write this paper, temozolomide have been stopped for 8 months, and the patient presented no signs of prolactinoma relapse and restarted his work again.
Figure 2.
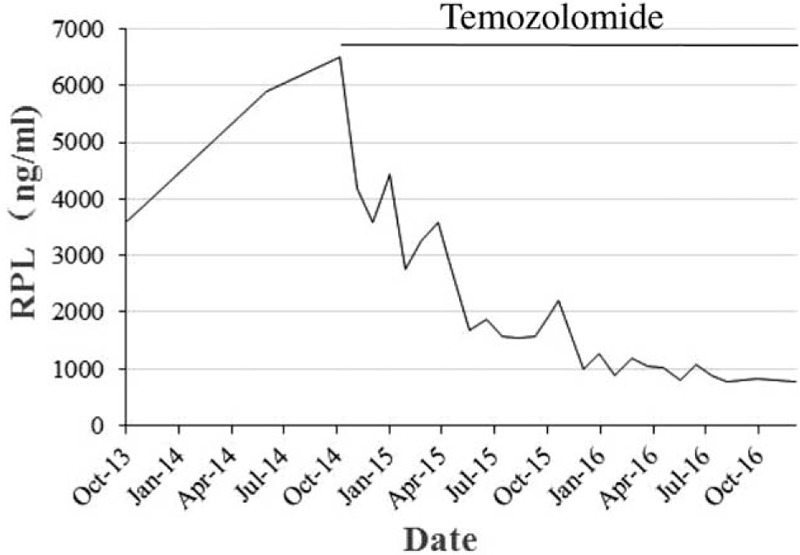
Graph showing the serum Prolactin concentration [ng/ml] at approximately 1-month interval. Prolactin dropped rapidly after the commencement of temozolomide.
3. A review of the literature
3.1. Data collection
Carrying the purpose of temozolomide treatment in aggressive pituitary adenomas or pituitary carcinomas that refractory to conventional therapies, a literature research was carried out with PubMed database. We used the combination of key phrases: temozolomide, pituitary carcinoma, pituitary adenoma, pituitary tumor, aggressive, atypical, and no restrictions were imposed in an attempt to maximize the papers enrolled. This identified 129 relevant papers, and 87 papers were removed after evaluation of abstract. The full-texts of the rest of 42 articles were assessed for eligibility and subsequent examination of the references was undertaken to increase the sensibility. We included a case series that contained 3 or more patients. It should be aware that there are 12 patients in the study by Losa et al,[14] which have been reported before and we had insufficient detail to eliminate those duplicate cases from our review. We tabulated the extracted results of each case in the incorporated literature as far as possible in order to determine whether types of tumor, MGMT status, Ki-67 labeling index, and p53 protein were associated with outcome of temozolomide treatment. We categorized the outcomes into complete/partial response or stable/progression of disease when hypothesis testing was needed to establish an R × C or fourfold table. In all tumors, a complete response was defined as a complete regression of tumor mass and a partial response was defined as a decrease of tumor volume ≥30% and progression of disease or stable disease meant radiological disease progressed or stabilized.[15]
3.2. Statistical analyses
By using Chi-square test and Student t test, we investigated the potential factors that could related to the efficacy of temozolomide in SPSS statistical program (Version 18.0, SPSS Inc., Chicago, IL).
4. Results
We included 12 eligible case-series published from 2009 to 2016,[14–25] giving a total of 113 temozolomide-treated patients (see Table 1). The majority of patients treated with temozolomide were man (59.4% male vs 40.6% female). On average, patients received 10.0 cycles of temozolomide treatment (range from 1 month to 29 months). All patients had evidence of aggressive behaviors despite multiple previous therapeutic measures and surgery remained the first choice for aggressive pituitary adenomas and pituitary carcinomas. All patients had been treated with surgery (craniotomy or transsphenoidal surgery) before the initiation of temozolomide treatment, 27.9% received 1 time of operation, 22.9% received 2 times of operation, 19.7% received 3 times of operation, and 29.5% received more than 3 times of operation. Radiotherapy was another choice for refractory pituitary tumors, 77% of patients treated with radiation before temozolomide treatment, and 21.3% received a least 2 cycles of radiation. Furthermore, a variety of chemotherapeutic agents, including dopamine agonists, somatostatin analogs, lomustine, ketoconazole, pegvisomant, bevacizumab, trinotecan, had been tried as a sequential therapy or alleviative treatment at different stages of disease. A great measure of cases (94%) used the routine temozolomide regimen (150–200 mg/m2 for 5 days every 28 days), while Bush et al[23] took 75 mg/m2 for 21 days followed by 7 days off therapy. We also noticed that 5 cases received radiotherapy and 6 cases received capecitabine at the same time.
Table 1.
Summary of collected studies.

Among all the cases comprised (see Table 2), ACTH-secreting tumors (41 cases, including functioning or silent corticotroph adenomas, carcinomas, and Nelson syndrome) were the most frequent ones treated with temozolomide, followed by prolatinomas (32 cases), nonfunctioning tumors (29 cases, including null cell or immunonegative adenomas, carcinomas), and other subtypes (10 cases and 1 case for GH-secreting tumors and TSH-secreting tumor, respectively). Response (complete or partial) rates differed from each other by tumor subtypes (P = .037, Chi-square test); adrenocorticotroph tumors and prolatinomas showed a higher response rate (61.0% and 56.3% respectively) than nonfunctioning tumors, which showed a radiological temozolomide response in 31.0% of cases. As you can see, we did not bring the response rates of GH-secreting tumors and thyroid-stimulating hormone-secreting tumor into a comparison as a result of it was less representative and lack of persuasiveness.
Table 2.
Tumor response to temozolomide treatment according to the type of hormone.

Due to the rareness of pituitary carcinomas, aggressive pituitary adenomas were more common in temozolomide-treated cases (see Table 3). Nevertheless, when we examined for a direct correlation between temozolomide efficacy and tumor types (aggressive pituitary adenomas and pituitary carcinomas), we found no relationship between groups (P = .901, Chi-square test).
Table 3.
Summary of temozolomide treatment outcomes for APA or PC.

Among those with available data regarding histology and biomarkers, we conducted an investigation to discuss whether temozolomide response was correlated with expression of MGMT, Ki-67 labeling index, and p53 protein (see Table 4). MGMT status was determined in 68 published cases treated with temozolomide in all contexts and MGMT immunopositivity was classified into positive and negative by the cutoff value at 10% of cells.[24] Overall, we found that there was a significant correlation between MGMT status and the effectiveness of temozolomide (P = .008, Chi-square test) and it made MGMT staining a strong predictor to the outcome of temozolomide treatment. We identified 61 cases that provided us with Ki-67 labeling index data (presented as a percentage) when excluded the ambiguous ones (no specific figures), and there was no significant correlation between Ki-67 labeling index and the effectiveness of temozolomide (P = .953, Student t test). As for p53 protein, 44 samples were pointed out after we thoroughly reviewed the literature, and it was considered immunopositive if p53 index≧10%.[15] Finally, we detected that there was no statistical significance between p53 and temozolomide efficacy (P = .820, Chi-square test).
Table 4.
Summary of temozolomide treatment outcomes associated with MGMT and Ki-67 status.

5. Discussion
The treatment of aggressive pituitary adenomas and pituitary carcinomas frequently requires multiple approaches, including surgery, radiotherapy, and medical therapy to control tumor growth and normalize pituitary hormone hypersecretion, yet, some cases yielded disappointed results despite repeated attempts.[25,26] Temozolomide is an alkylating agent, which is usually used as a standard chemotherapy for glioblastoma multiforme.[8] In recent years, several cases or cases series emerged about the use of temozolomide as a possible salvage treatment for refractory aggressive pituitary adenomas or pituitary carcinomas, since the first uses of temozolomide treatment in pituitary tumors in 2006.[12,13]
The standard therapeutic dose is the sequential delivery of 150 to 200 mg/m2 of body surface/ day during 5 days every 28 days and most of the cases in the presented studies followed the administration method (including our case) except Bush et al[23] took 75 mg/m2 for 21 days followed by 7 days off therapy. The common adverse effects of temozolomide include fatigue, dizziness, nausea, vomiting, headaches, constipation, and diarrhea. Most of them are transient and tolerable and seldom lead to treatment cessation, thus temozolomide can be used for a long term of treatment. Hematologic toxicities such as thrombocytopenia, leukocytopenia, and lymphocytopenia are also noticed in some cases and these unfrequent side effects usually lead to the cessation of temozolomide treatment or a reduction in the temozolomide dose or an increase in the interval between cycles.[17,18,22]
The case we presented had previously progressed despite tumor resection, repeated gamma knife therapy, and 2 types of dopamine agonist (bromocriptine and cabergoline) and this is accordant with the aggressive nature that we conclude from literature review (All patients had been treated with surgery and 77% of patients treated with radiation before temozolomide remedy). Temozolomide had induced tumor mass shrinkage and prolactin suppression after 22 cycles of treatment and sustained control of disease for a long period after therapy termination.
In our review, ACTH-secreting tumors are the most frequently presented tumor type, followed by prolactinoma, nonfunctioning tumors, GH-secreting tumors, and TSH-secreting tumor. This distribution of tumor type differs slightly from the literature recently published,[27] which found that nonfunctioning pituitary tumors are the less frequent one. We also noticed that GH-secreting tumors and TSH-secreting tumor are less represented, and the possible explanation is that they are unfrequent in epidemiology aspect and are less inclined to develop malignant behaviors than other tumor types.
According to World Health Organization, elevated mitotic index, Ki67 labeling index > 3%, and extensive p53 expression indicate the aggressive nature of pituitary tumor.[28] Mitotic index was no reported in the majority of cases and we have no adequate resource to extrapolate any conclusion about the relationship between mitosis and temozolomide efficacy. Some authors proposed that an elevated Ki-67 labeling index related to a higher rate of invasion[29] and others described it as a valuable bio-predictor for pituitary tumor recurrence.[30] Dudziak et al[31] suggested that Ki-67 labeling index exceeding 10% should always raise the suspicion of malignancy. Regarding to the relationship between elevated Ki-67 labeling index and the efficacy of temozolomide therapy, our study suggests that an increased Ki-67 immunopositivity of tumor cells is not responsible for resistance to temozolomide.
The tumor suppressor protein p53 plays a broader role in the pathogenesis or progression of a wide range of cancers, and p53 is thought to be a transcription factor activated in response to several forms of cellular stress, including DNA damage, hypoxia, viral infection, heat shock, and mitogenic or oncogenic stresses.[32] Moreover, inactivation of the p53 pathway occurs in the majority of human cancers and usually causes resistance to therapy and poor survival.[33] Still, some authors put a high value of p53 expression and described that there was a significant correlation between the expression of p53 and aggressive pituitary tumor behavior.[34] It is reported that MGMT expression is downregulated by wild-type p53 and p53 immunohistochemical activity is associated with the effectiveness of temozolomide.[35] Nevertheless, we discover that there is no statistical significance between p53 and temozolomide efficacy.
MGMT overexpress in many types of human tumors[11] and assessment of MGMT status should be performed by immunohistochemistry, which evaluates the level of protein expression.[25] MGMT can counteract the effect of temozolomide by removing methyl groups from O6-guanine and low MGMT expression is considered a predictor of tumor response to temozolomide in glioblastomas.[36] Low expression of MGMT seems to better correlate with favorable therapeutic response than intermediate to high MGMT expression according to Raverot et al.[25] Generally, we suggest that there is a significant correlation between MGMT expression and the effectiveness of temozolomide in refractory pituitary tumors, and in an effort to anticipate likelihood of a temozolomide response, all cases are recommended to assess MGMT expression before starting alkylating agents therapy. MGMT status is a significative predictor to temozolomide efficacy, but it should be noted that MGMT might not be the sole molecular factor determining sensitivity to temozolomide.
According to Hegi et al,[37] MGMT expression was a result of promoter methylation of the gene and epigenetic inactivation of the gene for the DNA repair enzyme MGMT via promoter hypermethylation had been shown to predict therapeutic response to temozolomide in glioblastomas. However, other authors reported that there were no relationship between MGMT promoter methylation status and MGMT immunoexpression and MGMT promoter methylation was not clinically useful in predicting tumor response to temozolomide therapy.[23] It remains a matter of speculation whether MGMT promoter methylation status contributes to MGMT expression and eventually alters temozolomide responsiveness.
Inactivated mutations in the DNA mismatch repair gene MSH6 and loss of MSH6 expression are associated with temozolomide resistance in glioblastomas multiforme.[38] Hirohata et al[20] reported that lack of MSH6 immunopositivity had a significant correlation with resistance to temozolomide treatment and preserving MSH6 function was important for the effectiveness of temozolomide on malignant pituitary tumors.
It is reported that other chemotherapeutic agents in combination with temozolomide are useful and effective. Zacharia et al[19] reported 4 cases treated with a novel chemotherapeutic regimen of capecitabine and temozolomide , originally designed in their laboratory, and achieved dramatic antineoplastic effects against corticotrophic pituitary tumors and 3 of 4 patients demonstrated tumor response. Bode et al[39] reported an ACTH-secreting pituitary carcinoma with widespread intracranial, spinal, and systemic metastases, which received pasireotide and temozolomide together. A sustained tumor control was achieved after 12 months of combination therapy and for more than 9 months on monotherapy with pasireotide. Vieira et al[40] described a case with silent somatotroph pituitary carcinoma that had been treated with temozolomide and zoledronic for 7 months and this led to stable disease of primary tumor as well as metastases.
Anti-angiogenic therapy is also described as an option in the treatment of aggressive pituitary tumors. Ortiz et al[41] provided us with a case evolved from aggressive silent corticotroph adenoma to carcinoma after temozolomide treatment and anti-vascular endothelial growth factor (anti-VEGF) therapy reached a long-term control of the tumor mass. We also noticed that there are 3 cases that received reduction in tumor size after first course of temozolomide therapy but failed in second course of treatment when disease progressed.[16]
The clinical data are still limited about temozolomide efficacy to aggressive pituitary adenomas and pituitary carcinomas, and multicenter cooperation is demanded to provide us with more therapy strategies and establish clinical guidelines for these refractory tumors.
Acknowledgment
The authors sincerely thank Prof. Guanjian Liu (Department of Evidence-Based Center, West China School of Medicine, Sichuan University, China) for reviewing our statistical analyses.
Footnotes
Abbreviations: ACTH = adrenocorticotrophic hormone, FSH = follicle-stimulating hormone, GH = growth hormone, MGMT = O(6)-methylguanine-DNA methyltransferase, MRI = magnetic resonance imaging, PRL = prolactin, TSH = thyroid-stimulating hormone, VEGF = vascular endothelial growth factor.
Drs CC, SY, and SZ contributed equally in this study as the cofirst authors.
Funding/support: This research was supported by Foundation of the Science and Technology Department of Sichuan Province (Grant NO. 2015SZ0120).
The authors report no conflicts of interest.
References
- [1].Daly AF, Tichomirowa MA, Beckers A. The epidemiology and genetics of pituitary adenomas. Best Pract Res Clin Endocrinol Metab 2009;23:543–54. [DOI] [PubMed] [Google Scholar]
- [2].Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neurooncology 2012;14(Suppl 5):v1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA 2017;317:516–24. [DOI] [PubMed] [Google Scholar]
- [4].Di Ieva A, Rotondo F, Syro LV, et al. Aggressive pituitary adenomas: diagnosis and emerging treatments. Nat Rev Endocrinol 2014;10:423–35. [DOI] [PubMed] [Google Scholar]
- [5].Chatzellis E, Alexandraki KI, Androulakis II, et al. Aggressive pituitary tumors. Neuroendocrinology 2015;101:87–104. [DOI] [PubMed] [Google Scholar]
- [6].Kaltsas GA, Nomikos P, Kontogeorgos G, et al. Clinical review: diagnosis and management of pituitary carcinomas. J Clin Endocrinol Metab 2005;90:3089–99. [DOI] [PubMed] [Google Scholar]
- [7].O’Reilly SM, Newlands ES, Glaser MG, et al. Temozolomide: a new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumours. Eur J Cancer 1993;29A:940–2. [DOI] [PubMed] [Google Scholar]
- [8].Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- [9].Tatar Z, Thivat E, Planchat E, et al. Temozolomide and unusual indications: review of literature. Cancer Treat Rev 2013;39:125–35. [DOI] [PubMed] [Google Scholar]
- [10].Sheehan J, Rainey J, Nguyen J, et al. Temozolomide-induced inhibition of pituitary adenoma cells. J Neurosurg 2011;114:354–8. [DOI] [PubMed] [Google Scholar]
- [11].Christmann M, Verbeek B, Roos WP, et al. O(6)-Methylguanine-DNA methyltransferase (MGMT) in normal tissues and tumors: enzyme activity, promoter methylation and immunohistochemistry. Biochim Biophys Acta 2011;1816:179–90. [DOI] [PubMed] [Google Scholar]
- [12].Fadul CE, Kominsky AL, Meyer LP, et al. Long-term response of pituitary carcinoma to temozolomide. Report of two cases. J Neurosurg 2006;105:621–6. [DOI] [PubMed] [Google Scholar]
- [13].Lim S, Shahinian H, Maya MM, et al. Temozolomide: a novel treatment for pituitary carcinoma. Lancet Oncol 2006;7:518–20. [DOI] [PubMed] [Google Scholar]
- [14].Losa M, Bogazzi F, Cannavo S, et al. Temozolomide therapy in patients with aggressive pituitary adenomas or carcinomas. J Neurooncol 2016;126:519–25. [DOI] [PubMed] [Google Scholar]
- [15].Bengtsson D, Schroder HD, Andersen M, et al. Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J Clin Endocrinol Metab 2015;100:1689–98. [DOI] [PubMed] [Google Scholar]
- [16].Campdera M, Palacios N, Aller J, et al. Temozolomide for aggressive ACTH pituitary tumors: failure of a second course of treatment. Pituitary 2016;19:158–66. [DOI] [PubMed] [Google Scholar]
- [17].Bruno OD, Juarez-Allen L, Christiansen SB, et al. Temozolomide therapy for aggressive pituitary tumors: results in a small series of patients from Argentina. Int J Endocrinol 2015;2015:587893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ceccato F, Lombardi G, Manara R, et al. Temozolomide and pasireotide treatment for aggressive pituitary adenoma: expertise at a tertiary care center. J Neurooncol 2015;122:189–96. [DOI] [PubMed] [Google Scholar]
- [19].Zacharia BE, Gulati AP, Bruce JN, et al. High response rates and prolonged survival in patients with corticotroph pituitary tumors and refractory Cushing disease from capecitabine and temozolomide (CAPTEM): a case series. Neurosurgery 2014;74:E447–55. discussion E55. [DOI] [PubMed] [Google Scholar]
- [20].Hirohata T, Asano K, Ogawa Y, et al. DNA mismatch repair protein (MSH6) correlated with the responses of atypical pituitary adenomas and pituitary carcinomas to temozolomide: the national cooperative study by the Japan Society for Hypothalamic and Pituitary Tumors. J Clin Endocrinol Metab 2013;98:1130–6. [DOI] [PubMed] [Google Scholar]
- [21].Whitelaw BC, Dworakowska D, Thomas NW, et al. Temozolomide in the management of dopamine agonist-resistant prolactinomas. Clin Endocrinol 2012;76:877–86. [DOI] [PubMed] [Google Scholar]
- [22].Hagen C, Schroeder HD, Hansen S, et al. Temozolomide treatment of a pituitary carcinoma and two pituitary macroadenomas resistant to conventional therapy. Eur J Endocrinol 2009;161:631–7. [DOI] [PubMed] [Google Scholar]
- [23].Bush ZM, Longtine JA, Cunningham T, et al. Temozolomide treatment for aggressive pituitary tumors: correlation of clinical outcome with O(6)-methylguanine methyltransferase (MGMT) promoter methylation and expression. J Clin Endocrinol Metab 2010;95:E280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Losa M, Mazza E, Terreni MR, et al. Salvage therapy with temozolomide in patients with aggressive or metastatic pituitary adenomas: experience in six cases. Eur J Endocrinol 2010;163:843–51. [DOI] [PubMed] [Google Scholar]
- [25].Raverot G, Castinetti F, Jouanneau E, et al. Pituitary carcinomas and aggressive pituitary tumours: merits and pitfalls of temozolomide treatment. Clin Endocrinol 2012;76:769–75. [DOI] [PubMed] [Google Scholar]
- [26].Heaney AP. Clinical review: pituitary carcinoma: difficult diagnosis and treatment. J Clin Endocrinol Metab 2011;96:3649–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ji Y, Vogel RI, Lou E. Temozolomide treatment of pituitary carcinomas and atypical adenomas: systematic review of case reports. Neurooncol Pract 2016;3:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Laws ER, Jr, Lopes MB. The new WHO classification of pituitary tumors: highlights and areas of controversy. Acta Neuropathol 2006;111:80–1. [DOI] [PubMed] [Google Scholar]
- [29].Pizarro CB, Oliveira MC, Coutinho LB, et al. Measurement of Ki-67 antigen in 159 pituitary adenomas using the MIB-1 monoclonal antibody. Braz J Med Biol Res 2004;37:235–43. [DOI] [PubMed] [Google Scholar]
- [30].Ramirez C, Cheng S, Vargas G, et al. Expression of Ki-67, PTTG1, FGFR4, and SSTR 2, 3, and 5 in nonfunctioning pituitary adenomas: a high throughput TMA, immunohistochemical study. J Clin Endocrinol Metab 2012;97:1745–51. [DOI] [PubMed] [Google Scholar]
- [31].Dudziak K, Honegger J, Bornemann A, et al. Pituitary carcinoma with malignant growth from first presentation and fulminant clinical course: case report and review of the literature. J Clin Endocrinol Metab 2011;96:2665–9. [DOI] [PubMed] [Google Scholar]
- [32].Ashcroft M, Kubbutat MH, Vousden KH. Regulation of p53 function and stability by phosphorylation. Mol Cell Biol 1999;19:1751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Herrero AB, Rojas EA, Misiewicz-Krzeminska I, et al. Molecular mechanisms of p53 deregulation in cancer: an overview in multiple myeloma. Int J Mol Sci 2016;17:pii: E2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Thapar K, Scheithauer BW, Kovacs K, et al. p53 expression in pituitary adenomas and carcinomas: correlation with invasiveness and tumor growth fractions. Neurosurgery 1996;38:765–70. discussion 770–1. [PubMed] [Google Scholar]
- [35].Bocangel D, Sengupta S, Mitra S, et al. p53-Mediated down-regulation of the human DNA repair gene O6-methylguanine-DNA methyltransferase (MGMT) via interaction with Sp1 transcription factor. Anticancer Res 2009;29:3741–50. [PMC free article] [PubMed] [Google Scholar]
- [36].Cao VT, Jung TY, Jung S, et al. The correlation and prognostic significance of MGMT promoter methylation and MGMT protein in glioblastomas. Neurosurgery 2009;65:866–75. discussion 75. [DOI] [PubMed] [Google Scholar]
- [37].Hegi ME, Diserens AC, Godard S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res 2004;10:1871–4. [DOI] [PubMed] [Google Scholar]
- [38].Cahill DP, Levine KK, Betensky RA, et al. Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res 2007;13:2038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bode H, Seiz M, Lammert A, et al. SOM230 (pasireotide) and temozolomide achieve sustained control of tumour progression and ACTH secretion in pituitary carcinoma with widespread metastases. Exp Clin Endocrinol Diabetes 2010;118:760–3. [DOI] [PubMed] [Google Scholar]
- [40].Vieira Neto L, Chimelli L, Pereira PJ, et al. The role of temozolomide in the treatment of a patient with a pure silent pituitary somatotroph carcinoma. Endocr Pract 2013;19:e145–9. [DOI] [PubMed] [Google Scholar]
- [41].Ortiz LD, Syro LV, Scheithauer BW, et al. Anti-VEGF therapy in pituitary carcinoma. Pituitary 2012;15:445–9. [DOI] [PubMed] [Google Scholar]


